Abstract
Dysregulation of B cell receptor (BCR) signalling is a hallmark of chronic lymphocytic leukaemia (CLL) pathology, and targeting BCR pathway kinases has brought great therapeutic advances. Activation of the BCR in lymphoid organs has been associated with CLL cell proliferation and survival, leading to progressive disease. While these responses are mediated predominantly by IgM, the role of IgD is less clear. Seeking to uncover downstream consequences of individual and combined stimulation of the two BCR isotypes, we found an amplification of IgD expression and IgD-mediated calcium signalling by previous stimulation of IgM in CLL. Furthermore, no heterologous downmodulation of the isotypes, as observed in healthy donors, was present. Only marginal downregulation of the expression of various chemokine receptors by α-IgM and α-IgD stimulation was found as compared to normal B cells. Consistently, calcium responses of CLL cells to different chemokines were only weakly affected by preceding BCR activation. In contrast, migration towards the two homeostatic chemokines CXCL12 and CCL21 was differentially regulated by IgM and IgD. While IgM activation reduced migration of CLL cells towards CXCL12, but not CCL21, IgD activation predominantly impacted on CCL21 but not CXCL12-mediated chemotaxis. This indicates that the preference for one chemokine over the other may depend on the functional presence of the two isotypes in CLL. Inhibitors against the kinases Syk, Lyn, and Btk antagonised both BCR- and chemokine-induced calcium signals.
Electronic supplementary material
The online version of this article (doi:10.1007/s00277-016-2788-6) contains supplementary material, which is available to authorized users.
Keywords: CLL, IgM, IgD, BCR signalling, Chemokines
Introduction
Chronic lymphocytic leukaemia (CLL) is characterised by the progressive accumulation of malignant monoclonal B lymphocytes in blood and primary and secondary lymphoid organs. CLL cells are phenotypically mature B cells [1] usually expressing both IgM and IgD on their surface. CLL cells display various degrees of anergy, linked to reduced IgM expression and signalling capacity [2, 3]. High B cell receptor (BCR) signalling capacity in response to IgM [4] as well as IgD [5] stimulation has been associated with adverse prognosis and progressive disease. However, little is known about the functional differences between these two isotypes, and their combined role in CLL.
The importance of antigen stimulation for CLL is further supported by the biased IGHV gene usage in CLL [6, 7]. Unmutated CLL (u-CLL) tend to express low affinity poly- and autoreactive BCRs, while high affinity monoreactive BCRs occur mainly in mutated CLL (m-CLL) and have been shown in certain cases to be specific for bacterial [8] or fungal pathogens [9, 10]. Autonomous BCR signalling was described as a hallmark of CLL [11]. Recently, on a basis of transgenic murine CLL models, it was suggested that both cell autonomous and cell external low affinity BCR interactions contribute to CLL pathogenesis [12]. This obvious reliance of CLL cells on both ligand-dependent and ligand-independent BCR signals has led to the establishment of inhibitors against BCR pathway kinases, targeting Syk, Btk and Lyn for CLL treatment, with great clinical success [13–16]. A prominent effect of Ibrutinib and other inhibitors in vivo is a lymphocytosis caused by the redistribution of CLL cells from lymphoid organs into the periphery [17], based on antagonisation of migration and retention signals [18]. Consistently, chemokine-mediated migration and integrin activation of CLL cells were efficiently inhibited in vitro by targeting Syk [19, 20] and Btk [21]. Collectively, this indicates that the effectiveness of these novel agents is, at least in part, due to a block in the interaction of CLL cells with protective signals from their direct lymphoid microenvironment. The chemokine receptor CXCR4 and its ligand CXCL12 are of particular significance for CLL cell migration and survival [22]. CXCL12 stimulation leads to phosphorylation of Syk [19] and Btk [23]. Other chemokine receptors robustly expressed on CLL cells are CXCR5, CCR7, and the atypical chemokine receptor CCRL2, a presumed regulator of CCR7 activity [24]. Furthermore, CXCR3 is expressed on CLL in variable amounts, in contrast to other B cell lymphomas. High CXCR3 expression levels are associated with indolent disease, exerting a negative functional regulation on CXCR4 [25]. In healthy B cells, activation of the BCR fundamentally alters the expression and function of CXCR4 [26], CCR7, and CXCR5 [27]. In CLL, downregulation of CXCR4 [28] and CXCR5 [29] was observed predominantly in high-risk cases after stimulation with immobilised α-IgM antibodies. In contrast, the impact of IgD activation on the expression pattern of these receptors has not been evaluated yet.
In this study, we examined how IgM and IgD cross-respond to stimulation in CLL and how BCR stimulation impacts on chemokine receptor expression and function, also in the context of therapeutic BCR inhibition.
Material and methods
Patient samples and cell isolation
Blood samples were collected from CLL patients after informed consent and ethical approval at the Freiburg Medical University Center and the Third Medical Department, Paracelsus Medical University Salzburg. The samples collected in Salzburg were used for the chemotaxis assays during revision of the manuscript; for all other experiments, the Freiburg cohort was used. A summary of all patients used including IGHV mutation status and BCR expression is given in Supplementary Table 1. Peripheral blood mononuclear cells (PBMCs) from CLL patients and healthy donors were isolated by density gradient centrifugation over Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). Samples were cytometrically analysed for the quantity of CLL cells and included in this study if more than 85 % of cells were CD19 positive.
Reagents
Murine monoclonal antibody against CCR7 and CCRL2 and PE-labelled antibodies against CXCR5 and CXCR3 were purchased from R&D Systems. PE-labelled antibodies against CXCR4, PE and FITC-labelled antibodies against CD19, and FITC and PE-labelled antibodies against IgD were purchased from BD. PE-labelled antibodies against IgM were purchased from Biolegend and Beckman Coulter. PE-labelled rabbit α-mouse IgG was obtained from Dako Cytomation. Goat F(ab’)2 anti-human IgM (α-IgM) and IgD (α-IgD) for stimulations and F(ab’)2 of irrelevant specificity as a control were purchased from Southern Biotech. Chemokines were purchased from Peprotech and R&D. Ibrutinib (PCI-32765) was bought from Selleckchem, Bafetinib (INNO 406) from Adooq, and R406 from Riegel Pharmaceuticals. Annexin V-FITC and 7-aminoactinomycin D (7AAD) for determination of cell viability were from Beckman Coulter.
