Abstract
Background
It is well known that HOX transcript antisense intergenic ribonucleic acid (HOTAIR) plays an important role in breast cancer (BC). However, whether circulating HOTAIR in plasma could be used for BC diagnosis and dynamic monitoring are unclear.
Methods
We tested the expression levels of HOTAIR in 30 pairs of tissue samples and 148 plasma samples from BC patients by quantitative real time‐polymerase chain reaction, and the correlation between plasma HOTAIR levels and clinical features were analyzed. Receiver operating characteristic curve (ROC) was used to assess the diagnostic power of plasma HOTAIR for BC. Furthermore, we explored the monitoring values of plasma HOTAIR for BC and analyzed the correlation of HOTAIR levels between plasma and corresponding tissues of the same patients.
Results
The expression levels of HOTAIR were significantly higher in BC tissues and plasma than in the control (P < 0.05). The expression levels of plasma HOTAIR were correlated with lymph node metastasis (P = 0.018), estrogen receptor (ER) (P = 0.012), c‐erbB‐2 (P = 0.006) and triple positive (P = 0.015). The area under the ROC curve of plasma HOTAIR was 0.80 (sensitivity 69.2%; specificity 93.3%), which was higher than the carcinoembryonic antigen and carbohydrate antigen 15‐3 values obtained. Moreover, plasma HOTAIR expression levels in postoperative patients were lower than those in preoperative patients (P = 0.029) and were moderately correlated with the corresponding tissue levels of the same patients (r = 0.68, P < 0.0001).
Conclusion
These results indicated that HOTAIR may be a potential biomarker for the diagnosis of BC.
Keywords: Biomarker, breast cancer, HOTAIR, plasma
Introduction
Breast cancer (BC) is the most common type of malignant tumor, is the leading cause of death, and has the highest incidence rate of new cancers in women.1 As there are no obvious symptoms in early stages, a large number of patients are diagnosed in middle and terminal stages, with poor prognosis and poor treatment effect. Therefore, early diagnosis and therapy are key measures to decrease BC mortality. Although some traditional markers are used for the early screening and prognostic monitoring of BC, such as cancer antigen (CA)15‐3 and carcinoembryonic antigen (CEA), their diagnostic value is limited because of lower sensitivity and specificity.2 Therefore, determining the ideal biomarker for BC is essential for early diagnosis and treatment.
Long non‐coding ribonucleic acids (lncRNAs) are RNAs longer than 200 nucleotides in length without the capability of encoding protein. There has been great interest in lncRNAs in the last few years as they play a crucial role in biological regulation and disease occurrence.3, 4 HOX transcript antisense intergenic RNA (HOTAIR) is a polyadenylated RNA with 2158 nucleotides and six exons.
It has been demonstrated that HOTAIR can promote chromatin relocalization through Polycomb‐repressive complex 2 (PRC2), contributing to the carcinogenesis of BC.5 According to a preliminary study, HOTAIR expression is significantly increased in BC tissue. A high level of HOTAIR promotes cell invasion and metastasis of BC, which seems to indicate a poor prognosis.6 In addition, HOTAIR could upregulate calcium‐binding protein (CaBP) S100A4 and couple estrogen receptor1 (GPER1) by interacting with micro RNA (miR)‐568 and miR‐148a to accelerate tumor progression.7, 8 Recently, a study found that serum HOTAIR was significantly increased in BC patients, which could be regarded as a potential diagnostic marker of early stage.9 However, the detection HOTAIR expression in plasma or serum as a biomarker for diagnosis and dynamic monitoring is infrequent.
In the current study, we detected the expression of HOTAIR in the plasma of BC patients and examined its association with clinical features to determine its proficiency as a biomarker for BC.
