Abstract
In this study, we investigated whether the single nucleotide polymorphisms (SNPs) associated with telomere length (TL) were associated with the incidence of hypertension (HTN)/coronary heart disease (CHD) and cardiovascular risk factors in the Korean population. Data from 5,705 (ages 39–70) participants in the Korean Genome Epidemiology Study (rural Ansung and urban Ansan cohorts) were studied. Twelve SNPs known to be associated with telomere biology were tested for an association with HTN/CHD. As results, no significant associations were found between the selected TL-related SNPs and prevalence of HTN and CHD. Among non-alcohol users, subjects with minor alleles in rs1269304 and rs10936601 (TERC and LRRC34, respectively) exhibited a higher rate of CHD occurrence (odds ratio [OR], 1.862; 95% confidence intervals [CIs], 1.137, 3.049; OR, 1.855; 95% CIs, 1.111, 2.985; respectively). However, alcohol users with minor alleles in rs398652 (PELI2) were significantly associated with higher HTN prevalence (OR, 1.179; 95% CIs, 1.040, 1.336). Of the 3 SNPs related to disease outcomes, rs1296304 was significantly associated with increased levels of diastolic blood pressure (β estimate, 0.470; 95% CIs, 0.013, 0.926). The minor allele in rs398652 was significantly associated with higher levels of body mass index (OR, 0.128; 95% CIs, 0.010, 0.246) and γ-glutamyl transpeptidase (OR, 0.013; 95% CIs, 0.001, 0.024). In conclusion, there were no significant associations between the selected TL-related SNPs and the occurrence of HTN/CHD in Koreans. However, the results suggest the presence of a possible interaction between related SNPs and alcohol behavior associated with HTN/CHD occurrence.
Keywords: Telomere length, Genetic variants, Hypertension, Coronary heart disease, Korean Genome Epidemiology Study
INTRODUCTION
In humans, telomere shortening is regarded as a predictor of aging and aging-related diseases [1]. Telomeres are nucleoprotein complexes composed of short DNA repeats found at the ends of eukaryotic chromosomes. They are made up of TTAGGG sequence repeats and associated with sequence-specific binding proteins in humans [2]. These specialized ribonucleoprotein complexes protect the chromosome from degradation by nucleases, and inhibit abnormal DNA repair. Additionally, complexed structure of telomere plays a major role in genomic stability [3]. The loss of telomeric DNA occurs whenever the genome is replicated during cell division; this degradation proceeds progressively until the telomeres become critically shortened [4].
Many previous studies have suggested that a relatively short telomere length (TL) is associated with a higher risk of developing aging-related chronic diseases, particularly cardiovascular dysfunctions [5,6,7,8]. These results indicate that increased telomere shortening is a risk factor for the manifestation of aging-related cardiovascular disease (CVD), and is indicative of an active inflammatory response to oxidative stress [9]. Furthermore, genetic variants implicated in TL have been reported to be associated with the incidence of aging-related chronic diseases [10]. For example, two single nucleotide polymorphisms (SNPs) in OBFC1 (rs10786775 C > G, rs11591710 A > C) were found to be associated with a higher risk of developing coronary heart disease (CHD), and three SNPs in TERC (rs12696304 C > G, rs10936601 G > T and rs16847897 G > C) were correlated with a higher risk of CHD and the type II diabetes mellitus (T2DM) [11]. It was also reported that genetic variations in BICD1 (rs2630578, rs1151026) and near TERC (rs16847897) were correlated with the manifestation of CVD. In a Japanese case study, genetic variations in TERT were significantly correlated with coronary artery disease (CAD) [12]. Moreover, in recent clinical studies, it was shown that subjects with a variant allele of rs398652 (associated with longer telomeres) have a significantly reduced risk of developing bladder cancer [13]. In addition, rs398652 genetic variants have an obvious impact on TL and esophageal squamous cell carcinoma susceptibility in Chinese populations [14]. While evidence for the causal role of TL variation in some chronic diseases has accumulated [15], the link between SNPs involved in telomere biology and the risk of hypertension (HTN), CHD, and its risk factors, has not been extensively studied in Korean populations. In this study, we examined whether SNPs previously reported to be associated with TL in humans are related to the incidence of HTN/CHD and risk factors of CVD, in a Korean population.
MATERIALS AND METHODS
Study population
Study subjects were obtained from two cohorts within the Korean Genome Epidemiology Study (KoGES): the rural Ansung and urban Ansan cohorts. The details of the KoGES have been described elsewhere [16]. Briefly, the Ansung and Ansan cohorts were designed as longitudinal prospective studies and initiated in 2001, with the aim of investigating risk factors for chronic diseases in Koreans. The KoGES included a health examination, interviews, and laboratory tests; it recruited 10,038 participants from each area (5,018 in Ansung and 5,020 in Ansan aged 39–70) using a two-stage cluster sampling method. Of the recruited participants, 1,196 individuals with poor genotyping data were excluded from the baseline data. This study was based on the baseline data collected in 2001, with subjects providing data for important analytic variables such as HTN, CHD, and 12 primary SNPs. Overall, 5,705 subjects were included in the statistical analysis. All participants provided written consent forms, a process approved by the Human Subjects Review Committee at Korea University Asan Hospital and the Ajou University Medical Center. This study was approved by the Institutional Review Board of Korea University (KU-IRB-14-EX-153-A-1).
General characteristics
Socio-demographic information including age, sex, residential area, educational level, physical activity, smoking status, and alcohol use were obtained from KoGES. Residential area was divided into a rural area, Ansung, and an urban area, Ansan. Education level was categorized into four levels, defined by the highest level of education received: elementary school, middle school, high school, and university. Physical activity was classified into two groups according to whether or not the individual performed any of the following daily activities: at least 20 minutes of intense physical activity, at least 30 minutes of moderate physical activity, or at least 30 minutes of walking. Subjects were divided into three groups according to their smoking status during the survey period, as follows: “nonsmoker,” “ex-smoker,” or “current smoker.” Similarly, a current drinker was defined as a subject who drank alcoholic beverages regularly during the survey period.
