Abstract
Background
Epidermal growth factor receptor‐tyrosine kinase inhibitors (EGFR‐TKIs) are remarkably effective for treating EGFR‐mutant non‐small cell lung cancer (NSCLC). However, the individual role of EGFR‐TKIs in patients with brain metastasis (BM) arising from EGFR‐mutant NSCLC remains unclear.
Methods
Patients with BM secondary to NSCLC and harboring EGFR‐activating mutations were retrospectively screened. Patients who received gefitinib or erlotinib to control both extracranial lesions (ECLs) and intracranial lesions (ICLs) were eligible. If ECLs remained stable or remissive while ICLs progressed; asymptomatic BM progressed to symptomatic BM; BM symptoms were not alleviated within two weeks; or BM symptoms deteriorated after initial release, patients received brain radiotherapy or other local treatments and continued taking TKIs until ECLs progression occurred.
Results
In 43 eligible patients, the objective response and disease control rates for ICLs were 57% and 91%, respectively. Median progression‐free survival (PFS) was 9.3 months. The median PFS for ICLs and ECLs was 9.7 and 13.7 months, respectively. Non‐smokers and second‐line TKIs were found to be independent positive prognostic factors for PFS and overall survival (OS) respectively, with a hazard ratio of 0.29 (95% confidence interval [CI] 0.14–0.61; P = 0.001) and 0.34 (95% CI 0.16–0.70; P = 0.003). No significant difference in median OS was observed between patients who did or did not receive brain radiotherapy (23.6 vs. 18.7 months; P = 0.317).
Conclusion
EGFR‐TKIs alone are effective for treating BM arising from EGFR‐mutant NSCLC. The efficacy of TKIs in ICLs and ECLs should be evaluated separately.
Keywords: Brain metastasis, epidermal growth factor receptor, non‐small cell lung cancer, tyrosine kinase inhibitors
Introduction
Lung cancer is the leading cause of cancer death in many countries, including China.1, 2 The brain is one of the most common sites affected by metastases in lung cancer and approximately 40% of patients with non‐small cell lung cancer (NSCLC) develop brain metastasis (BM) during the course of their disease.3 BM arising from lung cancer is usually associated with a poor prognosis because of the limited number of novel treatment options. There are few traditional treatments for BM in patients with NSCLC: radiotherapy‐based local therapy, whole‐brain radiotherapy (WBRT), stereotactic radiosurgery, and radiotherapy combined with surgery; however, these treatments carry a median survival rate of only three to six months.4
Although WBRT can attenuate neurologic symptoms, it can also reduce patients’ performance status (PS) and delay further systemic treatment.5, 6 Systemic chemotherapy can be employed with radiotherapy in combined modality strategies or as a sensitizer for radiotherapy, but the role of systemic chemotherapy is often considered to be limited because the blood–brain barrier renders the brain a pharmacologic sanctuary.7, 8
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR‐TKIs), gefitinib and erlotinib, have been proven to be effective for advanced NSCLC, especially for patients with tumors carrying activating mutations in the EGFR‐TK domain.9, 10, 11 An important prospective phase III study published in the New England Journal of Medicine in 2009 reported the efficacy of first‐line gefitinib in advanced NSCLC in a predominantly Asian population. The objective response rate (ORR) for gefitinib was 71.2% in the mutation‐positive subgroup.10 However, just like most clinical trials involving TKIs, this study did not explore the role of gefitinib in patients with BM, as cases of newly diagnosed BM not yet treated with radiation or surgery were excluded. Although objective responses of intracranial diseases to TKI treatment have been reported in some studies and individual case reports, limitations of these studies included unknown EGFR mutation status of participating patients and the fact that most patients had received WBRT prior to or along with TKIs.12, 13, 14, 15, 16, 17 Therefore, the individual role of TKIs in patients with BM arising from EGFR‐mutant NSCLC has been unclear until recently. As reported in the CTONG‐0803 (NCT00663689) trial conducted by Wu et al. in Mainland China, NSCLC patients with asymptomatic BM could benefit from erlotinib alone, with a median PFS of 10.1 months for intracranial progression.18 Another phase II study, reported by Iuchi et al., indicated that gefitinib alone could be highly effective in patients with BM, especially for those with exon 19 deletions.19 Since late 2004, we started to pay closer attention to EGFR mutation analysis in our cancer center. From our data, a very small portion of patients with BM arising from EGFR‐mutant NSCLC did not receive standard brain radiotherapy as the initial treatment, because of patient refusal, poor PS, or other reasons, but received EGFR‐TKIs. In this study, we retrospectively summarized the efficacy of EGFR‐TKIs in these patients to evaluate the individual role of TKIs in patients with BM arising from EGFR‐mutant NSCLC.