Methods
Patients and samples
In this study, we employed BC patients and age and gender matched healthy volunteers at the Affiliated Hospital of Southwest Medical University from January 2014 to September 2015. Thirty paired tissue samples (tumor tissue and matched adjacent non‐tumorous tissue) were obtained from surgeries. Blood samples were collected from BC patients and healthy volunteers, including 88 preoperative BC (including 79 infiltrating ductal carcinoma, 7 ductal carcinoma, and 2 mucinous carcinoma), 100 healthy, and 30 paired blood samples (preoperative and postoperative blood in 7 days after surgery). None of the patients had received any clinical treatment before sample collection and each specimen was confirmed as BC by histopathological examination. The age and gender matched healthy volunteers had not been exposed to any disease or injury. The Ethical Review Committee of the Affiliated Hospital of Southwest Medical University approved the study protocol and all of the participants provided informed consent. The tumor and corresponding non‐cancerous tissues were immediately soaked in liquid nitrogen and then stored at −80°C. Peripheral blood samples were collected in ethylenediaminetetraacetic acid‐2K vacutainer tubes and centrifuged at 1500 g for 10 minutes. The supernatant was then transferred into RNAse free tubes and stored at −80°C.
Ribonucleic acid (RNA) extraction and quantitative real‐time polymerase chain reaction
Total RNA from tissues (50 mg) was isolated by Trizol Reagent (TaKaRa, Tokyo, Japan) and plasma samples (200 μL) were isolated using the Blood Total RNA Rapid Extraction Kit (BioTeke, China). Total RNAs were then reverse transcribed using a PrimeScriptRT Reagent Kit with gDNA Eraser (TaKaRa) following the manufacturer's protocol. HOTAIR expression was measured by real time‐polymerase chain reaction (RT‐PCR) using SYBR Green assays (Takara) in a 7500 Fast Real‐Time PCR System (Applied Biosystems, Foster City, CA, USA). β‐actin was used to normalize the relative levels of lncRNA. Primers were designed with Primer 5, synthesized by Sangon Biotech (Shanghai, China), and sequenced as follows: 5′‐GGTAGAAAAAGCAACCACGAAGC‐3′(forward) and 5′‐GCACGAAGGCTCA TCATTCA‐3′ (reverse) for HOTAIR;5′‐TCCTCTCCCAAGCCACACA‐3′ (forward) and 5′‐GCACGAAGGCTCATCATTCA‐3′ (reverse) for β‐actin. The relative expression was calculated using 2 − ΔΔCt methods.10
Detection of serological tumor marker
The levels of CA15‐3 and CEA were measured using electro‐chemiluminescence immunoassay in Roche cobas e 601 (Roche, Basel, Switzerland).
Statistical analysis
Data were compared by Mann–Whitney U test to distinguish significant differences between the two groups. A receiver operating characteristic (ROC) curve was established to evaluate the diagnostic value for discriminating between BC patients and healthy controls. All statistical analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA) and P < 0.05 was considered statistically significant.
Results
Expression of HOX transcript antisense intergenic RNA (HOTAIR) in breast cancer (BC) plasma
We performed quantitative RT‐PCR to analyze 30 pairs of tissues to validate HOTAIR expression in BC tissues. The levels of HOTAIR expression in tumor tissues were higher than in adjacent non‐cancerous tissues (Fig 1, P = 0.002). Further, we tested HOTAIR expression levels in plasma samples from 88 BC patients and 100 healthy controls and found that levels were significantly increased in BC samples (Fig 2, P < 0.0001).
Figure 1.
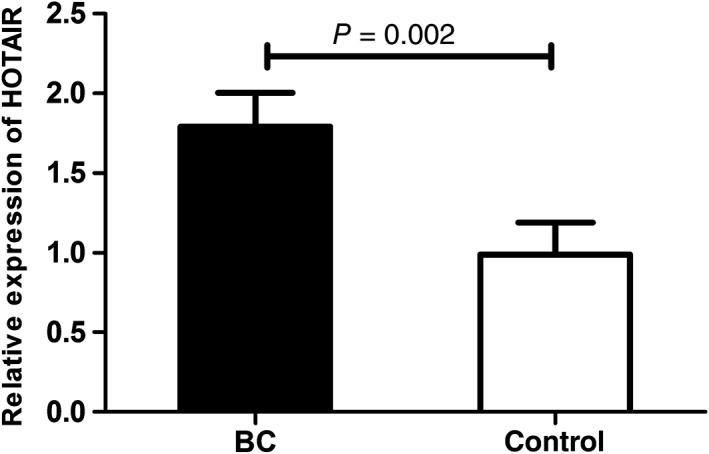
Relative expression of HOX transcript antisense intergenic ribonucleic acid (HOTAIR) in in tumor tissues and adjacent non‐cancerous tissues. BC, breast cancer.