Anthropometric and biochemical measurements
Information on anthropometric and biochemical measurements was obtained from health examination data in KoGES, and originally collected by trained experts. Body mass index (BMI, kg/m2) was calculated by dividing the weight (kg) of an individual by their height squared (m2), which was measured to the nearest 0.1 cm or 0.1 kg, respectively. Waist circumference (WC, cm) was measured at the narrowest point between the lower rib and the iliac crest (measured to the nearest 0.1 cm), and the average of three repeated measurements was recorded. Blood pressure was measured while the individual was in a sitting position, using a mercury sphygmomanometer. The average of two repeated measurements was used. For laboratory tests, all participants fasted for at least an 8 hours period prior to blood collection. The levels of total cholesterol (TC, mg/dL), triglycerides (TG, mg/dL), HDL cholesterol (HDLC, mg/dL), fasting blood glucose (FBG, mg/dL), γ-glutamyl transpeptidase (GTP, IU/L), and high sensitivity C reactive protein (hs-CRP) were measured by Seoul Clinical Laboratories (Seoul, Korea). LDL cholesterol (LDLC, mg/dL) was calculated using the Friedewald formula in subjects with TG less than 400 mg/dL, as follows [17]:
LDL cholesterol = total cholesterol − HDL cholesterol − (triglycerides / 5.0)
For subjects with TG of 400 mg/dL or more, the LDLC value was marked as being unavailable.
Disease outcomes
Diagnoses of HTN and CHD were based on an evaluation of self-reported medical history. Subjects who were diagnosed with HTN, used blood pressure medicine, or presented with a systolic blood pressure reading greater than 140 mmHg or a diastolic blood pressure (DBP) reading greater than 90 mmHg were considered to exhibit HTN. CHD was diagnosed by clinical doctors or using self-reported questionnaire information on myocardial infarction (MI), and CAD.
DNA genotyping and imputation
Detailed information on genotyping is provided elsewhere [16]. Briefly, DNA samples were isolated from the peripheral blood of participants and genotyped using the Affymetrix Genome-Wide Human SNP array 5.0 (Affymetrix, Inc., Santa Clara, CA, USA). The accuracy of the genotyping was calculated via Bayesian Robust Linear Modelling using the Mahalnobis Distance genotyping algorithm. Participants with a high missing genotype call rate, high heterozygosity, any kind of diagnosed cancer, and sex inconsistencies were excluded from the study. Furthermore, SNPs with high missing genotype call rates (> 5%), minor allele frequencies (MAF) < 0.01, and not in Hardy-Weinberg equilibrium (HWE, p value < 1 × 10-6) were excluded. Ultimately, a total of 352,228 SNPs in 8,842 participants were used in the analysis. To obtain additional genotype information, SNP markers were imputed from a HapMap reference panel consisting of 3.99 million SNPs (HapMap release 22) in 90 individuals from Japanese individuals in Tokyo (JPT) and Han Chinese individuals from Beijing, China (CHB), using the IMPUTE software program (University of Oxford, Oxford, United Kingdom). In total, 1,804,397 SNPs remained after this process of quality control, from which SNPs with a minor allele frequency < 0.01 or an information score < 0.30 were removed.
Telomere length in relation to SNP selection
Previous genome-wide association study (GWAS) publications focusing on telomeres were found using “telomere” or “telomere length” as keywords in the GWAS Catalog (https://www.genome.gov/26525384, 31 SNPs). Based on these previous studies, SNPs in “TERT” and “TERC,” which are well known as TL-related genes, were also extracted from the GWAS Catalog (30 and 7 SNPs, respectively). We then searched two of the most recent GWAS publications in NCBI PubMed (http://www.ncbi.nlm.nih.gov/pubmed/), using the same keywords (2 SNPs). Of the 70 SNPs discovered during the previous GWAS studies [13,18,19,20,21,22,23,24,25,26], 8 genotyped SNPs and 4 imputed SNPs in independent genes were presented in our genotype data.
Statistical analyses
Statistical analyses were performed using Stata SE 12.0 (Stata Corp., College Station, TX, USA). First, the distribution of variable values was investigated. FBG, HDLC, TG, γ-GTP, and hs-CRP were log-transformed. The general characteristics of the subjects were described as mean ± standard error (SE) for continuous variables, and as a frequency (%, n) for categorical variables. Differences in characteristics associated with disease status were determined using Student’s t-test for continuous variables, and using χ2 tests for categorical variables. The association between selected SNPs and disease prevalence (HTN and CHD) was assessed using a logistic regression in an additive scale model, with adjustments for age, sex, area, BMI, education level, physical activity, smoking status, and alcohol use. The results are presented as the estimated odds ratio (OR) with 95% confidence intervals (95% CIs). To estimate the effect of alcohol use on these associations, the association between SNPs and disease outcomes was assessed using the same logistic regression model to analyze the data from alcohol users and non-users separately. Of the 12 SNPs, we selected three SNPs of interest in order to estimate the size of their effect size on cardiovascular risk factors. A linear regression model was used after adjustment for important covariates. Results are presented as estimated regression coefficients β with 95% CI. All statistical differences were determined at levels of p < 0.05.
RESULTS
General characteristics of the subjects
The general characteristics of the subjects are described in Table 1, according to the presence of HTN and CHD. The mean age was 48.2 ± 0.1 year, and nearly half of the subjects (49%) were male. HTN patients were older, and included higher proportions of subjects who were male, rural residents, alcohol users, ex-smokers, and less educated, compared to the control group (p < 0.05). HTN patients also had a higher average blood pressure, BMI, WC, FBG, LDLC, TG, γ-GTP, and hs-CRP, and lower average HDLC than the control group (p < 0.05). CHD patients were older, and had a higher average blood pressure, WC, TC, TG, and hs-CRP than the control group (p < 0.05). The genotype frequencies of selected SNPs are presented in Table 1.