BCR stimulation
CLL cells and healthy donor PBMCs were thawed and incubated at 37 °C overnight before use. BCR stimulation was performed in 48-well plates. Cells were suspended at 4 × 106 cells per ml, 250 μl of the suspension were applied per well, and α-Ig F(ab’)2 was added at a concentration of 20 μg/ml. Immobilisation of the F(ab’)2 in wells was performed by dilution in PBS and incubation over night at room temperature. The cell suspension was subsequently added after extensive washing with PBS and RPMI.
Flow cytometric detection of chemokine receptors and BCR expression
A total of 2.5 × 105 cells were stained with the appropriate antibody for 30 min at 4 °C in PBS containing 0.5 % BSA. Prior to adding the secondary antibody, excess antibody was removed by washing. A minimum of 2 × 104 cells were measured by flow cytometry on a FACSCalibur. Viable cells were identified by SSC-FSC, and the geometric mean of fluorescence intensities (MFI) was determined using FlowJo analysis software. Mean fluorescence intensity ratios (MFIR) were calculated using the appropriate isotypes.
Intracellular calcium measurement
For measurement of intracellular calcium mobilisation, cells were prepared as described in Quiroga et al. [20]. A total of 1 × 107 cells were incubated in 1 ml complete RPMI with 4 μM Fluo-3-AM (Invitrogen) at 37 °C for 30 min. Cells were then resuspended at 5 × 106/ml and incubated for another 10 min at 37 °C. After washing in complete RPMI, cells were resuspended in medium containing 1.5 mM CaCl2 and in case of the inhibitor studies R406, Bafetinib, or Ibrutinib at a concentration of 5 μM. After an incubation of 30 min at 37°, the cells were put on ice. Five minutes before each measurement, 100 μl of cell suspension was added into 400 μl of prewarmed RPMI with CaCl2 and, where applicable, with an inhibitor. After 15 s of baseline acquisition, α-IgM F(ab’)2, α-IgD F(ab’)2 (10 μg/ml), CXCL13 (500 ng/ml), CCL19, CCL21, or CXCL12 (200 ng/ml) were added and the fluorescence intensity was recorded for 2 min. For quantification, the baseline fluorescence intensity was subtracted from the peak intensity after stimulation. The resulting value was termed “calcium response”.
Chemotaxis
CLL cells were, after 24 h of BCR stimulation, transferred into transwell inserts (Corning Costar) with 5-μm pores. Either medium alone or medium containing CXCL12 (100 ng/ml) or CCL21 (200 ng/ml) was added to the lower well, and the cells were allowed to migrate for 2 h at 37 °C. Migrated cells were then stained for CD5/CD19 and counted using Flow Count Fluorospheres (Beckmann Coulter) as a reference.
Statistical analysis
Calculation of statistical significance was done using Graph Pad Prism Version 5.03. After assessing the datasets for normal distribution, significances were analysed using paired t test in case of normally distributed samples, and Wilcoxon matched pairs test in not normally distributed samples. Differences were considered significant with p < 0.05. P < 0.05 is marked as *, p < 0.01 as **, and p < 0.001 as ***.
Results
IgM stimulation of CLL cells results in increased calcium mobilisation in response to IgD
We investigated BCR and chemokine responses upon α-IgM and α-IgD stimulation in peripheral blood CLL samples. Analysing IgM and IgD surface expression in a cohort of 36 samples, we found a considerable inter-patient variability. Except for eight samples harbouring negligible levels of IgM and IgD on their surface (MFIR = 1), all other samples displayed clearly detectable IgM and IgD expressions (Fig. 1a). We did not observe any association of IgM/IgD expression and the IGHV mutational status in this cohort (data not shown).
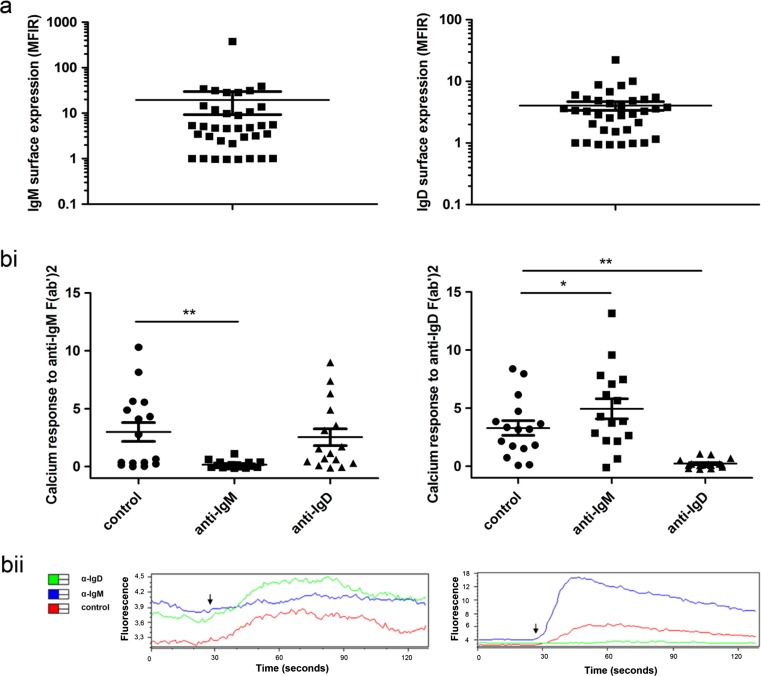
Fig. 1.
IgM- and IgD-mediated calcium mobilisation in CLL. a Surface expression of IgM and IgD was determined by flow cytometry (n = 36). A logarithmic scale is used due to the high inter-patient variation. b CLL cells were prestimulated by α-IgM or α-IgD antibodies (20 μg/ml) for 24 h, and calcium mobilisation upon a second BCR stimulation was measured by flow cytometry (n = 16). Exemplary fluorescence courses are given in bii. The arrows indicate the time of stimulation
In functional studies, we first evaluated the impact of IgM or IgD stimulation on further BCR-mediated calcium mobilisation in CLL samples expressing IgM and IgD. In agreement with the findings of Mockridge et al. [2], calcium responses to IgM and IgD stimulation were, like BCR surface expression, highly variable. Fifty-three percent of investigated CLL cases showed a very weak or no calcium response to α-IgM treatment despite detectable surface IgM levels (Fig. 1b, unstimulated controls). In contrast, the overall response of CLL cells to stimulation with α-IgD was higher, with only 12 % of cases displaying no calcium flux. As expected, preincubation with α-IgM or α-IgD resulted in desensitisation of the prestimulated isotype and thus an abolishment of further BCR-triggered calcium releases. However, we did not observe any cross-desensitisation of the other isotypes by IgM or IgD pre-stimulation. In contrast, the response invoked by α-IgD administration was significantly reinforced by previous incubation with α-IgM.
