Key Clinical Message
Primary gastric lymphoma is a rare malignant tumor that can sometimes present as spontaneous perforation. We present below a case of spontaneous primary gastric lymphoma perforation that was managed in our institution followed by a brief review of the literature and discussion.
Keywords: Gastric perforation, malignant ulcer management, primary gastric lymphoma
Introduction
Primary gastric lymphoma is a rare malignant tumor. It occurs most commonly in the stomach when it hits the gastrointestinal system 1. Whether low‐grade or high‐grade disease is present, the treatment of this tumor varies according to the type, location, and patient's presentation. In some instances, it might present as spontaneous perforation of the stomach or perforation related to chemotherapy 2, 3. At this point, surgical intervention is warranted in most cases. We present below a case of spontaneous primary gastric lymphoma perforation that was managed in our institution followed by a brief review of the literature and discussion.
Case Report
This is the case of an 80‐year‐old male patient who is presenting for abdominal pain. History goes back to the same day of presentation, diffuse, postprandial, and crampy in nature, associated with vomiting of food content as well as fever of 39°C and chills. He reports no hematochezia, melena, weight changes, or night sweats. Furthermore, there is no documented history of Helicobacter pylori infection, dyspepsia, or previous PPI treatment. No endoscopy, gastric biopsy, or pH‐metry were performed beforehand.
His past surgical history is positive for perforated gastric ulcer, postsurgical repair, and coronary artery bypass grafting, both done 10 years prior to presentation. He has a past medical history of coronary artery disease, hypertension, dyslipidemia, benign prostatic hyperplasia, and paroxysmal atrial fibrillation. The patient has no reported allergies, and his family history is noncontributory.
On admission, he was lethargic, febrile (38.5°C, rectal), and tachycardic (HR 110 bpm). Physical examination of the abdomen showed rigidity, diffuse tenderness to palpation, and rebound tenderness.
Laboratory showed the following: white blood cell count 10.2/mm3, neutrophils 73%, C‐reactive protein 7.37 U, HCO3 ‐ 17.5, prothrombin time international normalized ratio 1.97 IU.
Abdominal‐pelvic computed tomography scan was performed showing evidence of perforated gastric ulcer on the anterior antral wall of the stomach (Figs 1 and 2).
Figure 1.
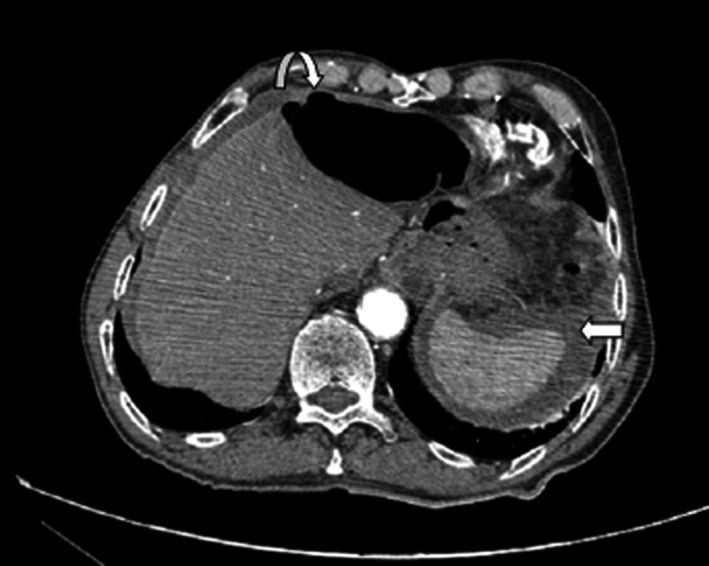
Axial view of abdomen CT scan with contrast showing perforation of the anterior antral wall of the stomach (curved arrow) as well as fluid in the perisplenic space (straight arrow).
Figure 2.
