Key Clinical Message
Orbital inflammatory pseudotumor is a rare complication of systemic lupus erythematosus. It may present a challenge for differential diagnosis, especially in the context of treatment with hydroxychloroquine, although dosage and duration of the treatment may guide us. Although high antibody titers can be found, this is not specific.
Keywords: Optic neuritis, optic perineuritis, orbital myositis, orbital pseudotumor, systemic lupus erythematosus
Case
A 49‐year‐old woman suspected of having a right optic neuritis was referred to the Neurology Department. She had been diagnosed with systemic lupus erythematosus (SLE) 10 years earlier, and, at the time of presentation, she was receiving azathioprine 50 mg three times a day, prednisone 5 mg daily, and belimumab 640 milligrams intravenously every 4 weeks.
Four months earlier, during an ophthalmologist screening test for retinal toxicity, an optic coherence tomography (OCT) had shown a reduction in the thickness of the macular and retinal nerve fiber layer in the right eye. She was recommended to stop treatment with hydroxychloroquine, which the patient had been receiving since the initial diagnosis of SLE 10 years before (200 mg orally per day). An MRI showed a high‐intensity signal in her right optic nerve at that moment (Fig. 1). Despite treatment withdrawal, follow‐up showed an inferior nasal scotoma in her right eye, not present in previous examinations, and she was referred to the Neurology Department.
Figure 1.
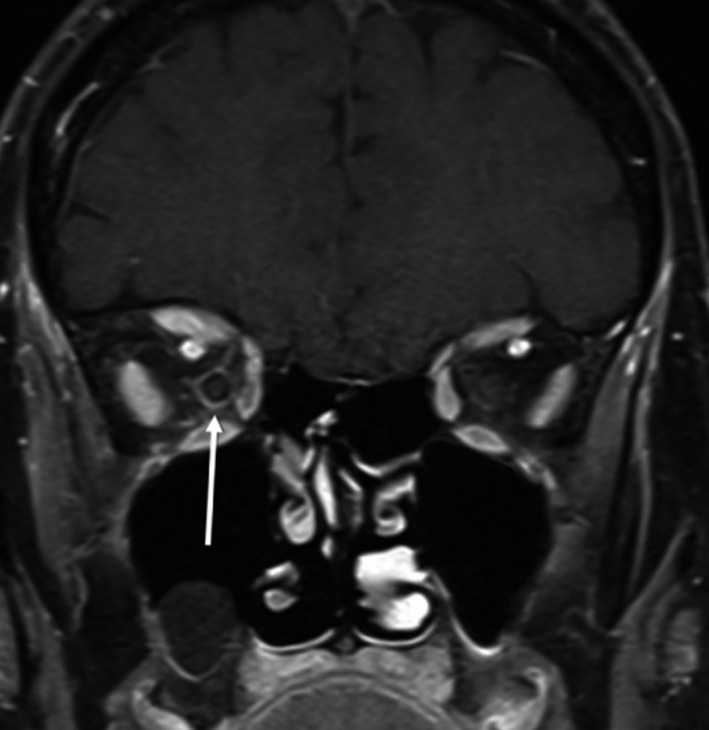
T1‐weighted MRI with gadolinium contrast. A perineural contrast enhancement can be seen on the right optic nerve, suggesting inflammation of the nerve sheath.
During the first visit to the Neurologist, physical examination did not reveal other pathological findings, so ancillary tests (visual evoked potentials and both orbital and cerebral MRI) were requested. Before the tests could be carried out, the patient developed vertical diplopia on the primary gaze position that was accentuated when looking up. A paralysis of the right eye in upgaze position was found on physical examination then.
MRI showed signs of inflammation in the right orbit with affected the orbital fat and conditioned a slight proptosis of the eyeball, not visible to the naked eye on physical examination. A mild posterior perineural signal could still be seen in the optic nerve, but also, both the right superior rectus and superior oblique muscles were thickened (Fig. 2B and C). Upon further examination, we found that these alterations in the muscles were already but subtly present in the first MRI, long before the diplopia appeared (Fig. 2A). Visual evoked potentials demonstrated prolonged latencies after visual stimulus, consistent with a moderate demyelinating neuropathy of the right eye. Extensive laboratory tests, including blood cell count, renal function, liver function, thyroid function, anti‐aquaporin‐4 antibodies, angiotensin‐converting enzyme levels, and serologic tests for syphilis, HIV, herpesvirus, and borrelia burgdoferi, were negative or normal. Anticardiolipin and antibeta‐2‐glycoprotein antibodies were also negative. Anti‐dsDNA levels were 167 UI/mL, and serum complement levels were low, with C3 0.76 g/L and C4 0.07 g/L; these results were similar to the results of previous blood tests.
Figure 2.

T2‐weighted brain and orbital MRI. (A) Coronal view of the MRI performed nine months before the patient developed the symptoms. A slight thickening of the superior rectus, which appears hyperintense compared to the contralateral muscle, is patent, as well as mild infiltration of the surrounding fat. (B) Coronal view of the MRI performed after developing diplopia. These alterations can be observed more clearly. (C) Axial cuts of the same MRI showing also slight proptosis of the right eye.