Figure 2.
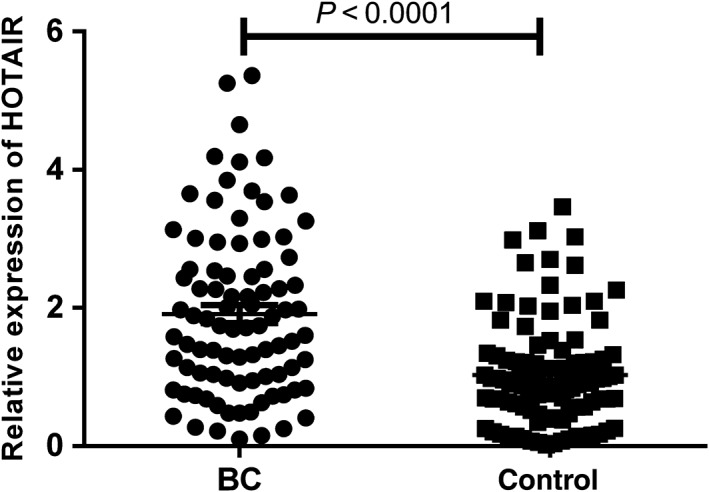
Relative expression of HOX transcript antisense intergenic ribonucleic acid (HOTAIR) in plasma of breast cancer (BC) patients and normal controls.
Correlation between HOTAIR expression in plasma and clinical features in BC patients
To evaluate the association of HOTAIR expression in plasma with BC development, we analyzed its relationship with clinical features. As shown in Table 1, HOTAIR expression significantly correlated with lymph node metastasis (P = 0.018), estrogen receptor (ER; P = 0.012), c‐erbB‐2 (P = 0.006) and triple positive (P = 0.015). However, there was no marked difference in statistics between HOTAIR levels and clinical characteristics in tumor node metastasis stage, age or Ki‐67.
Table 1.
Plasma HOTAIR and clinical characteristic correlations in breast cancer patients
| Characteristics | Patients (n = 88) | HOTAIR levels† (mean ± SEM) | P |
|---|---|---|---|
| Age | |||
| <50 | 51 | 1.79 ± 0.15 | 0.276 |
| ≥50 | 37 | 2.08 ± 0.23 | |
| TNM stage | |||
| I–II | 65 | 1.82 ± 0.15 | 0.231 |
| III–IV | 23 | 2.17 ± 0.25 | |
| Lymphatic metastasis | |||
| Metastasis | 50 | 2.18 ± 0.18 | 0.018 |
| No metastasis | 38 | 1.56 ± 0.17 | |
| ER | |||
| + | 59 | 2.10 ± 0.15 | 0.012 |
| − | 29 | 1.52 ± 0.23 | |
| PR | |||
| + | 50 | 1.95 ± 0.17 | 0.643 |
| − | 38 | 1.86 ± 0.21 | |
| c‐erbB‐2 | |||
| + | 68 | 2.10 ± 0.15 | 0.006 |
| − | 20 | 1.29 ± 0.22 | |
| Ki67 | |||
| ≤10% | 21 | 2.08 ± 0.30 | 0.641 |
| >10% | 67 | 1.86 ± 0.14 | |
| ER, PR and c‐erbB‐2 | |||
| + | 43 | 2.12 ± 0.26 | 0.015 |
| − | 28 | 1.59 ± 0.31 | |
HOX transcript antisense intergenic ribonucleic acid (HOTAIR) level refers to HOTAIR relative expression, calculated using 2 − ΔΔCt method. ER, estrogen receptor; PR, progesterone receptor; SEM, standard error of the mean; TNM, tumor node metastasis.