Table 1. Baseline characteristics of the subjects.
| Characteristics | All (n = 5,705) | Hypertension | p value† | Coronary heart disease | p value† | |||
|---|---|---|---|---|---|---|---|---|
| Control (n = 4,113) | Case (n = 1,592) | Control (n = 5,640) | Case (n = 65) | |||||
| Age, yr* | 48.2 ± 0.1 | 47.3 ± 0.1 | 50.5 ± 0.2 | < 0.0001 | 48.2 ± 0.1 | 53.0 ± 0.6 | < 0.0001 | |
| Males, % (No.) | 48.9 (2,792) | 47.1 (1,936) | 53.8 (856) | < 0.0001 | 48.8 (2,754) | 48.5 (38) | 0.1220 | |
| Area, % (No.) | < 0.0001 | < 0.0001 | ||||||
| Rural (Ansung) | 38.6 (2,202) | 33.5 (1,379) | 51.7 (823) | 38.3 (2,159) | 66.2 (43) | |||
| Urban (Ansan) | 61.4 (3,503) | 66.5 (2,734) | 48.3 (769) | 61.7 (3,481) | 33.9 (22) | |||
| Education level, % (No.)‡ | < 0.0001 | 0.0440 | ||||||
| ≤ Elementary | 22.4 (1,269) | 18.9 (775) | 31.4 (494) | 22.3 (1,249) | 30.8 (20) | |||
| ≤ Middle | 25.1 (1,422) | 24.8 (1,018) | 25.6 (404) | 25.0 (1,402) | 30.8 (20) | |||
| ≤ High | 36.5 (2,074) | 39.7 (1,628) | 28.3 (446) | 36.7 (2,061) | 20.0 (13) | |||
| ≥ University | 16.1 (912) | 16.6 (680) | 14.7 (232) | 16.0 (900) | 18.5 (12) | |||
| Physical activity, % (No.) | 84.4 (4,814) | 84.3 (3,467) | 84.7 (1,347) | 0.7440 | 84.4 (4,757) | 87.7 (57) | 0.4630 | |
| Alcohol use, % (No.) | 0.0390 | 0.0640 | ||||||
| Never | 43.0 (2,441) | 43.9 (1,797) | 40.8 (644) | 43.0 (2,414) | 42.2 (27) | |||
| Previous | 5.8 (328) | 5.4 (222) | 6.7 (106) | 5.7 (320) | 12.5 (8) | |||
| Current | 51.2 (2,905) | 50.7 (2,077) | 52.5 (828) | 51.3 (2,876) | 45.3 (29) | |||
| Smoking, % (No.) | < 0.0001 | 0.0300 | ||||||
| Never | 58.0 (3,271) | 58.8 (2,399) | 55.6 (872) | 58.1 (3,243) | 43.1 (28) | |||
| Previous | 15.3 (864) | 13.9 (566) | 19.0 (298) | 15.2 (848) | 24.6 (16) | |||
| Current | 26.8 (1,510) | 27.3 (1,113) | 26.3 (397) | 26.7 (1,489) | 32.3 (21) | |||
| SBP, mmHg | 118.9 ± 0.2 | 111.3 ± 0.2 | 138.5 ± 0.4 | < 0.0001 | 118.8 ± 0.2 | 126.7 ± 2.1 | 0.0003 | |
| DBP, mmHg | 79.6 ± 0.2 | 74.7 ± 0.1 | 92.3 ± 0.2 | < 0.0001 | 79.6 ± 0.2 | 83.1 ± 1.3 | 0.0159 | |
| BMI, kg/m2 | 24.7 ± 0.0 | 24.3 ± 0.0 | 25.9 ± 0.1 | < 0.0001 | 24.7 ± 0.0 | 25.4 ± 0.3 | 0.0618 | |
| WC, cm | 82.0 ± 0.1 | 80.5 ± 0.1 | 86.0 ± 0.2 | < 0.0001 | 82.0 ± 0.1 | 86.2 ± 1.0 | 0.0001 | |
| FBG, log (mg/dL) | 87.8 ± 0.3 | 86.0 ± 0.3 | 92.4 ± 0.7 | < 0.0001 | 87.7 ± 0.3 | 90.7 ± 2.6 | 0.1464 | |
| TC, mg/dL | 191.8 ± 35.9 | 189.4 ± 0.5 | 198.1 ± 1.0 | < 0.0001 | 191.7 ± 0.5 | 201.0 ± 4.7 | 0.0376 | |
| HDLC, log (mg/dL) | 44.8 ± 0.1 | 45.2 ± 0.2 | 43.7 ± 0.2 | < 0.0001 | 44.8 ± 0.1 | 42.5 ± 1.1 | 0.0560 | |
| LDLC, mg/dL | 116.1 ± 0.4 | 115.0 ± 0.5 | 118.8 ± 0.9 | 0.0001 | 116.0 ± 0.4 | 122.8 ± 4.0 | 0.0996 | |
| TG, log (mg/dL) | 162.1 ± 1.4 | 151.9 ± 1.6 | 188.3 ± 3.0 | < 0.0001 | 161.8 ± 1.4 | 183.6 ± 13.7 | 0.0437 | |
| γ-GTP, log (IU/L) | 36.5 ± 0.9 | 31.4 ± 0.8 | 49.6 ± 2.4 | < 0.0001 | 36.4 ± 0.9 | 39.0 ± 5.2 | 0.0763 | |
| hs-CRP, log (mg/dL) | 0.219 ± 0.007 | 0.202 ± 0.007 | 0.262 ± 0.018 | < 0.0001 | 0.218 ± 0.007 | 0.232 ± 0.028 | 0.0099 | |
| Genotype, %§ | ||||||||
| rs11125529 | 72.6 / 25.0 / 2.5 | 72.2 / 25.2 / 2.6 | 73.4 / 24.4 / 2.1 | 0.4900 | 72.6 / 25.0 / 2.5 | 72.3 / 27.7 / 0.0 | 0.4050 | |
| rs12696304 | 54.4 / 38.7 / 6.9 | 55.1 / 38.3 / 6.7 | 53.0 / 39.5 / 7.5 | 0.3050 | 54.5 / 38.6 / 6.9 | 44.6 / 44.6 / 0.8 | 0.2080 | |