Methods
Patients
We reviewed clinical data from the electronic medical record database of the Guangdong Lung Cancer Institute from January 2009 to April 2012. Eligible patients included those: (i) pathologically diagnosed with NSCLC; (ii) suffering from asymptomatic or symptomatic BM, confirmed by brain magnetic resonance imaging (MRI); (iii) carrying EGFR‐activating mutations in exon 18, 19, or 21, but not in exon 20, of the EGFR gene in their tumor tissues; and (iv) who had not received brain radiotherapy, surgery, or radiosurgery for any reason, but were instead treated with an EGFR‐TKI (gefitinib 250 mg once daily or erlotinib 150 mg once daily), to control both extracranial lesions (ECLs) and intracranial lesions (ICLs).
The main reason that patients did not undergo brain radiotherapy was refusal because of fear of the side effects. Other patients did not receive radiotherapy because of poor PS or old age. Since 2007, the following uniform treatment strategy has been applied for these patients at our cancer center.
Patients with asymptomatic BM were administered an oral EGFR‐TKI (gefitinib 250 mg once daily or erlotinib 150 mg once daily) until ECLs progressed, intolerable toxicity was observed, or refusal to continue treatment. According to Response Evaluation Criteria in Solid Tumors (RECIST), if ICLs progressed alone, with stable or remissive ECLs, or an asymptomatic BM progressed to a symptomatic BM (as defined by the presence of one or more of the following symptoms: signs of increased intracranial pressure, headache, nausea and vomiting, cognitive or affective disturbances, seizures, and focal neurologic symptoms), patients received brain radiotherapy and continued taking a TKI until their ECLs progressed. Radiotherapy for BM included WBRT, stereotactic radiosurgery, or both.
Patients with symptomatic BM were administered an oral TKI, together with corticosteroid and other symptomatic treatments. If the symptoms were alleviated within two weeks, TKI was continued without brain radiotherapy. If the symptoms were not relieved within two weeks, the symptoms deteriorated again after initial relief, or ICLs progressed prior to ECLs according to RECIST criteria, brain radiotherapy was commenced. Patients also continued taking a TKI until their ECLs progressed, intolerable toxicity occurred, or they refused subsequent treatment.
For all patients, if the ECLs progressed before the ICLs, or both progressed in parallel, TKI treatment was halted. Physicians adopted subsequent systemic and local brain treatments.