IgM stimulation increases IgD expression in CLL but not healthy donor-derived B cells
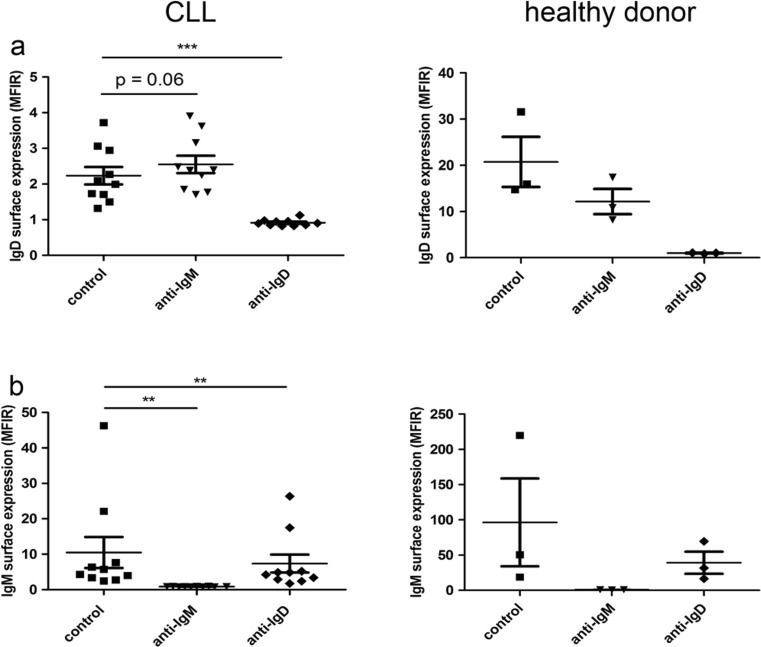
The observation of increased IgD-mediated calcium mobilisation after IgM stimulation raised the question whether this was caused by modulation of IgD surface expression. Indeed, increased IgD-mediated calcium mobilisation in CLL cells upon stimulation with α-IgM was paralleled by a slight increase in IgD surface expression, while a cross-desensitisation was observed in healthy B cells, with a reduction of IgD surface expression after IgM stimulation (Fig. 2a). In contrast, IgD stimulation reduced IgM expression levels in CLL as well as healthy B cells (Fig. 2b).
Fig. 2.
Modulation of BCR surface expression by BCR activation. a IgD and b IgM surface expression was measured by flow cytometry upon BCR activation by 20 μg/ml α-IgM and α-IgD antibodies for 24 h, compared to the appropriate negative control F(ab’)2 in CLL (n = 10) and healthy B cells (n = 3)
Regulation of chemokine receptor surface expression and chemokine-induced calcium responses by α-IgM and α-IgD stimulation is impaired in CLL
The regulation of chemokine receptors is an important process involved in B cell development and activation. Cases of aggressive CLL are characterised by the massive infiltration of bone marrow and lymphoid organs, and altered regulation of homing receptors is involved in disease progression [30]. Therefore, we systematically compared IgM- and IgD-mediated alterations in chemokine receptor expression in CLL and normal B cells.
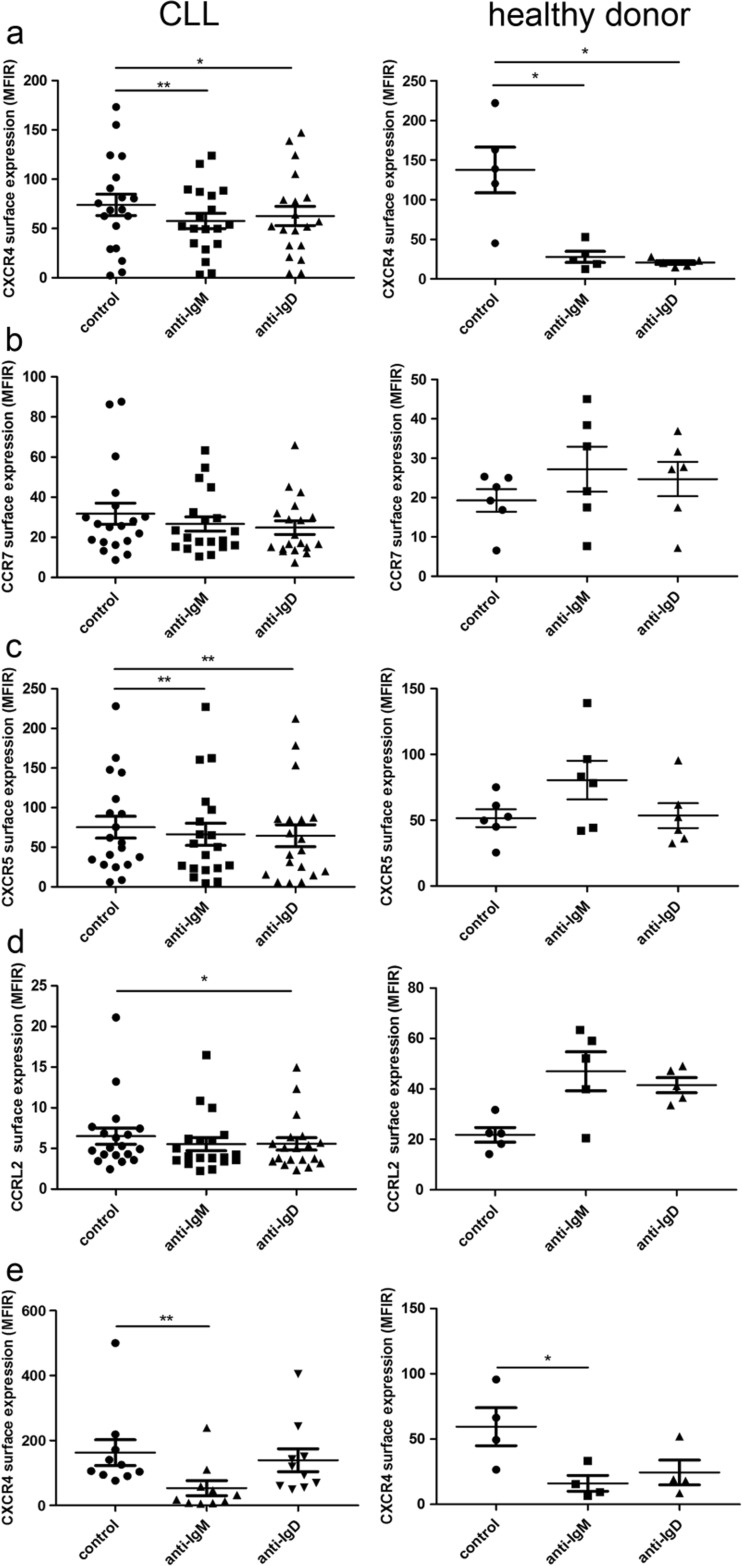
The extent of downregulation of CXCR4 expression in CLL by both soluble IgM and IgD stimulation (decrease to 78 and 85 % of the basal expression) was substantially lower than that observed in healthy donor-derived B cells (decrease to 20 and 15 % of the basal expression, Fig. 3a). Also, no significant regulation of CCR7 expression by IgM and IgD stimulation was observed (Fig. 3b). CXCR5 expression was significantly reduced upon stimulation of both isotypes in CLL cells but upregulated by IgM stimulation in healthy donor-derived B cells (Fig. 3c). Notably, CCRL2 was upregulated after stimulation of both isotypes in healthy donor-derived B cells (Fig. 3d), but slightly downregulated in CLL. Surface expression of the chemokine receptor CXCR3 and the B cell marker CD19 (as a control) were not changed by stimulation of IgM or IgD on CLL cells under the same conditions (data not shown).
Fig. 3.