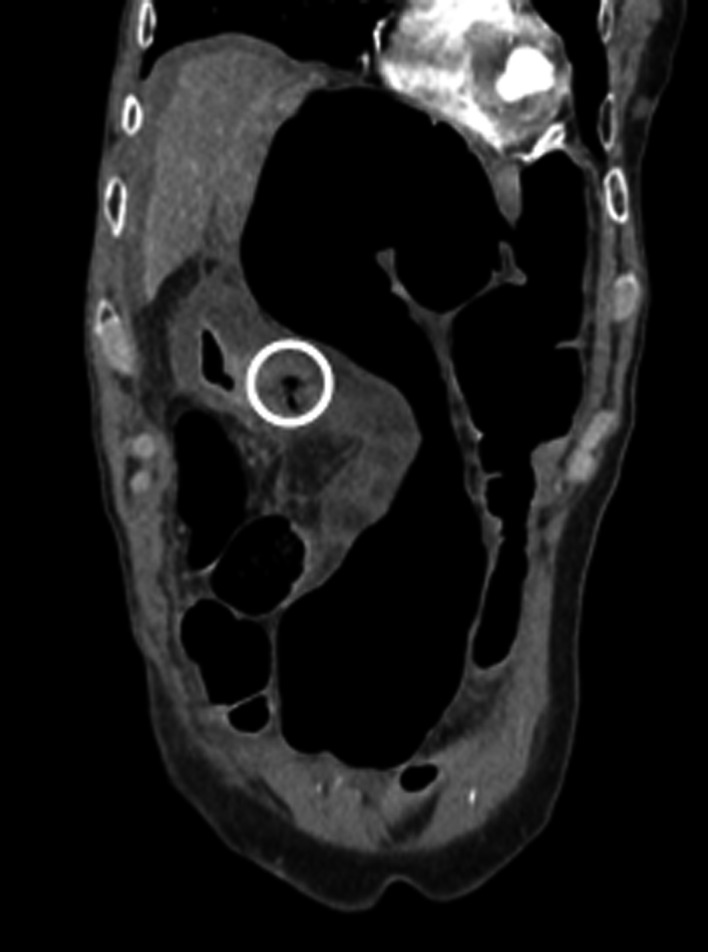
Sagittal view of abdomen CT scan showing the perforation in the antral anterior wall.
Patient was started on antibiotics and antifungals (ampicillin, ciprofloxacin, metronidazole, fluconazole). He was also given two fresh frozen plasma units and vitamin K, and urgent laparotomy was decided (Figs 3 and 4).
Figure 3.
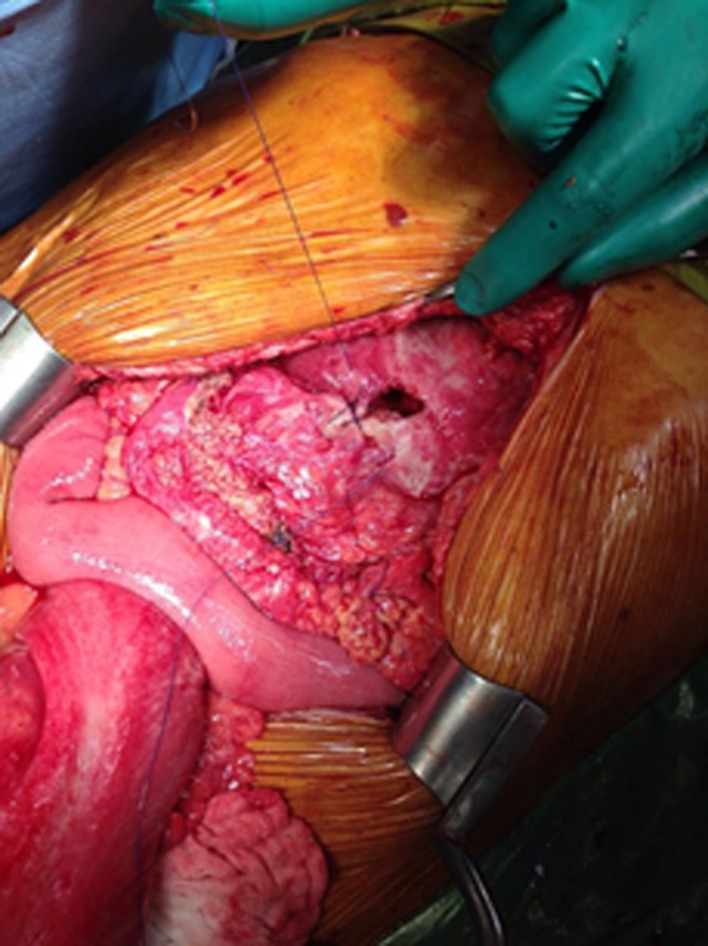
2 × 1 cm perforation at the anterior antral wall being repaired while awaiting the frozen result.