Evaluation of the diagnostic value of plasma HOTAIR as a potential biomarker for BC
In order to evaluate HOTAIR in plasma as a potential diagnostic biomarker for BC, an ROC curve was performed to evaluate the diagnostic value of plasma CEA, CA15‐3, and HOTAIR from 88 patients with BC and 100 controls. The results demonstrated that the diagnostic power of HOTAIR (area under the ROC curve [AUC] = 0.80; sensitivity 69.2%; specificity 93.3%) is higher than CEA (AUC = 0.50; sensitivity 65.4%; specificity 50.0%) and CA15‐3 (AUC = 0.65; sensitivity 73.1%; specificity 60.0%), which indicated that HOTAIR had the strongest ability to distinguish BC from healthy individuals. Moreover, combined detection of the three plasma indexes could enhance the diagnostic power (AUC = 0.82; sensitivity 73.1%; specificity 90.0%), as shown in Fig 3 and Table 2.
Figure 3.
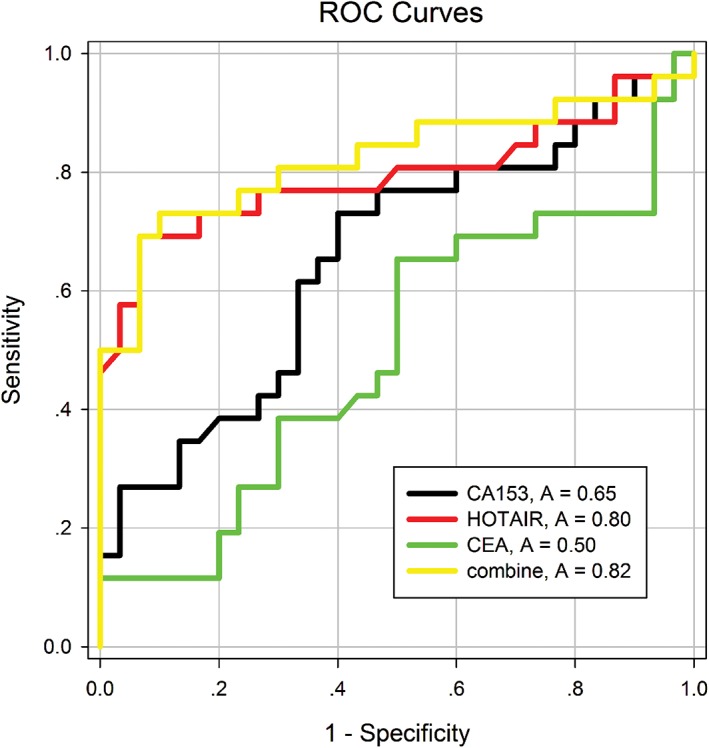
Receiver operating characteristic curves compare the diagnostic power of HOX transcript antisense intergenic ribonucleic acid (HOTAIR), cancer antigen (CA)153, carcinoembryonic antigen (CEA) and a combination of the three indexes to discriminate breast cancer patients from healthy controls.
Table 2.
HOTAIR, CA15‐3, CEA, and combined index sensitivity and specificity for breast cancer diagnosis
| Index | Sensitivity | 95% CI | Specificity | 95% CI |
|---|---|---|---|---|
| HOTAIR | 0.692 | 0.482–0.857 | 0.933 | 0.779–0.992 |
| CA15‐3 | 0.731 | 0.522–0.884 | 0.600 | 0.406–0.773 |
| CEA | 0.654 | 0.443–0.828 | 0.500 | 0.313–0.687 |
| Combination | 0.731 | 0.522–0.884 | 0.900 | 0.735–0.979 |
CA15, cancer antigen; CEA, carcinoembryonic antigen; CI, confidence interval; HOTAIR, HOX transcript antisense intergenic ribonucleic acid.