Regulation of chemokine receptors by BCR activation. Surface expression of a CXCR4, b CCR7, c CXCR5, and d CCRL2 was measured on CLL (n = 19) and healthy donor-derived (n = 5) B cells after 24-h stimulation with soluble α-IgM and α-IgD (20 μg/ml) antibodies. e CXCR4 expression after 24-h stimulation with immobilised α-IgM and α-IgD (20 μg/ml) was assessed on CLL (n = 10) and healthy B cells (n = 4). In all experiments, controls were incubated for the same amount of time with the appropriate negative control F(ab’)2
A stronger BCR stimulus is provided by stimulation with immobilised antibodies, and next, we tested whether this could induce a more distinct chemokine receptor regulation. Indeed, stimulation with immobilised α-IgM significantly reduced CXCR4 expression in CLL cells (Fig. 3e). The extent of downregulation was comparable to that in healthy donor-derived B cells (to 33 % of the original expression in CLL cells and to 27 % in healthy B cells). Immobilised α-IgD exerted no significant effect on CXCR4 expression in CLL, while in healthy B cells, stimulation of IgM and IgD resulted in a comparable CXCR4 downregulation. An IgM-induced reduction of expression was also observed for CXCR5 and CXCR3 (data not shown).
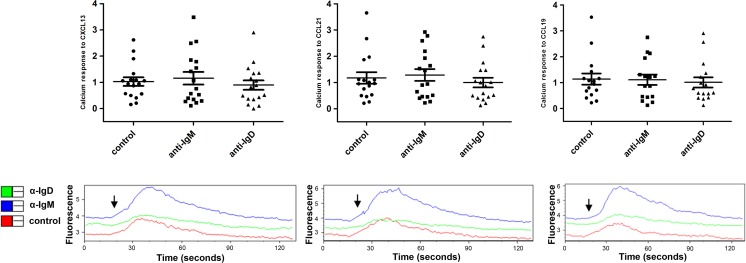
To assess the impact of IgM and IgD stimulation on the chemokine receptor system on a more functional basis, we measured the calcium release induced by chemokines. All samples tested showed a significant calcium response to the chemokines CXCL13, CCL19, and CCL21 (Fig. 4). However, this calcium flux was generally lower than that observed after IgM or IgD stimulation. Moreover, the chemokine-induced calcium flux could not be altered by prior IgM or IgD stimulation (Fig. 4).
Fig. 4.
Calcium mobilisation in response to chemokines. CLL cells (n = 16) were incubated for 24 h with soluble α-IgM or α-IgD antibodies. Calcium mobilisation was induced by the chemokines CXCL13 (500 ng/ml), CCL21 (200 ng/ml), and CCL19 (200 ng/ml) and determined using the Fluo-3 dye as described in materials and methods. The lower panels show representative examples for the kinetics of Fluo-3 fluorescence. The arrows indicate the time at which the chemokine was added
Chemotaxis towards CXCL12 and CCL21 is differentially regulated by IgM and IgD activation
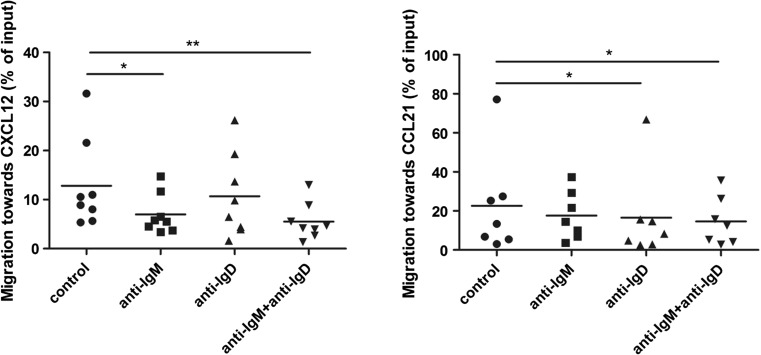
In contrast to calcium mobilisation, chemotaxis towards CXCL12 was significantly reduced upon IgM stimulation but not affected by IgD stimulation. A double stimulation did not reduce chemotaxis towards CXCL12 beyond the reduction seen after IgM stimulation alone (Fig. 5) and also did not further decrease CXCR4 expression compared to single IgM stimulation (data not shown). In contrast, chemotaxis towards the CCR7 ligand CCL21 was reduced by IgD but not IgM stimulation. A reduction similar to IgD stimulation alone was also observed after IgM/IgD double stimulation.