Hydroxychloroquine‐related toxicity was ruled out due to both clinical and radiologic progression after suspension of the treatment. The patient was diagnosed with orbital inflammatory pseudotumor secondary to SLE, and high‐dose corticosteroid therapy was initiated (oral metilpredinsolone one gram daily for 5 days), followed by slowly decreasing doses of prednisone to 10 mg daily. This therapy achieved to improve the symptoms and diplopia resolved, without permanent damage to the visual field. Afterward, immunosuppressive treatment with rituximab was started. The patient has remained asymptomatic ever since, and both anti‐dsDNA and complement levels have returned to normal values.
Discussion
Ocular manifestations are fairly common in SLE, mostly due to keratoconjunctivitis sicca. Neuro‐ophthalmic manifestations, however, are less frequent, with a prevalence of 3.6% in adults; among these, optic neuritis is the most common form of presentation and also one of the most visually compromising complications of SLE 1.
The implication of orbital structures in SLE and, specifically, inflammatory pseudotumor is very rare and therefore may go unnoticed 1, 2, 3. It usually presents with proptosis, pain, and diplopia, due to infiltration of the ocular muscles. Although increases in the titers of anti‐dsDNA antibodies and decreases in the complement levels may accompany disease relapses, this is not true for all SLE patients 4, 5. In our case, although high levels of anti‐dsDNA and low levels of C3 and C4 were found, these results were similar to the results of previous blood tests. Visual loss due to inflammatory infiltration of the optic nerve is uncommon; however, it may be a preceding sign, as in this case. 2, 6. Response to corticosteroid therapy is excellent in most patients, but sometimes progression of the disease could lead to major damage 2. Other causes such as infections or additional autoimmune disease should be excluded first. Although SLE‐associated retinopathy has been associated with elevated levels of antiphospholipid antibodies 2, this does not seem to be the case in inflammatory pseudotumor, and due to its low incidence, no risk factors that could help early diagnosis have been identified.
Hydroxychloroquine‐related toxicity was suspected at first in this case. Retinopathy and maculopathy are widely known complications of hydroxychloroquine treatment, although inflammatory pseudotumor has never been described in this setting. Although visual loss may be irreversible and even worsen after suspending treatment with hydroxychloroquine, this is more likely if high doses of hydroxychloroquine are used for more than 5 years or if there is impaired renal or liver function 7, 8. Although our patient had been treated with hydroxychloroquine for 10 years, the daily doses were below 6, 5 mg/kg, which are associated with very low risk of retinopathy. Besides, she never presented impaired renal or liver function in successive blood tests. Taking all this into account, hydroxychloroquine did not seem to be the cause for the pseudotumor.
Suspecting inflammatory pseudotumor and its relation to the SLE is imperative to avoid further complications. In this case, the slow progression of the disorder, probably due to simultaneous immunosuppressive therapy and chronic treatment with low doses of oral corticosteroids, as well as the interference of confounding factors (treatment with hydroxychloroquine) delayed the diagnosis. However, when the patient developed diplopia a review of the previous MRI showed that mild signs had been already present months before the symptoms appeared. Therefore, after a new finding appears, going back to previous studies may help to put things into perspective.
Conflict of Interest
None declared.
References
- 1. Silpa‐Archa, S. , Lee J. J., and Foster C. S.. 2016. Ocular manifestations in systemic lupus erythematosus. Br. J. Ophthalmol. 100:135–141. [DOI] [PubMed] [Google Scholar]
- 2. Palejwala, N. V. , Walia H. S., and Yeh S.. 2012. Ocular manifestations of systemic lupus erythematosus: a review of the literature. Autoimmune Dis. 2012:290898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uhlig, T. , Kvien T. K., Jensen J. L., Axell T. 1999. Sicca symptoms, saliva and tear production, and disease variables in 636 patients with rheumatoid arthritis. Ann. Rheum. Dis. 58:415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kavanaugh, A. , Tomar R., Reveille J., Solomon D. H., Homburger H. A. 2000. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. American College of Pathologists. Archives of pathology & laboratory medicine 124:71–81. [DOI] [PubMed] [Google Scholar]
- 5. Chaudhry, I. A. , Al‐Obaisi S., Al‐Sheikh O., et al. 2012. Unilateral optic neuritis, scleritis and exudative retinal detachment due to recurrent orbital pseudotumor. Saudi J. Ophthalmol. 26:449–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lloyd, W. , and Schur P. H.. 1981. Immune complexes, complement, and anti‐DNA in exacerbations of systemic lupus erythematosus (SLE). Medicine (Baltimore) 60:208–217. [DOI] [PubMed] [Google Scholar]
- 7. Moschos, M. M. , Nitoda E., Chatziralli I. P., et al. 2015. Assessment of hydroxychloroquine maculopathy after cessation of treatment: an optical coherence tomography and multifocal electroretinography study. Drug Des. Devel. Ther. 9:2993–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hobbs, H. E. , Sorsby A., and Freedman A.. 1959. Retinopathy following chloroquine therapy. Lancet 2:478–480. [DOI] [PubMed] [Google Scholar]