| rs10936601 | 54.2 / 38.4 / 7.4 | 54.8 / 38.1 / 7.2 | 52.9 / 39.2 / 7.9 | 0.3660 | 54.3 / 38.3 / 7.3 | 44.6 / 44.6 / 10.8 | 0.2440 | |
| rs2098713 | 24.2 / 48.8 / 17.0 | 34.6 / 48.3 / 17.1 | 33.2 / 48.8 / 17.0 | 0.4920 | 34.3 / 48.7 / 17.0 | 26.2 / 56.9 /16.9 | 0.3440 | |
| rs654128 | 93.3 / /6.7 / 0.1 | 93.3 / 6.7 / 0.1 | 92.5 / 7.5 / 0.0 | 0.3580 | 93.1 / 6.9 / 0.04 | 70.8 / 9.2 / 0.0 | 0.7540 | |
| rs17635722 | 75.6 / 22.9 / 1.5 | 75.5 / 23.0 / 1.5 | 75.6 / 22.7 / 1.7 | 0.7950 | 75.5 / 23.0 / 1.5 | 80.0 / 18.5 / 1.5 | 0.6890 | |
| rs398652 | 41.7 / 45.6 / 12.8 | 42.5 / 44.8 / 12.7 | 39.6 / 47.5 / 12.9 | 0.1320 | 41.8 / 45.5 / 12.8 | 33.9 / 52.3 / 13.9 | 0.4300 | |
| rs4902100 | 80.8 / 18.1 / 1.1 | 80.6 / 18.3 / 1.1 | 81.3 / 17.4 / 1.3 | 0.6100 | 80.9 / 18.0 / 1.1 | 76.9 / 23.1 / 0.0 | 0.4120 | |
| rs2535913 | 69.7 / 27.4 / 2.9 | 69.5 / 27.7 / 2.9 | 70.4 / 26.7 / 3.0 | 0.7580 | 69.8 / 27.3 / 2.9 | 63.1 / 35.4 / 1.5 | 0.3070 | |
| rs2162440 | 57.5 / 36.4 / 6.1 | 57.8 / 36.0 / 6.2 | 56.5 / 37.5 / 6.0 | 0.5630 | 57.5 / 36.4 / 6.1 | 55.4 / 40.0 / 4.6 | 0.7690 | |
| rs1975174 | 50.7 / 41.0 / 8.3 | 50.8 / 40.8 / 8.4 | 50.3 / 41.5 / 8.2 | 0.8920 | 50.7 / 40.9 / 8.4 | 52.3 / 44.6 / 3.1 | 0.2960 | |
| rs401681 | 45.4 / 44.1 / 10.5 | 46.3 / 43.3 / 10.4 | 43.0 / 46.2 / 10.8 | 0.0840 | 45.4 / 44.1 / 10.5 | 43.1 / 46.2 / 10.8 | 0.9310 | |
SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; WC, waist circumference; FBG, fasting blood glucose; TC, total cholesterol; HDLC, high density lipoprotein cholesterol; LDLC, low density lipoprotein cholesterol; TG, triglycerides; γ-GTP, gamma-glutamyl transpeptidase; hs-CRP, high sensitivity C reactive protein; n, number.
*Values are represented as mean ± standard error for continuous variables and number of counts and percentage for categorical variables. †p values are from student’s t-test for continuous variables and χ2 test for categorical variables assessing the difference between the groups. ‡Apart from major dependent variables (e.g. hypertension and coronary heart disease) and major independent variables (e.g. genotype), some variables included missing data points. §The values were represented as percentage of the AA / Aa / aa genotype, respectively.
Association between telomere length-related SNPs and disease outcomes
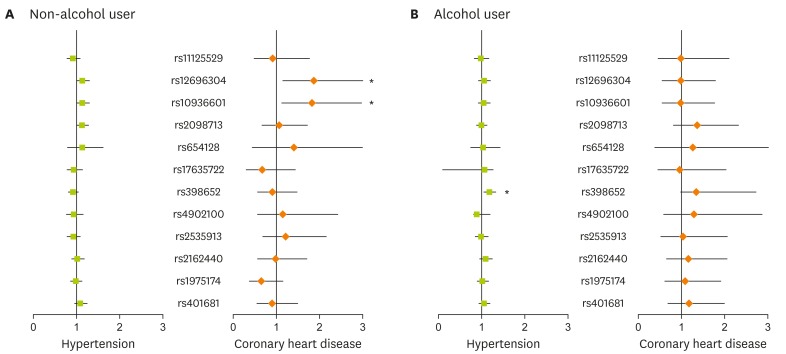
The associations between selected SNPs and HTN or CHD incidence are shown in Table 2. No significant associations between selected SNPs and disease outcome were observed in the overall population. Subjects who have minor alleles in rs12696304 of TERC exhibited a slightly higher, but not statistically significant OR for CHD. We further examined whether the association differed according to differences in lifestyle factors, such as alcohol consumption. Changes in association relative to alcohol status are presented in Figure 1. Of the non-alcohol users, subjects with minor alleles in rs1269304 and rs10936601 (TERC and LRRC34, respectively) were at greatest risk of CHD (OR, 1.862; 95% CIs, 1.137, 3.049; OR, 1.855; 95% CIs, 1.111, 2.985; respectively). However, alcohol users with minor alleles in rs398652 (PELI2) were significantly associated with a higher rate of HTN incidence (OR, 1.179; 95% CIs, 1.040, 1.336).