Assessment of HOTAIR value for monitoring tumor dynamics in BC patients
The expression levels of HOTAIR were analyzed in 30 paired preoperative and postoperative plasma samples from BC patients. HOTAIR expression levels were remarkably reduced in the postoperative samples (P = 0.029; Fig 4). Moreover, we conducted Pearson correlation analysis to explore the correlation between levels of HOTAIR expression in plasma and in the corresponding tissues of the same patients. A moderate correlation was observed for HOTAIR (r = 0.68; p < 0.0001; Fig 5).
Figure 4.
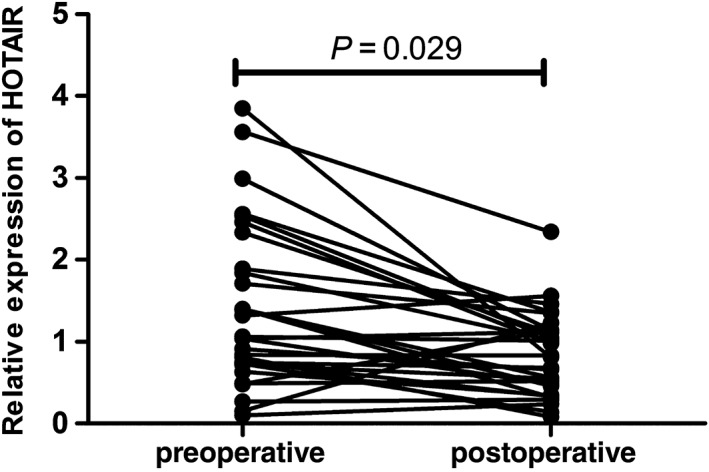
Relative expression levels of plasma HOX transcript antisense intergenic ribonucleic acid (HOTAIR) between postoperative and preoperative samples.
Figure 5.
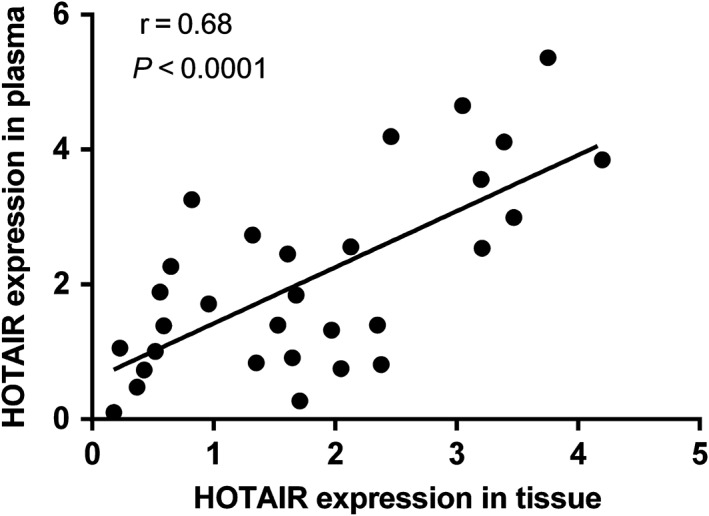
Spearman's rank correlation scatter plot of HOX transcript antisense intergenic ribonucleic acid (HOTAIR) levels in tumor tissues and plasma.
Discussion
Breast cancer has the highest incidence and morbidity of all female cancers in China.11 However, there is a lack of non‐invasive and early indicators for BC screening. Increasing studies have revealed that circulating nucleic acid (CNA) extensively exists in human fluid, such as blood, urine, and cerebrospinal fluid, and detection of CNA in plasma or serum may act as a biomarker for diagnosis and dynamic monitoring for cancer, including BC.12, 13, 14 Numerous studies have focused on miRNAs as potential tumor biomarkers for cancer diagnosis and prognosis prediction.15, 16, 17 However, few studies have investigated the diagnostic value of plasma lncRNAs in BC and, particularly, the value of tumor monitoring has never been studied.