Figure 4.
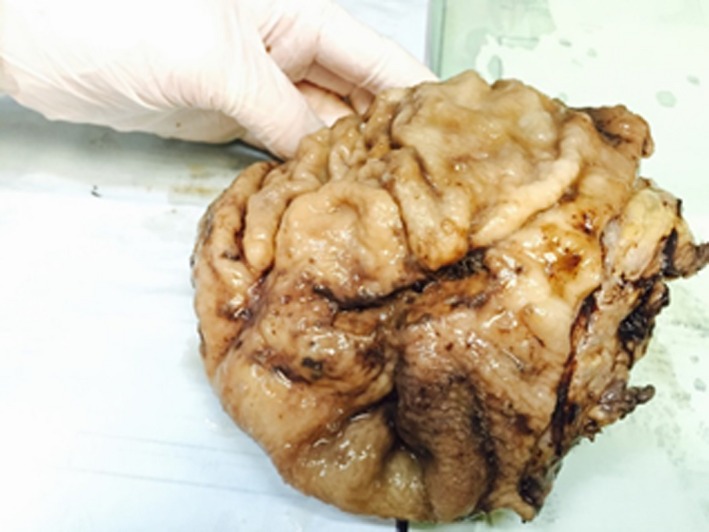
Gastrectomy specimen, gross view.
Midline laparotomy was performed. An extensively distended colon was encountered with the sigmoid adherent to the stomach at the level of the perforation. Abdominal fluid culture taken and irrigation was carried out. Liberation of the sigmoid colon and identification of 2 × 1 cm gastric perforation at the level of the antrum anteriorly were made. The relatively big perforation encountered, the fact that the patient was 80 years old and the relative thickness in the gastric mucosa was intriguing to us. Therefore, ulcer edges excised and sent to frozen section. Meanwhile closure of the ulcer was made using polyglactin 910, absorbable, synthetic, braided 2‐0 suture, and graham patch placed.
Frozen result came back as lymphoma, and the oncology and anesthesia teams were consulted on the spot. The best choice regarding the patient's condition, versus his clinical stability, was the main issue of debate. At this point, decision was taken to proceed for a subtotal gastrectomy. Gastrectomy was performed, and latero‐lateral gastrojejunal anastomosis was performed. Rectal tube was inserted, and decompression of the colon was performed. Four drains were placed, and the abdomen was closed.
The patient was then transferred to the intensive care unit where his condition was stabilized. The nasogastric tube was removed. On day 5 post‐op, an upper GI series was performed, showing no anastomotic leak, no stenosis, and feeding was initiated.
Final pathology revealed as follows: lymphocytes positive for CD20, BC12.
Diagnosis: Gastric B‐cell lymphoma of MALT, low grade with:
Free surgical margin
Measuring 5 × 9 × 1.5 cm
Tumor invades submucosa reaching muscular layer
Free serosa
Absence of Helicobacter pylori
Moderate chronic active gastritis in non‐neoplastic gastric mucosa
Free omentum
No evidence of invasion in 10 lymph nodes (epigastric)
Absence of Helicobacter pylori‐like organisms
Patient was then referred to the oncology team for follow‐up.
Discussion
In a time 40% of all non‐Hodgkin lymphomas (NHL) occur in extranodal location in the body, and the predominant site is the gastrointestinal tract 4, it constitutes merely 1–5% of this system's malignancies 5, 6. The stomach in particular is the most common affected organ 1. In 40% of the cases, it presents as low‐grade mucosa‐associated lymphoid tissue (MALT), and in 60% as high‐grade diffuse large B‐cell lymphoma (DLBCL) 7, 8, 9. It usually presents as nonspecific abdominal pain, dyspepsia, heartburn, and B symptoms being uncommon in this case, and the diagnosis is delayed 10.