Table 2. Available reported SNPs and their association with telomere length related SNP and disease prevalence.
| Nearest gene | Reported SNP | Reference | Availability | OA | MA | MAF | Hypertension | Coronary heart disease | ||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI)* | p value† | OR (95% CI)* | p value† | |||||||
| ACYP2 | rs11125529 | Codd et al. [18] | Genotyped | C | A | 0.15 | 0.948 (0.837, 1.074) | 0.403 | 0.938 (0.566, 1.554) | 0.804 |
| SDHDP3-TERC | rs12696304 | Prescott et al. [19] | Genotyped | G | C | 0.29 | 1.091 (0.987, 1.206) | 0.088 | 1.431 (0.984, 2.080) | 0.061 |
| LRRC34 | rs10936601 | Pooley et al. [20] | Genotyped | T | C | 0.29 | 1.091 (0.988, 1.204) | 0.085 | 1.409 (0.971, 2.045) | 0.071 |
| C5orf42 | rs2098713 | Saxena et al. [21] | Imputed | C | T | 0.45 | 1.055 (0.964, 1.155) | 0.244 | 1.200 (0.840, 1.715) | 0.317 |
| FAM162B | rs654128 | Gu et al. [13] | Genotyped | C | A | 0.04 | 1.064 (0.835, 1.356) | 0.613 | 1.331 (0.572, 3.094) | 0.507 |
| PELI2 | rs398652 | Gu et al. [13] | Genotyped | A | G | 0.39 | 1.059 (0.965, 1.161) | 0.225 | 1.196 (0.839, 1.703) | 0.322 |
| KRT80 | rs17635722 | Liu et al. [22] | Genotyped | C | T | 0.14 | 1.001 (0.877, 1.143) | 0.985 | 0.815 (0.465, 1.429) | 0.476 |
| SYT16 | rs4902100 | Lee et al. [23] | Imputed | A | G | 0.11 | 0.955 (0.824, 1.106) | 0.538 | 1.205 (0.700, 2.075) | 0.502 |
| DCAF4 | rs2535913 | Mangino et al. [24] | Genotyped | G | A | 0.18 | 0.957 (0.850, 1.078) | 0.468 | 1.118 (0.714, 1.750) | 0.626 |
| CELF4-MIR4318 | rs2162440 | Mangino et al. [24] | Imputed | G | A | 0.27 | 1.057 (0.954, 1.172) | 0.289 | 1.037 (0.63, 1.553) | 0.859 |
| VN1R859-ZNF676 | rs1975174 | Levy et al. [25] | Imputed | T | G | 0.32 | 1.000 (0.907, 1.103) | 0.999 | 0.932 (0.553, 1.252) | 0.379 |
| TERT-CLPTM1L | rs401681 | Stacey et al. [26] | Genotyped | C | T | 0.36 | 1.072 (0.975, 1.178) | 0.149 | 0.994 (0.685, 1.444) | 0.977 |
SNP, single nucleotide polymorphism; OA, other allele; MA, minor allele; MAF, minor allele frequencies; OR, odds ratio; CI, confidence interval.
*Effect size is described with ORs are described with 95% CI and p value at a 0.05 significance level for each SNPs. †Differences were tested using logistic regression model for disease prevalence after adjusting for sex, age, area, body mass index, education level, physical activity, smoking, and drinking.
Figure 1.
Association between disease prevalence and telomere related genotypes according to alcohol status. The forest plot was based on estimates from logistic regressions after adjustment for sex, age, area, body mass index, education level, physical activity, and smoking. In case of analysis within the non-alcohol users, previous alcohol use was additionally included in the regression model. *Indicated statistical significance at level of p < 0.05.
Association between interesting SNPs and cardiovascular risk factors
SNPs significantly associated with disease outcomes (rs1296304, rs10936601, and 398652) were selected, and their associations with cardiovascular risk factors were further evaluated (Table 3). Of 3 SNPs related to disease outcomes, rs1296304 was significantly associated with increased levels of DBP (β estimate, 0.470; 95% CIs, 0.013, 0.926). A minor allele in rs398652 was significantly associated with higher levels of BMI and γ-GTP (OR, 0.128; 95% CIs, 0.010, 0.246; OR, 0.013; 95% CIs, 0.001, 0.024). No association was observed between rs1093601 and tested risk factors.
Table 3. Difference of biochemical markers among the subjects according to telomere related genotype.
| Cardiovascular risk factor | rs12696304 | rs10936601 | rs398652 | |||
|---|---|---|---|---|---|---|
| Estimate* | p value | Estimate* | p value | Estimate* | p value | |
| SBP, mmHg | 0.500 (−0.187, 1.186) | 0.154 | 0.449 (−0.230, 1.129) | 0.195 | 0.426 (−0.204, 1.057) | 0.185 |
| DBP, mmHg | 0.470 (0.013, 0.926) | 0.044 | 0.424 (−0.028, 0.876) | 0.066 | 0.302 (−0.118, 0.721) | 0.159 |
| BMI, kg/m2 | 0.046 (−0.082, 0.175) | 0.481 | 0.045 (−0.082, 0.172) | 0.483 | 0.128 (0.010, 0.246) | 0.034 |
| WC, cm | 0.102 (−0.242, 0.445) | 0.561 | 0.074 (−0.266, 0.414) | 0.669 | 0.186 (−0.129, 0.501) | 0.247 |
| Log-transformed FBG, log (mg/dL)† | 0.002 (−0.002, 0.005) | 0.343 | 0.001 (−0.002, 0.004) | 0.511 | 0.001 (−0.002, 0.004) | 0.547 |
| TC, mg/dL | 0.561 (−0.911, 2.033) | 0.455 | 0.423 (−1.034, 1.880) | 0.569 | 1.088 (−0.264, 2.439) | 0.115 |
| Log-transformed HDLC, log (mg/dL)† | 0.003 (−0.000, 0.007) | 0.080 | 0.003 (−0.001, 0.007) | 0.125 | 0.001 (−0.003, 0.004) | 0.765 |
| LDLC, mg/dL | 0.385 (−0.952, 1.722) | 0.572 | 0.293 (−1.031, 1.616) | 0.665 | 0.774 (−0.457, 2.005) | 0.218 |
| Log transformed TG, log (mg/dL)† | −0.004 (−0.013, 0.005) | 0.406 | −0.005 (−0.013, 0.004) | 0.316 | 0.003 (−0.005, 0.011) | 0.488 |
| Log-transformed γ-GTP, log (IU/L)† | −0.003 (−0.016, 0.009) | 0.593 | −0.003 (−0.016, 0.009) | 0.578 | 0.013 (0.001, 0.024) | 0.027 |
| Log-transformed hs-CRP, log (mg/dL)† | 0.007 (−0.016, 0.029) | 0.562 | 0.004 (−0.018, 0.026) | 0.732 | 0.007 (−0.014, 0.027) | 0.513 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; WC, waist circumference; FBG, fasting blood glucose; TC, total cholesterol; HDLC, high density lipoprotein cholesterol; LDLC, low density lipoprotein cholesterol; TG, triglycerides; γ-GTP, gamma-glutamyl transpeptidase; hs-CRP, high sensitivity C reactive protein.