HOX transcript antisense intergenic RNA, first identified in 2007, is transcribed from the HOXC locus but represses expression of the distal HOXD locus and genes on other chromosomes.5 It is one of the first lncRNAs reported to be associated with cancer.18 Increasing evidence indicates that HOTAIR is overexpressed in several types of malignancies, including liver, gastric, and breast cancers.19, 20, 21 A high level of HOTAIR expression in primary breast tumors is a prospective predictor of subsequent metastasis. In this study, we found that the expression levels of HOTAIR, not only in tissues but also in plasma, were obviously higher than those in controls. High expression levels of HOTAIR in patients with BC were associated with lymph node metastasis (P = 0.018), ER (P = 0.012), and c‐erbB‐2 (P = 0.006).
It is well known that the ER expression is an important component of the occurrence and development of BC.22 ER levels greatly affect clinical efficacy and even often lead to the failure of clinical BC treatment.23 HOTAIR promoter contains multiple functional estrogen response elements (EREs). Studies have demonstrated that ER, along with ER regulators, could bind to the promoter of HOTAIR to enhance HOTAIR expression in cells.24 Previous studies have suggested that HOTAIR is upregulated in ER‐positive BC and could drive an estrogen‐independent ER transcriptional program.25 Another study revealed that HOTAIR is an independent prognostic marker of metastasis in primary ER‐positive BC patients.26 In our study, the expression level of plasma HOTAIR in ER‐negative patients was far lower than in ER‐positive patients. This result confirmed the relationship between HOTAIR expression and ER‐positivity. Conversely, Gökmen‐Polar et al. found that the prognostic role of HOTAIR expression is restricted to ER‐negative BC.27 Thus, further research to explore the relationship between ER and HOTAIR must be conducted.
Tumor metastasis is the main characteristic of malignant tumors and is a significant cause of death in most cancer patients. It has been demonstrated that high levels of HOTAIR tissue promote BC metastasis, which indicates poor prognosis.6 C‐erbB‐2 (Her‐2/neu) is a member of the epidermal growth factor family; C‐erbB‐2 (Her‐2/neu) overexpression in BC has been shown to correlate with a poor prognosis. C‐erbB‐2 is amplified multiple times in 30% of human BC and its concentrations are related with disease behavior.28, 29 Thor et al. found that 12% of c‐cerbB‐2‐positive patients with BC were associated with more aggressive tumors and worse lymph node metastasis.30 Our results revealed that plasma HOTAIR was associated with lymph node metastasis and c‐cerbB‐2 status; thus, it may be a potential marker for lymph node metastasis.
We also assessed the ability of HOTAIR, CEA, and CA15‐3 to distinguish BC from normal individuals in 88 patients that were newly diagnosed and 100 healthy donors. Our results demonstrated that HOTAIR provided the highest diagnostic capability for detection of BC (AUC = 0.80; sensitivity 69.2%; specificity 93.3%), which suggested that plasma HOTAIR could serve as a promising marker for BC detection. The use of the three plasma biomarkers in combination could improve differential diagnosis between BC patients and healthy controls compared with the use of HOTAIR alone (AUC = 0.82; sensitivity 73.1%; specificity 90.0%). The combined application of various detecting indexes may increase the BC detection rate.
Circulating nucleic acids are believed to result from apoptosis, and necrotic tumor cells from the tumor microenvironment release nucleic acids into the blood; circulating HOTAIR in the plasma of BC patients is unknown.31, 32 Zhou et al. showed that lncRNA H19 expression in plasma was overexpressed in preoperative compared with postoperative gastric cancer patients and found a correlation between H19 expression levels in tissue and plasma.33 Similarly, we explored 30 paired preoperative and postoperative plasma samples and analyzed the correlation between HOTAIR expression in tumor tissue and in plasma. Our results determined that concentrations of plasma HOTAIR were significantly reduced in postoperative patients. There was a medium correlation between tissue and plasma HOTAIR levels. These findings demonstrate that HOTAIR expression in plasma may be a biomarker for monitoring tumor status.