Efficacy and safety
The responses were evaluated according to the RECIST. At the baseline of TKI treatment, each patient routinely received a chest and upper abdomen computed tomography (CT) scan (covering the liver, gall bladder, pancreas, spleen, and adrenal glands) and brain MRI. The chest/upper abdomen CT and brain MRI were repeated every eight weeks to evaluate tumor response in the chest/upper abdomen and brain, respectively. Therefore, responses of ICLs and ECLs to TKIs were evaluated separately. Progression‐free survival (PFS) was also subclassified as PFS for ECLs and PFS for ICLs. PFS for ECLs was defined as the time from the commencement of TKI treatment to ECL progression. Similarly, PFS for ICLs was defined as the time from the commencement of TKI treatment to ICL progression or when a patient received brain radiotherapy for any reason. Overall survival (OS) was calculated from the commencement of TKI treatment to death from any cause. Symptoms were first evaluated two weeks after the commencement of TKI treatment, and thereafter, together with objective tumor response assessments. Adverse events were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
Analysis of epidermal growth factor receptor mutations
In our cancer center, we commenced EGFR mutation testing in NSCLC patients in late 2004, while all patients with advanced NSCLC were routinely subjected to EGFR mutation analysis since 2007, as long as sufficient tumor tissue could be obtained. Tumor samples were obtained during diagnostic procedures, mainly by fiber bronchoscopy, CT‐guided core biopsy of primary lung lesions or metastatic lesions, or lymphadenectomy. Direct sequencing was performed for EGFR mutational analysis. Genomic DNA was isolated from tumor tissues and exons 18, 19, 20, and 21 of the EGFR gene were amplified using four pairs of primers. Uncloned polymerase chain reaction (PCR) products were sequenced in both the sense and antisense directions. All sequencing reactions were performed using a Big Dye Terminator v3.1 Cycle Sequencing Kit and an ABI 3700 DNA analyzer (Applied Biosystems, Foster City, CA, USA). Sequence variation was confirmed by performing multiple independent PCR amplifications and repeating sequencing reactions.20
Statistical analysis
The PFS and OS rates were estimated using the Kaplan–Meier method and comparisons between subgroups were performed using the log‐rank test. Multivariate analysis was carried out using stepwise Cox regression. P values < 0.05 in two‐sided tests were considered to be statistically significant. Statistical analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
Forty‐three eligible patients were treated according to this strategy. The characteristics of these patients are summarized in Table 1. All patients were from Mainland China. Most were never‐smokers (74.4%), female (55.8%), with adenocarcinoma (97.7%), and had an Eastern Cooperative Oncology Group (ECOG) PS of 0–1 (74.4%). Only one patient with adenosquamous carcinoma carried an EGFR gene exon 19 deletion.
Table 1.
Patient characteristics (n = 43)
| Characteristic | Number of patients | % |
|---|---|---|
| Gender | ||
| Male | 19 | 44.2 |
| Female | 24 | 55.8 |
| Age (years) | ||
| Median (range) | 60 (27–76) | |
| Smoking status | ||
| Non‐smoker† | 32 | 74.4 |
| Current smoker | 11 | 25.6 |
| Pathology | ||
| Adenocarcinoma | 42 | 97.7 |
| Adenosquamous carcinoma | 1 | 2.3 |
| EGFR mutation | ||
| E19 deletion | 20 | 46.5 |
| E21‐L858R | 20 | 46.5 |
| E21‐L861Q | 1 | 2.3 |
| E19 deletion + E21‐L858R | 1 | 2.3 |
| E18‐G719X + E19 deletion + E20‐S768I | 1 | 2.3 |
| EGFR‐TKI therapy | ||
| First‐line | 27 | 62.8 |
| Second‐line | 16 | 37.2 |
| EGFR‐TKI administered | ||
| Gefitinib | 30 | 70.0 |
| Erlotinib | 13 | 30.0 |
| ECOG performance status | ||
| 0–1 | 32 | 74.4 |
| 2–3 | 11 | 25.6 |
| Brain metastasis | ||
| Asymptomatic | 33 | 76.7 |
| Symptomatic | 10 | 23.3 |
No history of smoking. ECOG, Eastern Cooperative Oncology Group; EGFR‐TKI, epidermal growth factor receptor‐tyrosine kinase inhibitor.
Efficacy
Out of the 43 patients studied, the ORR and was 72% and the disease control rate (DCR) was 93%. For the ICLs and ECLs, the ORR and DCR were 57% and 91%, and 69% and 95%, respectively. Three patients who showed a partial response in their ECLs achieved a complete response (CR) in their ICLs. Four patients had no intracranial target lesion because their largest ICLs in MRI results were less than 2 cm in diameter. Although all of their ICLs shrank after TKI treatment, the best ICL response was evaluated as stable disease (SD), while their extracranial target lesions were deemed to show a PR. After treatment with corticosteroids and TKIs, the symptoms of the 10 patients with symptomatic BM were relieved within two weeks and they all clinically benefited from the treatment, except for one who progressed after two months.