*Estimates are presented as regression coefficient β, 95% confidence interval (CI). Corresponding p values were provided separately. These estimates were obtained from linear regression regressions with an ordinary least squares estimation method. All regression models were adjusted for sex, age, area, body mass index, education level, physical activity, smoking, and drinking. †Values were used in statistical model after log-transformation to satisfy the assumption of normal distribution.
DISCUSSION
In this study, we hypothesized that some previously known genetic variants related to TL are associated with the incidence of HTN/CHD in the Korean population. The results show that no significant association between the selected TL-related SNPs and disease outcome was observed in this population; however, variations in TERC (rs12696304) and LRRC34 (rs10936601) tended to increase the ORs for HTN and CHD.
Several epidemiological studies have shown that age-dependent shortening of TL is associated with chronic diseases such as essential HTN, T2DM, CVD, and dementia [5,6,7,8,27]. It was reported that patients with CAD had a significantly shorter mean TL in their aortic cells, compared with subjects without heart disease [5]. Shorter TLs were also detected in patients with HTN, atherosclerosis, MI, chronic heart failure, stroke, even T2DM, than in control subjects [5,6,7]. In addition, prospective studies have reported a significantly shorter TL to be associated with increased risks of CAD, MI, and strokes [8]. These results raise the question as to whether genetic variations that affect TL should also be associated with disease risk.
So far, genetic variants at several loci have been found to be significantly associated with TL, through GWAS and meta-analyses [1,28,29,30,31]. Of these, the specific sequence variations within TERT and TERC genes were reported to cause variations in TL, with altered TL being a cause of some chronic diseases, including cancer and cardiac disease. This can be explained through the biology of telomeres. In humans, TL continuously decreases with aging because of a decrease in telomerase activity [32]. Telomerase is a large RNA-protein complex comprised of TERT and TERC. The enzyme maintains TL during DNA replication by catalyzing short telomere repeats, after recognizing a single stranded G-rich primer [18]. The downregulation of telomerase activity with aging leads to progressive telomere shortening, cell death, cellular senescence, or abnormal cell proliferation [33]. In line with this, a GWAS found that a SNP located within TERT (rs2736100, rs7705526, rs2736108) was significantly associated with TL [28]. Moreover, variations in TERT (rs2736122, rs2853668) affected the risk of CVD associated with TL in middle-aged adults [29], which was also validated in the Han Chinese population [30]. Although very little is known about the specific LRRC proteins, the LRRC superfamily includes proteins with a diverse array of structures, and involvement via protein–protein interactions in a variety of functions, including DNA repair, chromosomal stability, and heart development [18]. In contrast to previous findings, in this study, we failed to detect significant associations between selected TLs related SNPs and disease outcome, on the population level. This discrepancy might exist because of the different ethnic backgrounds of the study subjects, different and small sample sizes, and the different methods used to define CVD (self-reporting). Thus, a carefully designed large-scale study of well-phenotyped population would be needed in order to further investigate this discrepancy. However, we found that the associations between TL variations with HTN/CHD were modified by alcohol behavior. The association between SNPs in TERC and LRRC34 and the prevalence of CHD were evident only in non-alcohol users. In addition, the association between the presence of rs398652 at PELI2 and HTN prevalence was observed only in alcohol users, suggesting the presence of a potential interaction between TL-related SNPs and alcohol intake. Since cardiovascular dysfunctions are highly complex disease states correlated with both genetic and environmental factors, heritable variation would not be the only factor influencing TL. Accumulating data have shown that telomere shortening could be attributable to several risk factors for psychological stress, smoking, obesity, alcohol, chronic inflammation, and exposure to particulate air pollution [7,34]. Specifically, Pavanello et al. [34] reported that alcohol abuse was associated with shortened telomeres. Since telomeres are highly sensitive targets for damage due to oxidative stress [7], it could be hypothesized that high levels of 8-hydroxy-2'-deoxyguanosine (8-OH-dG), a marker for DNA damage, may be formed by the process of alcohol metabolism. Because TL is also affected by other aging-related risk factors for HTN/CAD, it is possible that the observed association between TL-related SNPs and disease outcomes was confounded by CVD risk factors such as blood pressure, obesity, and blood lipid profiles. Indeed, the presence of rs1296304 at SDHDP3-TERC was significantly associated with increased levels of DBP. In addition, a minor allele in rs398652, which exhibited an association with CAD in alcohol users, was significantly associated with higher levels of BMI and γ-GTP. Future studies to investigate whether the association between TL and aging-related disease is primarily or secondarily mediated via CVD risk factors are needed in order to confirm these observations.
In summary, we have demonstrated that both variations in several genes encoding proteins with known functions in telomere biology and other genes are not associated with the prevalence of HTN/CHD in the Korean population. However, potential interaction between related SNPs and alcohol behavior were observed, suggesting that chronic disease development is a complex process driven by many different factors. Confirmation of the presence of a causal association between TL and HTN/CHD, and further mechanistic investigations of this relationship are needed in order to verify our results.
ACKNOWLEDGEMENTS
This study was provided with biospecimens and data from the Korean Genome Analysis Project (4845-301), the Korean Genome and Epidemiology Study (4851-302), and Korea Biobank Project (4851-307, KBP-2014-062) that were supported by the Korea Center for Disease Control and Prevention, Republic of Korea.