In summary, our results showed that HOTAIR expression was significantly upregulated in both BC tissues and plasma. Furthermore, increased expression was associated with ER, c‐erbB‐2, and lymph node metastasis. The diagnostic capability of HOTAIR for BC detection is the most powerful among the three markers. At present, invasive biopsies, in which specimens are difficult to obtain and may induce the spread of cancer cells, are performed to diagnose BC. By contrast, the detection of plasma HOTAIR expression is a non‐invasive procedure that has high sensitivity and specificity and also correlates with BC oncogenesis and metastasis. Our results indicate that HOTAIR may serve as a potential biomarker for BC.
Disclosure
No authors report any conflict of interest.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Duffy MJ, Evoy D, McDermott EW. CA 15‐3: Uses and limitation as a biomarker for breast cancer. Clin Chim Acta 2010; 411: 1869–74. [DOI] [PubMed] [Google Scholar]
- 3. Guttman M, Donaghey J, Carey BW et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature 2011; 477: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tripathi V, Shen Z, Chakraborty A et al. Long noncoding RNA MALAT1 controls cell cycle progression by regulating the expression of oncogenic transcription factor B‐MYB. PLoS Genet 2013; 9(3): e1003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rinn JL, Kertesz M, Wang JK et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 2007; 129: 1311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gupta RA, Shah N, Wang KC et al. Long non‐coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010; 464: 1071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li JT, Wang LF, Zhao YL et al. Nuclear factor of activated T cells 5 maintained by Hotair suppression of miR‐568 upregulates S100 calcium binding protein A4 to promote breast cancer metastasis. Breast Cancer Res 2014; 16: 454. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8. Tao S, He H, Chen Q et al. Estradiol induces HOTAIR levels via GPER‐mediated miR‐148a inhibition in breast cancer. J Transl Med 2015; 13: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang L, Song X, Wang X et al. Circulating DNA of HOTAIR in serum is a novel biomarker for breast cancer. Breast Cancer Res Treat 2015; 152: 199–208. [DOI] [PubMed] [Google Scholar]
- 10. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2 − ΔΔCT method. Methods 2001; 25: 402–8. [DOI] [PubMed] [Google Scholar]
- 11. Chen W, Zheng R, Baade PD et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66 (2): 115–32. [DOI] [PubMed] [Google Scholar]
- 12. Dawson SJ, Tsui DW, Murtaza M et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med 2013; 368: 1199–209. [DOI] [PubMed] [Google Scholar]
- 13. De Leeneer K, Claes K. Non coding RNA molecules as potential biomarkers in breast cancer. Adv Exp Med Biol 2015; 867: 263–75. [DOI] [PubMed] [Google Scholar]
- 14. Schwarzenbach H, Hoon DS, Pantel K. Cell‐free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 2011; 11: 426–37. [DOI] [PubMed] [Google Scholar]
- 15. Bertoli G, Cava C, Castiglioni I. MicroRNAs: New biomarkers for diagnosis, prognosis, therapy prediction and therapeutic tools for breast cancer. Theranostics 2015; 5: 1122–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shaker O, Maher M, Nassar Y, Morcos G, Gad Z. Role of microRNAs ‐29b‐2, ‐155, ‐197 and ‐205 as diagnostic biomarkers in serum of breast cancer females. Gene 2015; 560: 77–82. [DOI] [PubMed] [Google Scholar]