Fig. 5.
Regulation of chemotaxis by BCR activation. Boyden chamber migration assays were performed towards CXCL12 (100 ng/ml; n = 8) and CCL21 (200 ng/ml; n = 7) after 24-h stimulation with α-IgM, α-IgD, or both (10 μg/ml). Cells were allowed to migrate for 2 h and subsequently stained for CD19/CD5 and for viability using Annexin-V-FITC and 7AAD. Only Annexin/7AAD double-negative and CD19/CD5 double-positive cells were counted. All experiments were performed as duplicates; results are shown as the percentage of input cells
Taken together, while chemokine receptor regulation in response to stimulation with α-IgM and α-IgD was reduced in CLL, with compensation when using stronger BCR stimulation by immobilised antigens, differential regulation of CXCL12- and CCR7-mediated chemotaxis was observed after IgM and IgD activation.
Inhibition of Syk, Lyn, and Btk strongly reduces chemokine-induced calcium responses
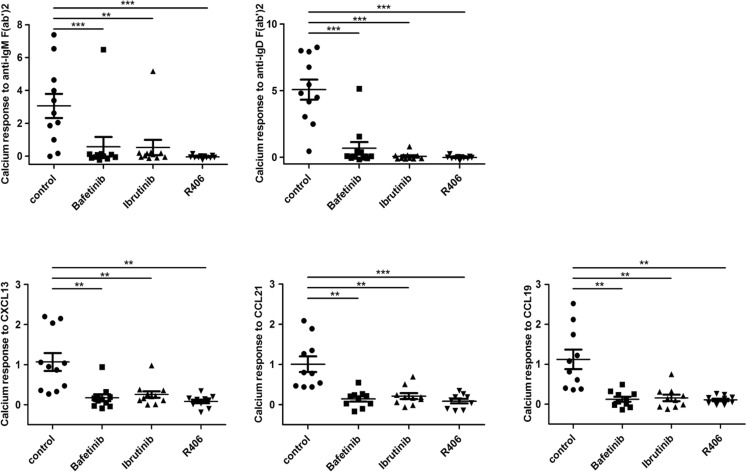
Using inhibitors against kinases mediating BCR signalling, remarkable effects on the anatomical localisation of the CLL cells have been observed in clinical trials, possibly by altering chemokine- and integrin-mediated signal transduction. We thus evaluated the impact of the Lyn/Abl inhibitor Bafetinib (INNO-406), the Btk inhibitor Ibrutinib (PCI-32765), and the Syk inhibitor R406 on BCR- and chemokine-mediated calcium mobilisation. Samples of ten patients showing a wide range of BCR-mediated calcium responses were used for this experiment. In all samples, R406 completely abolished BCR-mediated calcium release and strongly reduced the responses to the chemokines CXCL13, CCL19, and CCL21 (Fig. 6). Bafetinib treatment abolished IgM-mediated calcium release in all patient samples but one, and IgD-mediated response in all except for two cases. Responses to all three chemokines tested were strongly diminished, albeit not completely abrogated. Ibrutinib prevented calcium release after IgM and IgD activation in all cases but one (Fig. 6), also at lower concentrations (Supplementary Fig. 1). It also considerably reduced chemokine-mediated calcium mobilisation. Altogether, these therapeutics are highly effective in abrogating calcium mobilisation after BCR as well as chemokine receptor activation in the majority of CLL samples.
Fig. 6.
Impact of kinase inhibitors on BCR- and chemokine-mediated calcium mobilisation. Calcium mobilisation of CLL cells in response to α-IgM (10 μg/ml), α-IgD (10 μg/ml), CXCL13 (500 ng/ml), CCL21 (200 ng/ml), and CCL19 (200 ng/ml) was assessed as described. After loading with Fluo-3-AM, cells were additionally incubated for 30 min with 5 μM Bafetinib, Ibrutinib, R406, or an equal volume DMSO as a control (n = 11)
Discussion
CLL is a highly environment-dependent tumour with cells quickly dying when taken into solo cell culture [31]. BCR activation is a central stimulus driving CLL survival, proliferation, and pathogenesis [7]. Most CLL cells coexpress IgM and IgD, yet the ratio is highly variable and patient specific. The significance of this coexpression and individual functions for each isotype in normal and malignant B cells are still poorly understood. We thus aimed to determine their influence onto each other as well as on the chemokine system. Early papers reported heterologous desensitisation of IgD function by IgM prestimulation and vice versa in normal mouse and human B cells [32, 33]. In contrast to these findings in non-leukemic B cells, we found no desensitisation of the heterologous isotype in terms of calcium signalling or surface expression after selective stimulation of one BCR isotype in CLL. Notably, there was even an enforcement of IgD-invoked calcium responses by previous incubation with α-IgM antibodies. This went along with slightly increased IgD expression, while in healthy B cells a clear heterologous downmodulation was observed. Similar observations have previously been made in murine lymphoma cells [34], indicating that this phenomenon may be intrinsic to certain types of B cell malignancies. Taking into account the autonomous BCR signalling in CLL, the increase in calcium mobilisation and surface expression may reflect the inhibition of a constitutive internalisation and recycling process by IgM stimulation, leading to an accumulation of IgD at the cell surface.
BCR activation fundamentally alters the expression of several chemokine receptors on normal B cells in vivo, thereby assuring their correct localisation during the following steps of the immune response [35]. Our results confirm the previous observation of CXCR4 downmodulation in normal B cells upon IgM engagement [26] and add the observation of a comparable reduction upon IgD activation in these cells. In contrast, CLL cells were less susceptible to IgM- and IgD-induced CXCR4 and CXCR5 regulation than normal B cells, which may mirror the disrupted architecture and diffusion of the regular follicular chemokine gradients in CLL lymphoid organs [36, 37]. Notably, the impaired chemokine receptor regulation upon weak soluble BCR stimulation was independent of the extent of general IgM responsiveness and thus not attributable to general features of anergy. It was also uncoupled from functional regulation as we observed comparable effects to the reduction in migration by soluble stimulation to those reported by Vlad [28] using immobilised BCR stimulation.