Footnotes
Funding: This research was supported by Basic Science Research Program, through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (NRF-2015R1A2A1A15054758).
Conflict of Interest: The authors declare that they have no competing interests.
Author Contributions: None of the authors have any potential conflicts of interest associated with this research. Min-Jeong Shin and Jean Kyung Paik conceived the study. Min-Jeong Shin and Yoonsu Cho developed the statistical analysis. Yoonsu Cho analyzed the data. Jean Kyung Paik and Min-Jeong Shin prepared the first draft of the manuscript. Jean Kyung Paik, Ryungwoo Kang, Yoonsu Cho, and Min-Jeong Shin contributed to the writing of the manuscript. All authors reviewed and approved the manuscript.
References
- 1.Codd V, Mangino M, van der Harst P, Braund PS, Kaiser M, Beveridge AJ, Rafelt S, Moore J, Nelson C, Soranzo N, Zhai G, Valdes AM, Blackburn H, Mateo Leach I, de Boer RA, Kimura M, Aviv A, Goodall AH, Ouwehand W, van Veldhuisen DJ, van Gilst WH, Navis G, Burton PR, Tobin MD, Hall AS, Thompson JR, Spector T, Samani NJ, Wellcome Trust Case Control Consortium Common variants near TERC are associated with mean telomere length. Nat Genet. 2010;42:197–199. doi: 10.1038/ng.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Lange T. Protection of mammalian telomeres. Oncogene. 2002;21:532–540. doi: 10.1038/sj.onc.1205080. [DOI] [PubMed] [Google Scholar]
- 3.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 4.Portugal RD, Land MG, Svaiter BF. A computational model for telomere-dependent cell-replicative aging. Biosystems. 2008;91:262–267. doi: 10.1016/j.biosystems.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- 6.Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23:842–846. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 7.Salpea KD, Talmud PJ, Cooper JA, Maubaret CG, Stephens JW, Abelak K, Humphries SE. Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis. 2010;209:42–50. doi: 10.1016/j.atherosclerosis.2009.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weischer M, Bojesen SE, Cawthon RM, Freiberg JJ, Tybjærg-Hansen A, Nordestgaard BG. Short telomere length, myocardial infarction, ischemic heart disease, and early death. Arterioscler Thromb Vasc Biol. 2012;32:822–829. doi: 10.1161/ATVBAHA.111.237271. [DOI] [PubMed] [Google Scholar]
- 9.Aviv A. Leukocyte telomere length, hypertension, and atherosclerosis: are there potential mechanistic explanations? Hypertension. 2009;53:590–591. doi: 10.1161/HYPERTENSIONAHA.109.128926. [DOI] [PubMed] [Google Scholar]
- 10.Serrano AL, Andrés V. Telomeres and cardiovascular disease: does size matter? Circ Res. 2004;94:575–584. doi: 10.1161/01.RES.0000122141.18795.9C. [DOI] [PubMed] [Google Scholar]
- 11.Maubaret CG, Salpea KD, Romanoski CE, Folkersen L, Cooper JA, Stephanou C, Li KW, Palmen J, Hamsten A, Neil A, Stephens JW, Lusis AJ, Eriksson P, Talmud PJ, Humphries SE, Simon Broome Research Group. EARSII consortium Association of TERC and OBFC1 haplotypes with mean leukocyte telomere length and risk for coronary heart disease. PLoS One. 2013;8:e83122. doi: 10.1371/journal.pone.0083122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber M, Treszl A, Wehland M, Winther I, Zergibel I, Reibis R, Bolbrinker J, Stoll M, Schönfelder G, Wegscheider K, Völler H, Kreutz R. Genetic variants implicated in telomere length associated with left ventricular function in patients with hypertension and cardiac organ damage. J Mol Med (Berl) 2012;90:1059–1067. doi: 10.1007/s00109-012-0874-3. [DOI] [PubMed] [Google Scholar]
- 13.Gu J, Chen M, Shete S, Amos CI, Kamat A, Ye Y, Lin J, Dinney CP, Wu X. A genome-wide association study identifies a locus on chromosome 14q21 as a predictor of leukocyte telomere length and as a marker of susceptibility for bladder cancer. Cancer Prev Res (Phila) 2011;4:514–521. doi: 10.1158/1940-6207.CAPR-11-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi J, Sun F, Peng L, Li B, Liu L, Zhou C, Han J, Zhang L, Zhou L, Zhang X, Pu H, Tong L, Yuan Q, Song X, Yang M. Leukocyte telomere length-related genetic variants in 1p34.2 and 14q21 loci contribute to the risk of esophageal squamous cell carcinoma. Int J Cancer. 2013;132:2799–2807. doi: 10.1002/ijc.27959. [DOI] [PubMed] [Google Scholar]
- 15.Trudeau MA, Wong JM. Genetic variations in telomere maintenance, with implications on tissue renewal capacity and chronic disease pathologies. Curr Pharmacogenomics Person Med. 2010;8:7–24. doi: 10.2174/1875692111008010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho YS, Go MJ, Kim YJ, Heo JY, Oh JH, Ban HJ, Yoon D, Lee MH, Kim DJ, Park M, Cha SH, Kim JW, Han BG, Min H, Ahn Y, Park MS, Han HR, Jang HY, Cho EY, Lee JE, Cho NH, Shin C, Park T, Park JW, Lee JK, Cardon L, Clarke G, McCarthy MI, Lee JY, Lee JK, Oh B, Kim HL. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat Genet. 