- 17. Müller V, Gade S, Steinbach B et al. Changes in serum levels of miR‐21, miR‐210, and miR‐373 in HER2‐positive breast cancer patients undergoing neoadjuvant therapy: A translational research project within the Geparquinto trial. Breast Cancer Res Treat 2014; 147: 61–8. [DOI] [PubMed] [Google Scholar]
- 18. Woo CJ, Kingston RE. HOTAIR lifts noncoding RNAs to new levels. Cell 2007; 129: 1257–9. [DOI] [PubMed] [Google Scholar]
- 19. Yang Z, Zhou L, Wu LM et al. Overexpression of long non‐coding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann Surg Oncol 2011; 18: 1243–50. [DOI] [PubMed] [Google Scholar]
- 20. Endo H, Shiroki T, Nakagawa T et al. Enhanced expression of long non‐coding RNA HOTAIR is associated with the development of gastric cancer. PLoS One 2013; 8 (10): e77070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chisholm KM, Wan Y, Li R, Montgomery KD, Chang HY, West RB. Detection of long non‐coding RNA in archival tissue: Correlation with polycomb protein expression in primary and metastatic breast carcinoma. PLoS One 2012; 7 (10): e47998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oliveras‐Ferraros C, Vazquez‐Martin A, Cufi S et al. Inhibitor of apoptosis (IAP) survivin is indispensable for survival of HER2 gene‐amplified breast cancer cells with primary resistance to HER1/2‐targeted therapies. Biochem Biophys Res Commun 2011; 407: 412–9. [DOI] [PubMed] [Google Scholar]
- 23. Traub F, Feist H, Kreipe HH, Pich A. SELDI‐MS‐based expression profiling of ductal invasive and lobular invasive human breast carcinomas. Pathol Res Pract 2005; 201: 763–70. [DOI] [PubMed] [Google Scholar]
- 24. Bhan A, Hussain I, Ansari KI, Kasiri S, Bashyal A, Mandal SS. Antisense transcript long noncoding RNA (lncRNA) HOTAIR is transcriptionally induced by estradiol. J Mol Biol 2013; 425: 3707–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xue X, Yang YA, Zhang A et al. LncRNA HOTAIR enhances ER signaling and confers tamoxifen resistance in breast cancer. Oncogene 2016; 35 (21): 2746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sørensen KP, Thomassen M, Tan Q et al. Long non‐coding RNA HOTAIR is an independent prognostic marker of metastasis in estrogen receptor‐positive primary breast cancer. Breast Cancer Res Treat 2013; 142: 529–36. [DOI] [PubMed] [Google Scholar]
- 27. Gökmen‐Polar Y, Vladislav IT, Neelamraju Y, Janga SC, Badve S. Prognostic impact of HOTAIR expression is restricted to ER‐negative breast cancers. Sci Rep 2015; 5: 8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: Correlation of relapse and survival with amplification of the HER‐2/neu oncogene. Science 1987; 235: 177–82. [DOI] [PubMed] [Google Scholar]
- 29. Slamon DJ, Godolphin W, Jones LA et al. Studies of the HER‐2/neu proto‐oncogene in human breast and ovarian cancer. Science 1989; 244: 707–12. [DOI] [PubMed] [Google Scholar]
- 30. Thor AD, Liu S, Edgerton S et al. Activation (tyrosine phosphorylation) of ErbB‐2 (HER‐2/neu): A study of incidence and correlation with outcome in breast cancer. J Clin Oncol 2000; 18: 3230–9. [DOI] [PubMed] [Google Scholar]
- 31. Liu J, Gao J, Du Y et al. Combination of plasma microRNAs with serum CA19‐9 for early detection of pancreatic cancer. Int J Cancer 2012; 131: 683–91. [DOI] [PubMed] [Google Scholar]
- 32. Chen G, Wang J, Cui Q. Could circulating miRNAs contribute to cancer therapy? Trends Mol Med 2013; 19: 71–3. [DOI] [PubMed] [Google Scholar]
- 33. Zhou X, Yin C, Dang Y, Ye F, Zhang G. Identification of the long non‐coding RNA H19 in plasma as a novel biomarker for diagnosis of gastric cancer. Sci Rep 2015; 5: 11516. [DOI] [PMC free article] [PubMed] [Google Scholar]