When we collated our data for inclusion in this manuscript, 41 patients had progressive disease, one patient remained remissive or stable, and the other patient discontinued gefitinib by himself, although his ECLs remained a PR. ICLs progressed before ECLs in 20 patients and ECLs before ICLs in 11. In the one patient with adenosquamous carcinoma, the ICLs and ECLs progressed concurrently. The overall median PFS was 9.3 months (95% confidence interval [CI], 6.7–11.9). The median PFS for ICLs and ECLs were 9.7 (95% CI 8.0–11.4) and 13.7 months (95% CI 10.1–17.3), respectively. The individual PFS for ICLs and ECLs is shown in Figure 1. The overall median OS was 23.6 months (95% CI, 15.0–32.3). In the 20 patients whose ICLs progressed first, 17 received WBRT or other local treatments, while the others refused treatment. In the 11 patients whose ECLs progressed first, 10 did not receive WBRT because of refusal or deterioration in PS.
Figure 1.
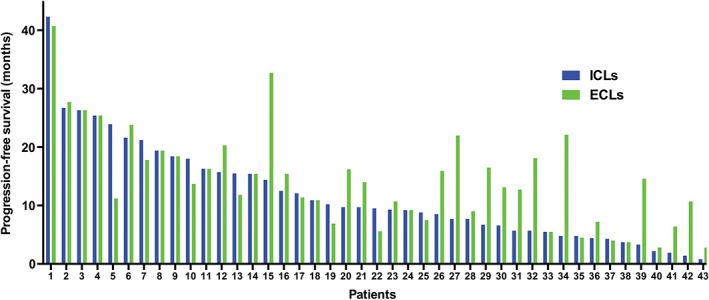
The individual duration time for intracranial lesions (ICLs) and extracranial lesions (ECLs).
The median OS in patients who did and did not receive WBRT was 23.6 (95% CI 12.8–34.4) and 18.7 months (95% CI 8.1–29.3), respectively (P = 0.317). Non‐smokers demonstrated longer PFS than smokers (10.9 vs. 4.4 months; P < 0.001), and a similar trend was observed in median OS, although borderline significance was obtained (24.4 vs. 16.8 months; P = 0.093). Second‐line treated patients exhibited prolonged OS compared with first‐line treated patients (27.8 vs. 17.7 months; P = 0.002), but no significant difference was obtained in median PFS (P = 0.521). No significant difference was observed in survival between gender (male vs. female), age (< 65 years vs. ≥ 65 years), ECOG PS (0–1 vs. 2–3), EGFR mutation (19 deletion vs. other mutations), and EGFR‐TKIs administered (gefitinib vs. erlotinib).
The only patient with adenosquamous carcinoma carrying an EGFR exon 19 deletion was female and had never smoked. The tissue used for pathologic diagnosis and EGFR mutation analysis was obtained from a primary tumor in a lung lobe by CT‐guided core biopsy. The patient received gemcitabine plus carboplatin as first‐line therapy. The response was SD and the PFS was 5.0 months. Brain metastasis developed before second‐line therapy (gefitinib) was initiated. Both ICLs and ECLs regressed initially (evaluated as SD) but progressed concurrently after 5.5 months. The patient refused any further treatment and died a month later.
Multivariate stepwise Cox regression analysis
Four variables, EGFR mutation, smoking history, line of TKIs, and ECOG PS, were included in Cox regression analysis for PFS and OS. Non‐smokers and second‐line treatments were found to be independent positive prognostic factors for PFS and OS, with hazard ratios of 0.29 (95% CI 0.14–0.61, P = 0.001) and 0.34 (95% CI 0.16–0.70, P = 0.003), respectively.
Toxicity
Rash and diarrhea were the most common adverse effects (21/43 [49%] and 16/43 [37%], respectively). Most rashes were grade 1 or 2. Three patients developed grade 3 rashes. Treatment was discontinued or the drug dose was reduced because of intolerable side effects. No interstitial lung disease or brain toxicity was found.