An interesting novel finding of our study is the differential influence of IgM versus IgD activation on the chemotactic preference of CLL cells. IgM-stimulated CLL cells retained chemotaxis towards the key lymph node chemokine CCL21 but displayed downregulated migration to CXCL12 suggesting a preferential role of CCR7 for migration of antigen-stimulated CLL cells within the lymphoid microenvironment. In contrast, the retained chemotaxis towards CXCL12 but not CCL21 upon IgD stimulation may suggest a different niche preference of these cells. Further studies should address whether the homing propensities of CLL cells to distinct organs depend on different IgD/IgM ratios of these cells.
Finally, we found inhibition of both chemokine- and BCR-mediated calcium responses by R406, Bafetinib, and Ibrutinib. While expected for BCR-induced calcium signalling, the strong dependence of chemokine receptor-induced calcium signals for all three kinases was unanticipated. This indicates a largely overlapping signalling cascade leading to the mobilisation of intracellular calcium, which probably converges on the common regulator Plcγ2. Residual Plcγ2 activity upon Lyn or Btk inhibition [38, 39] may account for intact calcium signals in exceptional patient samples (Fig. 6a) and could be caused by activating mutations, which should be addressed in future studies in detail.
Taken together, our data show that in addition to the generally reduced BCR responsiveness, CLL cells display defects in the regulation of chemokine receptors after BCR activation, as well as a specific regulation of migratory preferences by IgM and IgD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Characterisation of patient samples used for this study. IGHV mutation status (M = mutated; UM = unmutated; ND = not determined), the cohort (FR = Freiburg; S = Salzburg) and IgM and IgD surface expression as MFIR measured 2 h after thawing are summed up for all patient samples used in this study. All patients were untreated at the time of sample collection. Measurements of the Freiburg cohort were performed using α-IgM-PE antibody from Biolegend and α-IgD-FITC antibody from BD, measurements of the Salzburg cohort using α-IgM-PE antibody from Beckman Coulter and α-IgD-PE antibody from BD. (PDF 122 kb)
Effect of 1 μM Ibrutinib on BCR mediated calcium mobilisation. Calcium mobilisation of CLL cells in response to α-IgM (10 μg/ml), α-IgD (10 μg/ml) after incubation with 1 μM Ibrutinib was assessed (n = 3). Calcium responses after inhibitor treatment were normalised to the control responses. (TIF 27169 kb)
Acknowledgments
This research was funded by the German Federal Ministry of Education and Research (BMBF 01 EO 0803) and supported by the German Federal Ministry of Education and Research (BMBF 01EO1303) (to M.B.), the Monika-Kutzner foundation (to M.B. and T.N.H.), and Austrian Science Fund (FWF) P26421 to T.N.H. Open access funding provided by Paracelsus Medical University.
Compliance with ethical standards
Blood samples were collected from CLL patients after informed consent and ethical approval at the Freiburg Medical University Center and the Third Medical Department, Paracelsus Medical University Salzburg.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Tanja N. Hartmann and Meike Burger contributed equally to this work.
References
- 1.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mockridge CI, Potter KN, Wheatley I, Neville LA, Packham G, Stevenson FK. Reversible anergy of sIgM-mediated signaling in the two subsets of CLL defined by VH-gene mutational status. Blood. 2007;109(10):4424–4431. doi: 10.1182/blood-2006-11-056648. [DOI] [PubMed] [Google Scholar]
- 3.Muzio M, Apollonio B, Scielzo C, Frenquelli M, Vandoni I, Boussiotis V, Caligaris-Cappio F, Ghia P. Constitutive activation of distinct BCR-signaling pathways in a subset of CLL patients: a molecular signature of anergy. Blood. 2008;112(1):188–195. doi: 10.1182/blood-2007-09-111344. [DOI] [PubMed] [Google Scholar]
- 4.Lanham S, Hamblin T, Oscier D, Ibbotson R, Stevenson F, Packham G. Differential signaling via surface IgM is associated with VH gene mutational status and CD38 expression in chronic lymphocytic leukemia. Blood. 2003;101(3):1087–1093. doi: 10.1182/blood-2002-06-1822. [DOI] [PubMed] [Google Scholar]
- 5.Morabito F, Cutrona G, Gentile M, Fabbi M, Matis S, Colombo M, Reverberi D, Megna M, Spriano M, Callea V, Vigna E, Rossi E, Lucia E, Festini G, Zupo S, Molica S, Neri A, Ferrarini M. Prognostic relevance of in vitro response to cell stimulation via surface IgD in binet stage a CLL. Br J Haematol. 2010;149(1):160–163. doi: 10.1111/j.1365-2141.2009.08032.x. [DOI] [PubMed] [Google Scholar]
- 6.Stamatopoulos K, Belessi C, Moreno C, Boudjograh M, Guida G, Smilevska T, Belhoul L, Stella S, Stavroyianni N, Crespo M, Hadzidimitriou A, Sutton L, Bosch F, Laoutaris N, Anagnostopoulos A, Montserrat E, Fassas A, Dighiero G, Caligaris-Cappio F, Merle-Beral H, Ghia P, Davi F. Over 20% of patients with chronic lymphocytic leukemia carry stereotyped receptors: pathogenetic implications and clinical correlations. Blood. 2007;109(1):259–270. doi: 10.1182/blood-2006-03-012948. [DOI] [PubMed] [Google Scholar]
- 7.Stevenson FK, Caligaris-Cappio F. Chronic lymphocytic leukemia: revelations from the B-cell receptor. Blood. 2004;103(12):4389–4395. doi: 10.1182/blood-2003-12-4312. [DOI] [PubMed] [Google Scholar]