Nowadays, the treatment of primary gastric lymphoma has shifted away from surgery, toward chemotherapy regimens. In a time surgery is now limited to cases of perforation, hemorrhage, or obstruction due to the tumor, it is no longer the cornerstone of treatment with its mortality rate reaching up to 8% 11, 12. As shown in Table 1, chemotherapy has surpassed surgery in terms of benefits and complications 13, 14, 15, 16, 17, 18. Ohkura et al. summarized 15 cases requiring gastrectomy between 1985 and 2013 in Japan 19. They concluded that the mean tumor size was 9.13 cm, and the mean diameter of perforation was 1.45 cm. Our patient had a tumor of 9 cm and a diameter of 2 × 1 cm. Gastric lymphoma perforation, associated with chemotherapy, occurs mainly due to rapid tumor response, necrosis, lysis, and exuberant granulation 2. Spontaneous perforation in the context of lymphoma without chemotherapy occurs due to tumor necrosis reaching the subserosa, with or without a concomitant ulcer 3.
Table 1.
Surgery versus chemotherapy complications in the treatment of primary gastric lymphoma
| Treatment modality | Complications |
|---|---|
| Gastrectomy | 38% weight loss |
| 17% malabsorption syndromes | |
| 13% dumping syndrome | |
| Chemotherapy | 5% gastric perforation and gastrointestinal hemorrhage |
The most important step in the management of gastric perforation is the intraoperative decision making. Ergul et al. stated some cues that would ensue a high clinical suspicion for malignancy, such as an ulcer >6 cm, advanced patient's age, perforation >0.5 cm, and a high white cell count 20. Our patient's condition clearly met several of the abovementioned criteria that would raise one's clinical suspicion to malignant processes: perforation >0.5 cm, advanced age (80 years), high WBC (10.2 × 103). Thus, the intraoperative decision was to send a frozen section of the margins and consult the oncology team on the spot.
In the context of hemodynamic instability but high clinical suspicion for the presence of malignancy, the surgeon would be facing a double jeopardy, whether to undergo a primary repair with Graham patch or plug, versus a subtotal or total gastrectomy. However, a review of the literature and our center's experience demonstrate that an oncological approach is more appropriate. The risk of recurrent tumor perforation, whether spontaneous or in response to chemotherapy, along with the associated high mortality, makes it crucial to undergo a radical treatment, with an oncologic approach. At this point, it is important for the operating surgeon to be experienced in the field of oncological and upper GI surgery, so that the ideal operative management can be achieved in a time‐efficient manner.
Conflict of Interest
The authors declare no potential conflict of interest.
References
- 1. Lewin, K. J. , Ranchod M., and Dorfman R. F.. 1978. Lymphomas of the gastrointestinal tract. A study of 117 cases presenting with gastrointestinal disease. Cancer 42:693–707. [DOI] [PubMed] [Google Scholar]
- 2. Ono, K. , Matsumura S., Sakamoto K., Kobayashi S., Kamano T., and Iwasaki R.. 1997. [A case of gastric malignant lymphoma with perforation during chemotherapy]. Gan To Kagaku Ryoho. 24:105–108. [PubMed] [Google Scholar]
- 3. Shiomi, H. , Watanabe E., Umeda T., Haeuchi K., Sugimura Y., Okamoto Y., et al. 1997. A case report of perforated gastric malignant lymphoma. Jpn J. Canc. Clin. 43:25–28. [Google Scholar]
- 4. Paryani, S. , Hoppe R. T., Burke J. S., Sneed P., Dawley D., Cox R. S., et al. 1983. Extralymphatic involvement in diffuse non‐Hodgkin's lymphoma. J. Clin. Oncol. 1:682–688. [DOI] [PubMed] [Google Scholar]