Subsequent systemic treatment
At the final follow‐up, one patient was still receiving TKI treatment. In the remaining 42 patients who had ceased TKI treatment, eight were treated with platinum‐based doublet chemotherapy, four with single‐agent docetaxel, two with single‐agent pemetrexed, two with sorafenib, and one received immunotherapy. One patient was given best supportive care, while the remaining 24 patients refused further systemic therapy.
Discussion
The results of this study show that patients with BM arising from EGFR‐mutant NSCLC can benefit dramatically from EGFR‐TKI treatment (without WBRT or other local brain treatments): the ORR was 72% and DCR 93%. Median PFS was 9.3 months, with 9.7 for ICLs and 13.7 for ECLs. The results obtained in our study were similar to those found in previous studies by Wu et al. and Iuchi et al.18, 19 In other studies evaluating the efficacy of EGFR‐TKIs in BM arising from NSCLC, WBRT and/or radiosurgery were administered prior to or together with TKIs in most patients, indicating that the impact of TKIs on BM was masked by radiotherapy and the effects of TKIs alone in BM could not be properly evaluated.12, 14, 15, 16 In a study evaluating the efficacy of EGFR‐TKIs alone in BM in treatment‐naive patients, the recruited patients had never smoked, were Asian (all from Korea), and suffered from asymptomatic BM secondary to lung adenocarcinoma.13 Although the EGFR mutation status of these patients is unknown, 60% of patients with these clinical characteristics are likely to carry EGFR‐activating mutations, according to the results of the IPASS study.10 TKI treatment resulted in a response rate of 69.6%, an intracranial tumor response rate of 73.9%, and a median PFS of 7.1 months, which suggests that selected patients with BM would benefit greatly from TKI treatment. Because EGFR mutations have been shown to be better predictors of EGFR‐TKI efficacy than EGFR protein expression, EGFR gene copy number, and clinical characteristics, we selected patients according to EGFR mutation status.10, 21, 22 A dramatic benefit was observed in our study. The median PFS and OS were 9.3 and 23.6 months, respectively, which is better than those for WBRT in unselected patients in a previous study.4 In our study, no significant difference in median OS was observed between patients who did or did not receive WBRT, which suggests that WBRT may not be immediately essential for treating BM arising from EGFR‐mutant NSCLC, and that EGFR‐TKI alone may be a more effective and less toxic option in this setting. Because BM‐related symptoms were alleviated rapidly (within two weeks), and a similarly rapid response was observed in intracranial tumors treated with TKIs in patients selected according to clinical characteristics, TKIs were deemed an appropriate option for treating symptomatic BM arising from EGFR‐mutant NSCLC.13 Our results confirm that EGFR‐activating mutations are novel biomarkers for selecting patients who may benefit from TKI treatment, regardless of whether they have BM. Although ours is a retrospective study, all the clinical data were retrieved from the electronic medical record database at the Guangdong Lung Cancer Institute and we formulated a uniform treatment strategy for eligible patients in advance. These features increased the reliability of our results.
Another feature of our study was that the efficacy of TKIs in ICLs and ECLs was evaluated separately, which marks a departure from classic RECIST criteria. If ICLs progressed first while ECLs remained remissive, patients received brain radiotherapy and continued taking EGFR‐TKIs until ECLs progressed. This strategy was very different from those employed in most clinical trials, in which the test treatment must cease as soon as the disease is deemed to be progressive. The brain is regarded as a special case in systemic treatment because of the blood–brain barrier. However, several studies have demonstrated that EGFR‐TKIs are able to cross the blood–brain barrier and display efficacy against intracranial metastasis.13, 23 The efficacy of TKIs in ICLs usually mirrors or exceeds their efficacy in ECLs.23 In the present study, three patients who showed a PR in their ECLs achieved a CR in their ICLs. In spite of these results, the brain has been found to be a frequent site of disease recurrence after an initial response to EGFR‐TKIs for patients in whom ECLs remained stable.24 In 41 patients in our study who had PD during the course of TKI treatment, 20 displayed ICL progression first. We treated 17 of them with WBRT to control the ICLs and continued TKI treatment. The median PFS for ICLs and ECLs was 9.7 and 13.7 months, respectively, which means that patients received four more months of disease control for ECLs than for ICLs as a result of the treatment strategy we employed. Therefore, considering the difference in the response and the duration of response between ICLs and ECLs, we recommend the efficacy of TKIs for ICLs and ECLs to be evaluated separately, in order to maximize the clinical benefit achieved using TKIs.