- 8.Lanemo Myhrinder A, Hellqvist E, Sidorova E, Soderberg A, Baxendale H, Dahle C, Willander K, Tobin G, Backman E, Soderberg O, Rosenquist R, Horkko S, Rosen A. A new perspective: molecular motifs on oxidized LDL, apoptotic cells, and bacteria are targets for chronic lymphocytic leukemia antibodies. Blood. 2008;111(7):3838–3848. doi: 10.1182/blood-2007-11-125450. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Munoz R, Llorente L. Chronic lymphocytic leukaemia: could immunological tolerance mechanisms be the origin of lymphoid neoplasms? Immunology. 2014;142(4):536–550. doi: 10.1111/imm.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoogeboom R, van Kessel KP, Hochstenbach F, Wormhoudt TA, Reinten RJ, Wagner K, Kater AP, Guikema JE, Bende RJ, van Noesel CJ. A mutated B cell chronic lymphocytic leukemia subset that recognizes and responds to fungi. J Exp Med. 2013;210(1):59–70. doi: 10.1084/jem.20121801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duhren-Von Minden M, Ubelhart R, Schneider D, Wossning T, Bach MP, Buchner M, Hofmann D, Surova E, Follo M, Kohler F, Wardemann H, Zirlik K, Veelken H, Jumaa H. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature. 2012;489(7415):309–312. doi: 10.1038/nature11309. [DOI] [PubMed] [Google Scholar]
- 12.Iacovelli S, Hug E, Bennardo S, Duehren-Von Minden M, Gobessi S, Rinaldi A, Suljagic M, Bilbao D, Bolasco G, Eckl-Dorna J, Niederberger V, Autore F, Sica S, Laurenti L, Wang H, Cornall RJ, Clarke SH, Croce CM, Bertoni F, Jumaa H, Efremov DG. Two types of BCR interactions are positively selected during leukemia development in the Emu-TCL1 transgenic mouse model of CLL. Blood. 2015;125(10):1578–1588. doi: 10.1182/blood-2014-07-587790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, Bairey O, Hillmen P, Bartlett NL, Li J, Simpson D, Grosicki S, Devereux S, McCarthy H, Coutre S, Quach H, Gaidano G, Maslyak Z, Stevens DA, Janssens A, Offner F, Mayer J, O’Dwyer M, Hellmann A, Schuh A, Siddiqi T, Polliack A, Tam CS, Suri D, Cheng M, Clow F, Styles L, James DF, Kipps TJ, Investigators R. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–2437. doi: 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Sukbuntherng J, Chang BY, Clow F, Hedrick E, Buggy JJ, James DF, O’Brien S. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chanan-Khan A, Cramer P, Demirkan F, Fraser G, Silva RS, Grosicki S, Pristupa A, Janssens A, Mayer J, Bartlett NL, Dilhuydy MS, Pylypenko H, Loscertales J, Avigdor A, Rule S, Villa D, Samoilova O, Panagiotidis P, Goy A, Mato A, Pavlovsky MA, Karlsson C, Mahler M, Salman M, Sun S, Phelps C, Balasubramanian S, Howes A, Hallek M, Investigators H. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17(2):200–211. doi: 10.1016/S1470-2045(15)00465-9. [DOI] [PubMed] [Google Scholar]
- 16.Friedberg JW, Sharman J, Sweetenham J, Johnston PB, Vose JM, Lacasce A, Schaefer-Cutillo J, De Vos S, Sinha R, Leonard JP, Cripe LD, Gregory SA, Sterba MP, Lowe AM, Levy R, Shipp MA. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115(13):2578–2585. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burger JA, Montserrat E. Coming full circle: 70 years of chronic lymphocytic leukemia cell redistribution, from glucocorticoids to inhibitors of B-cell receptor signaling. Blood. 2013;121(9):1501–1509. doi: 10.1182/blood-2012-08-452607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herman SE, Mustafa RZ, Jones J, Wong DH, Farooqui M, Wiestner A. Treatment with ibrutinib inhibits BTK- and VLA-4-dependent adhesion of chronic lymphocytic leukemia cells in vivo. Clin Cancer Res. 2015;21(20):4642–4651. doi: 10.1158/1078-0432.CCR-15-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchner M, Baer C, Prinz G, Dierks C, Burger M, Zenz T, Stilgenbauer S, Jumaa H, Veelken H, Zirlik K. Spleen tyrosine kinase inhibition prevents chemokine- and integrin-mediated stromal protective effects in chronic lymphocytic leukemia. Blood. 2010;115(22):4497–4506. doi: 10.1182/blood-2009-07-233692. [DOI] [PubMed] [Google Scholar]
- 20.Quiroga MP, Balakrishnan K, Kurtova AV, Sivina M, Keating MJ, Wierda WG, Gandhi V, Burger JA. B-cell antigen receptor signaling enhances chronic lymphocytic leukemia cell migration and survival: specific targeting with a novel spleen tyrosine kinase inhibitor, R406. Blood. 2009;114(5):1029–1037. doi: 10.1182/blood-2009-03-212837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown JR. Ibrutinib (PCI-32765), the first BTK (Bruton’s tyrosine kinase) inhibitor in clinical trials. Curr Hematol Malig Rep. 2013;8(1):1–6. doi: 10.1007/s11899-012-0147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burger M, Hartmann T, Krome M, Rawluk J, Tamamura H, Fujii N, Kipps TJ, Burger JA. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood. 2005;106(5):1824–1830. doi: 10.1182/blood-2004-12-4918. [DOI] [PubMed] [Google Scholar]
- 23.de Gorter DJ, Beuling EA, Kersseboom R, Middendorp S, van Gils JM, Hendriks RW, Pals ST, Spaargaren M. Bruton’s tyrosine kinase and phospholipase Cgamma2 mediate chemokine-controlled B cell migration and homing. Immunity. 2007;26(1):93–104. doi: 10.1016/j.immuni.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Catusse J, Leick M, Groch M, Clark DJ, Buchner MV, Zirlik K, Burger M. Role of the atypical chemoattractant receptor CRAM in regulating CCL19 induced CCR7 responses in B-cell chronic lymphocytic leukemia. Mol Cancer. 2010;9:297. doi: 10.1186/1476-4598-9-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganghammer S, Gutjahr J, Hutterer E, Krenn PW, Pucher S, Zelle-Rieser C, Johrer K, Wijtmans M, Leurs R, Smit MJ, Gattei V, Greil R, Hartmann TN. Combined CXCR3/CXCR4 measurements are of high prognostic value in chronic lymphocytic leukemia due to negative co-operativity of the receptors. Haematologica. 2016;101(3):e99–e102. doi: 10.3324/haematol.2015.133470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guinamard R, Signoret N, Ishiai M, Marsh M, Kurosaki T, Ravetch JV. B cell antigen receptor engagement inhibits stromal cell-derived factor (SDF)-1alpha chemotaxis and promotes protein kinase C (PKC)-induced internalization of CXCR4. J Exp Med. 1999;189(9):1461–1466. doi: 10.1084/jem.189.9.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reif K, Ekland EH, Ohl L, Nakano H, Lipp M, Forster R, Cyster JG. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002;416(6876):94–99. doi: 10.1038/416094a. [DOI] [PubMed] [Google Scholar]