- 5. Dawson, I. M. P. , Cornes J. S., and Morson B. C.. 1961. Primary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br. J. Surg. 49:80–89. [DOI] [PubMed] [Google Scholar]
- 6. Ghai, S. , Pattison J., Ghai S., O'Malley M. E., Khalili K., and Stephens M.. 2007. Primary gastrointestinal lymphoma: spectrum of imaging findings with pathologic correlation 1. Radiographics 27:1371–1388. [DOI] [PubMed] [Google Scholar]
- 7. Sano, R. 1987. Classification of malignant gastric lymphoma. Pp. 257–275. Igakushoin, Tokyo. [Google Scholar]
- 8. Koch, P. , del Valle F., Berdel W. E., Willich N. A., Reers B., Hiddemann W., et al. 2001. Primary gastrointestinal non‐Hodgkin's lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J. Clin. Oncol. 19:3861–3873. [DOI] [PubMed] [Google Scholar]
- 9. Koch, P. , Probst A., Berdel W. E., Willich N. A., Reinartz G., Brockmann J., et al. 2005. Treatment results in localized primary gastric lymphoma: data of patients registered within the German multicenter study (GIT NHL 02/96). J. Clin. Oncol. 23:7050–7059. [DOI] [PubMed] [Google Scholar]
- 10. Brooks, J. J. , and Enterline H. T.. 1983. Primary gastric lymphomas: a clinicopathologic study of 58 cases with long‐term follow‐up and literature review. Cancer 51:701–711. [DOI] [PubMed] [Google Scholar]
- 11. Al‐Akwaa, A. M. , Siddiqui N., and Al‐Mofleh I. A.. 2004. Primary gastric lymphoma. World J. Gastroenterol. 10:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roukos, D. H. , Hottenrott C., Encke A., Baltogiannis G., and Casioumis D.. 1994. Primary gastric lymphomas: a clinicopathologic study with literature review. Surg. Oncol. 3:115–125. [DOI] [PubMed] [Google Scholar]
- 13. Avilés, A. , Díaz‐Maqueo J. C., de la Torre A., Rodriguez L., Guzmán R., Talavera A., et al. 1991. Is surgery necessary in the treatment of primary gastric non‐Hodgkin lymphoma? Leuk. Lymphoma 5:365–369. [DOI] [PubMed] [Google Scholar]
- 14. Avilés, A. , Nambo M. J., Neri N., Huerta‐Guzmán J., Cuadra I., Alvarado I., et al. 2004. The role of surgery in primary gastric lymphoma: results of a controlled clinical trial. Ann. Surg. 240:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maisey, N. , Norman A., Prior Y., and Cunningham D.. 2004. Chemotherapy for primary gastric lymphoma: does in‐patient observation prevent complications? Clin. Oncol. (R. Coll. Radiol.) 16:48–52. [DOI] [PubMed] [Google Scholar]
- 16. Sharma, S. , Singhal S., De S., Chander S., Rath G. K., Misra A., et al. 1990. Primary gastric lymphoma: a prospective analysis of 12 cases and review of the literature. J. Surg. Oncol. 43:231–238. [DOI] [PubMed] [Google Scholar]
- 17. Fleming, I. D. , Mitchell S., and Dilawari R. A.. 1982. The role of surgery in the management of gastric lymphoma. Cancer 49:1135–1141. [DOI] [PubMed] [Google Scholar]
- 18. Liu, H. T. , Hsu C., Chen C. L., Chiang I. P., Chen L. T., Chen Y. C., et al. 2000. Chemotherapy alone versus surgery followed by chemotherapy for stage I/IIE large‐cell lymphoma of the stomach. Am. J. Hematol. 64:175–179. [DOI] [PubMed] [Google Scholar]
- 19. Ohkura, Y. , Lee S., Kaji D., Ota Y., Haruta S., Takeji Y., et al. 2015. Spontaneous perforation of primary gastric malignant lymphoma: a case report and review of the literature. World J. Surg. Oncol. 13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ergul, E. , and Gozetlik E. O.. 2009. Emergency spontaneous gastric perforations: ulcus versus cancer. Langenbecks Arch. Surg. 394:643–646. [DOI] [PubMed] [Google Scholar]