In the present study, no significant differences were observed in PFS or OS between clinical characteristics, except for the difference that occurred between patients treated with first‐line and second‐line TKIs, and smokers and non‐smokers. As univariate and multivariate Cox regression analysis revealed, smoking was a risk factor for PFS and second‐line TKIs could be a good prognostic factor for OS compared with first‐line TKI therapy. Previous studies have shown that EGFR‐activating mutations arise somatically during tumor formation, with only a proportion of cancer cells in an individual patient carrying heterozygous activating mutations because of heterogeneity of genetic abnormalities, and that EGFR mutations were rarely found in patients with squamous cell carcinoma.9, 11, 20 Therefore, we assume that the EGFR exon 19 deletion was not present in all differentiated cancer cells in the patient with adenosquamous carcinoma. Thus, in addition to the type of EGFR mutation, the prevalence of EGFR mutant cells may determine the efficacy of EGFR‐TKI treatment in a particular patient.
There were some limitations to our study. The primary limitations were the retrospective nature and small sample size. Furthermore, we did not include patients treated with radiotherapy alone or with radiotherapy and EGFR‐TKIs, which might bring more implications into the management of EGFR‐mutant patients with BM.
In conclusion, this study shows that EGFR‐TKIs are active against both ICLs and ECLs in patients with EGFR‐mutant NSCLC. EGFR‐TKIs alone appear to be an effective and tolerable option for patients with BM arising from EGFR‐mutant NSCLC. Because of the special anatomic structure of the brain, the efficacy of TKIs in ICLs and ECLs should be evaluated separately to maximize the benefits of TKI treatment. Moreover, a prospective study comparing the efficacy between EGFR‐TKIs and WBRT in patients with EGFR‐mutant NSCLC with BM is warranted.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81172090), the Guangdong Provincial Key Laboratory of Lung Cancer Translational Medicine (No. 2012A061400006), the Special Fund for Research in the Public Interest from the National Health and Family Planning Commission of PRC (No. 201402031), and the Research Fund from the Guangzhou Science and Technology Bureau (No. 2014Y2‐00050).
Contributor Information
Yi‐Long Wu, Email: syylwu@live.cn.
Qing Zhou, Email: gzzhouqing@126.com.
References
- 1. Chen W, Zheng R, Baade PD et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–32. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30. [DOI] [PubMed] [Google Scholar]
- 3. Rizzi A, Tondini M, Rocco G et al. Lung cancer with a single brain metastasis: Therapeutic options. Tumori 1990; 76: 579–81. [DOI] [PubMed] [Google Scholar]
- 4. Lagerwaard FJ, Levendag PC, Nowak PJ, Eijkenboom WM, Hanssens PE, Schmitz PI. Identification of prognostic factors in patients with brain metastases: A review of 1292 patients. Int J Radiat Oncol Biol Phys 1999; 43: 795–803. [DOI] [PubMed] [Google Scholar]
- 5. Laack NN, Brown PD. Cognitive sequelae of brain radiation in adults. Semin Oncol 2004; 31: 702–13. [DOI] [PubMed] [Google Scholar]
- 6. Lee DH, Han JY, Kim HT et al. Primary chemotherapy for newly diagnosed nonsmall cell lung cancer patients with synchronous brain metastases compared with whole‐brain radiotherapy administered first: Result of a randomized pilot study. Cancer 2008; 113: 143–9. [DOI] [PubMed] [Google Scholar]
- 7. Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol 2005; 23: 6207–19. [DOI] [PubMed] [Google Scholar]