- 28.Vlad A, Deglesne PA, Letestu R, Saint-Georges S, Chevallier N, Baran-Marszak F, Varin-Blank N, Ajchenbaum-Cymbalista F, Ledoux D. Down-regulation of CXCR4 and CD62L in chronic lymphocytic leukemia cells is triggered by B-cell receptor ligation and associated with progressive disease. Cancer Res. 2009;69(16):6387–6395. doi: 10.1158/0008-5472.CAN-08-4750. [DOI] [PubMed] [Google Scholar]
- 29.Saint Georges S, Quettier M, Bouyaba M, Le Coquil S, Lauriente V, Guittat L, Levy V, Ajchenbaum-Cymbalista F, Varin-Blank N, Le Roy C, Ledoux D (2016) Protein kinase D-dependent CXCR4 down-regulation upon BCR triggering is linked to lymphadenopathy in chronic lymphocytic leukaemia. Oncotarget. doi:10.18632/oncotarget.9031 [DOI] [PMC free article] [PubMed]
- 30.Hartmann TN, Grabovsky V, Wang W, Desch P, Rubenzer G, Wollner S, Binsky I, Vallon-Eberhard A, Sapoznikov A, Burger M, Shachar I, Haran M, Honczarenko M, Greil R, Alon R. Circulating B-cell chronic lymphocytic leukemia cells display impaired migration to lymph nodes and bone marrow. Cancer Res. 2009;69(7):3121–3130. doi: 10.1158/0008-5472.CAN-08-4136. [DOI] [PubMed] [Google Scholar]
- 31.Collins RJ, Verschuer LA, Harmon BV, Prentice RL, Pope JH, Kerr JF. Spontaneous programmed death (apoptosis) of B-chronic lymphocytic leukaemia cells following their culture in vitro. Br J Haematol. 1989;71(3):343–350. doi: 10.1111/j.1365-2141.1989.tb04290.x. [DOI] [PubMed] [Google Scholar]
- 32.Cambier J, Chen ZZ, Pasternak J, Ransom J, Sandoval V, Pickles H. Ligand-induced desensitization of B-cell membrane immunoglobulin-mediated Ca2+ mobilization and protein kinase C translocation. Proc Natl Acad Sci U S A. 1988;85(17):6493–6497. doi: 10.1073/pnas.85.17.6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rijkers GT, Griffioen AW, Zegers BJ, Cambier JC. Ligation of membrane immunoglobulin leads to inactivation of the signal-transducing ability of membrane immunoglobulin, CD19, CD21, and B-cell gp95. Proc Natl Acad Sci U S A. 1990;87(22):8766–8770. doi: 10.1073/pnas.87.22.8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geisberger R, Konigsberger S, Achatz G. Membrane IgM influences membrane IgD mediated antigen internalization in the B cell line Bcl1. Immunol Lett. 2006;102(2):169–176. doi: 10.1016/j.imlet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Stein JV, Nombela-Arrieta C. Chemokine control of lymphocyte trafficking: a general overview. Immunology. 2005;116(1):1–12. doi: 10.1111/j.1365-2567.2005.02183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Girbl T, Hinterseer E, Grossinger EM, Asslaber D, Oberascher K, Weiss L, Hauser-Kronberger C, Neureiter D, Kerschbaum H, Naor D, Alon R, Greil R, Hartmann TN. CD40-mediated activation of chronic lymphocytic leukemia cells promotes their CD44-dependent adhesion to hyaluronan and restricts CCL21-induced motility. Cancer Res. 2013;73(2):561–570. doi: 10.1158/0008-5472.CAN-12-2749. [DOI] [PubMed] [Google Scholar]
- 37.Till KJ, Lin K, Zuzel M, Cawley JC. The chemokine receptor CCR7 and alpha4 integrin are important for migration of chronic lymphocytic leukemia cells into lymph nodes. Blood. 2002;99(8):2977–2984. doi: 10.1182/blood.V99.8.2977. [DOI] [PubMed] [Google Scholar]
- 38.Liu TM, Woyach JA, Zhong Y, Lozanski A, Lozanski G, Dong S, Strattan E, Lehman A, Zhang X, Jones JA, Flynn J, Andritsos LA, Maddocks K, Jaglowski SM, Blum KA, Byrd JC, Dubovsky JA, Johnson AJ. Hypermorphic mutation of phospholipase C, gamma2 acquired in ibrutinib-resistant CLL confers BTK independency upon B-cell receptor activation. Blood. 2015;126(1):61–68. doi: 10.1182/blood-2015-02-626846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woyach JA, Furman RR, Liu TM, Ozer HG, Zapatka M, Ruppert AS, Xue L, Li DH, Steggerda SM, Versele M, Dave SS, Zhang J, Yilmaz AS, Jaglowski SM, Blum KA, Lozanski A, Lozanski G, James DF, Barrientos JC, Lichter P, Stilgenbauer S, Buggy JJ, Chang BY, Johnson AJ, Byrd JC. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370(24):2286–2294. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characterisation of patient samples used for this study. IGHV mutation status (M = mutated; UM = unmutated; ND = not determined), the cohort (FR = Freiburg; S = Salzburg) and IgM and IgD surface expression as MFIR measured 2 h after thawing are summed up for all patient samples used in this study. All patients were untreated at the time of sample collection. Measurements of the Freiburg cohort were performed using α-IgM-PE antibody from Biolegend and α-IgD-FITC antibody from BD, measurements of the Salzburg cohort using α-IgM-PE antibody from Beckman Coulter and α-IgD-PE antibody from BD. (PDF 122 kb)
Effect of 1 μM Ibrutinib on BCR mediated calcium mobilisation. Calcium mobilisation of CLL cells in response to α-IgM (10 μg/ml), α-IgD (10 μg/ml) after incubation with 1 μM Ibrutinib was assessed (n = 3). Calcium responses after inhibitor treatment were normalised to the control responses. (TIF 27169 kb)