- 8. Khuntia D, Brown P, Li J, Mehta MP. Whole‐brain radiotherapy in the management of brain metastasis. J Clin Oncol 2006; 24: 1295–304. [DOI] [PubMed] [Google Scholar]
- 9. YL W, Zhong WZ, Li LY et al. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non‐small cell lung cancer: A meta‐analysis based on updated individual patient data from six medical centers in mainland China. J Thorac Oncol 2007; 2: 430–9. [DOI] [PubMed] [Google Scholar]
- 10. Mok TS, YL W, Thongprasert S et al. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–57. [DOI] [PubMed] [Google Scholar]
- 11. Rosell R, Moran T, Queralt C et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009; 361: 958–67. [DOI] [PubMed] [Google Scholar]
- 12. Hotta K, Kiura K, Ueoka H et al. Effect of gefitinib ('Iressa', ZD1839) on brain metastases in patients with advanced non‐small‐cell lung cancer. Lung Cancer 2004; 46: 255–61. [DOI] [PubMed] [Google Scholar]
- 13. Kim JE, Lee DH, Choi Y et al. Epidermal growth factor receptor tyrosine kinase inhibitors as a first‐line therapy for never‐smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer 2009; 65: 351–4. [DOI] [PubMed] [Google Scholar]
- 14. Altavilla G, Arrigo C, Santarpia MC et al. Erlotinib therapy in a patient with non‐small‐cell lung cancer and brain metastases. J Neurooncol 2008; 90: 31–3. [DOI] [PubMed] [Google Scholar]
- 15. Wu C, Li YL, Wang ZM, Li Z, Zhang TX, Wei Z. Gefitinib as palliative therapy for lung adenocarcinoma metastatic to the brain. Lung Cancer 2007; 57: 359–64. [DOI] [PubMed] [Google Scholar]
- 16. Namba Y, Kijima T, Yokota S et al. Gefitinib in patients with brain metastases from non‐small‐cell lung cancer: Review of 15 clinical cases. Clin Lung Cancer 2004; 6: 123–8. [DOI] [PubMed] [Google Scholar]
- 17. Chiu CH, Tsai CM, Chen YM, Chiang SC, Liou JL, Perng RP. Gefitinib is active in patients with brain metastases from non‐small cell lung cancer and response is related to skin toxicity. Lung Cancer 2005; 47: 129–38. [DOI] [PubMed] [Google Scholar]
- 18. YL W, Zhou C, Cheng Y et al. Erlotinib as second‐line treatment in patients with advanced non‐small‐cell lung cancer and asymptomatic brain metastases: A phase II study (CTONG‐0803). Ann Oncol 2013; 24: 993–9. [DOI] [PubMed] [Google Scholar]
- 19. Iuchi T, Shingyoji M, Sakaida T et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR‐mutant lung adenocarcinoma. Lung Cancer 2013; 82: 282–7. [DOI] [PubMed] [Google Scholar]
- 20. Lynch TJ, Bell DW, Sordella R et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non‐small‐cell lung cancer to gefitinib. N Engl J Med 2004; 350: 2129–39. [DOI] [PubMed] [Google Scholar]
- 21. Sholl LM, Xiao Y, Joshi V et al. EGFR mutation is a better predictor of response to tyrosine kinase inhibitors in non‐small cell lung carcinoma than FISH, CISH, and immunohistochemistry. Am J Clin Pathol 2010; 133: 922–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dahabreh IJ, Linardou H, Siannis F, Kosmidis P, Bafaloukos D, Murray S. Somatic EGFR mutation and gene copy gain as predictive biomarkers for response to tyrosine kinase inhibitors in non‐small cell lung cancer. Clin Cancer Res 2010; 16: 291–303. [DOI] [PubMed] [Google Scholar]
- 23. Porta R, Sánchez‐Torres JM, Paz‐Ares L et al. Brain metastases from lung cancer responding to erlotinib: The importance of EGFR mutation. Eur Respir J 2011; 37: 624–31. [DOI] [PubMed] [Google Scholar]
- 24. Omuro AM, Kris MG, Miller VA et al. High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer 2005; 103: 2344–8. [DOI] [PubMed] [Google Scholar]