2009;41:527–534. doi: 10.1038/ng.357. [DOI] [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18.Codd V, Nelson CP, Albrecht E, Mangino M, Deelen J, Buxton JL, Hottenga JJ, Fischer K, Esko T, Surakka I, Broer L, Nyholt DR, Mateo Leach I, Salo P, Hägg S, Matthews MK, Palmen J, Norata GD, O’Reilly PF, Saleheen D, Amin N, Balmforth AJ, Beekman M, de Boer RA, Böhringer S, Braund PS, Burton PR, de Craen AJ, Denniff M, Dong Y, Douroudis K, Dubinina E, Eriksson JG, Garlaschelli K, Guo D, Hartikainen AL, Henders AK, Houwing-Duistermaat JJ, Kananen L, Karssen LC, Kettunen J, Klopp N, Lagou V, van Leeuwen EM, Madden PA, Mägi R, Magnusson PK, Männistö S, McCarthy MI, Medland SE, Mihailov E, Montgomery GW, Oostra BA, Palotie A, Peters A, Pollard H, Pouta A, Prokopenko I, Ripatti S, Salomaa V, Suchiman HE, Valdes AM, Verweij N, Viñuela A, Wang X, Wichmann HE, Widen E, Willemsen G, Wright MJ, Xia K, Xiao X, van Veldhuisen DJ, Catapano AL, Tobin MD, Hall AS, Blakemore AI, van Gilst WH, Zhu H, Erdmann J, Reilly MP, Kathiresan S, Schunkert H, Talmud PJ, Pedersen NL, Perola M, Ouwehand W, Kaprio J, Martin NG, van Duijn CM, Hovatta I, Gieger C, Metspalu A, Boomsma DI, Jarvelin MR, Slagboom PE, Thompson JR, Spector TD, van der Harst P, Samani NJ CARDIoGRAM consortium. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45:422–427. 427e1–427e2. doi: 10.1038/ng.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prescott J, Kraft P, Chasman DI, Savage SA, Mirabello L, Berndt SI, Weissfeld JL, Han J, Hayes RB, Chanock SJ, Hunter DJ, De Vivo I. Genome-wide association study of relative telomere length. PLoS One. 2011;6:e19635. doi: 10.1371/journal.pone.0019635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pooley KA, Bojesen SE, Weischer M, Nielsen SF, Thompson D, Amin Al Olama A, Michailidou K, Tyrer JP, Benlloch S, Brown J, Audley T, Luben R, Khaw KT, Neal DE, Hamdy FC, Donovan JL, Kote-Jarai Z, Baynes C, Shah M, Bolla MK, Wang Q, Dennis J, Dicks E, Yang R, Rudolph A, Schildkraut J, Chang-Claude J, Burwinkel B, Chenevix-Trench G, Pharoah PD, Berchuck A, Eeles RA, Easton DF, Dunning AM, Nordestgaard BG. A genome-wide association scan (GWAS) for mean telomere length within the COGS project: identified loci show little association with hormone-related cancer risk. Hum Mol Genet. 2013;22:5056–5064. doi: 10.1093/hmg/ddt355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saxena R, Bjonnes A, Prescott J, Dib P, Natt P, Lane J, Lerner M, Cooper JA, Ye Y, Li KW, Maubaret CG, Codd V, Brackett D, Mirabello L, Kraft P, Dinney CP, Stowell D, Peyton M, Ralhan S, Wander GS, Mehra NK, Salpea KD, Gu J, Wu X, Mangino M, Hunter DJ, De Vivo I, Humphries SE, Samani NJ, Spector TD, Savage SA, Sanghera DK. Genome-wide association study identifies variants in casein kinase II (CSNK2A2) to be associated with leukocyte telomere length in a Punjabi Sikh diabetic cohort. Circ Cardiovasc Genet. 2014;7:287–295. doi: 10.1161/CIRCGENETICS.113.000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Cao L, Li Z, Zhou D, Liu W, Shen Q, Wu Y, Zhang D, Hu X, Wang T, Ye J, Weng X, Zhang H, Zhang D, Zhang Z, Liu F, He L, Shi Y. A genome-wide association study identifies a locus on TERT for mean telomere length in Han Chinese. PLoS One. 2014;9:e85043. doi: 10.1371/journal.pone.0085043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JH, Cheng R, Honig LS, Feitosa M, Kammerer CM, Kang MS, Schupf N, Lin SJ, Sanders JL, Bae H, Druley T, Perls T, Christensen K, Province M, Mayeux R. Genome wide association and linkage analyses identified three loci-4q25, 17q23.2, and 10q11.21-associated with variation in leukocyte telomere length: the Long Life Family Study. Front Genet. 2014;4:310. doi: 10.3389/fgene.2013.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mangino M, Christiansen L, Stone R, Hunt SC, Horvath K, Eisenberg DT, Kimura M, Petersen I, Kark JD, Herbig U, Reiner AP, Benetos A, Codd V, Nyholt DR, Sinnreich R, Christensen K, Nassar H, Hwang SJ, Levy D, Bataille V, Fitzpatrick AL, Chen W, Berenson GS, Samani NJ, Martin NG, Tishkoff S, Schork NJ, Kyvik KO, Dalgård C, Spector TD, Aviv A. DCAF4, a novel gene associated with leucocyte telomere length. J Med Genet. 2015;52:157–162. doi: 10.1136/jmedgenet-2014-102681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy D, Neuhausen SL, Hunt SC, Kimura M, Hwang SJ, Chen W, Bis JC, Fitzpatrick AL, Smith E, Johnson AD, Gardner JP, Srinivasan SR, Schork N, Rotter JI, Herbig U, Psaty BM, Sastrasinh M, Murray SS, Vasan RS, Province MA, Glazer NL, Lu X, Cao X, Kronmal R, Mangino M, Soranzo N, Spector TD, Berenson GS, Aviv A. Genome-wide association identifies OBFC1 as a locus involved in human leukocyte telomere biology. Proc Natl Acad Sci U S A. 2010;107:9293–9298. doi: 10.1073/pnas.0911494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stacey SN, Sulem P, Gudbjartsson DF, Jonasdottir A, Thorleifsson G, Gudjonsson SA, Masson G, Gudmundsson J, Sigurgeirsson B, Benediktsdottir KR, Thorisdottir K, Ragnarsson R, Fuentelsaz V, Corredera C, Grasa M, Planelles D, Sanmartin O, Rudnai P, Gurzau E, Koppova K, Hemminki K, Nexø BA, Tjønneland A, Overvad K, Johannsdottir H, Helgadottir HT, Thorsteinsdottir U, Kong A, Vogel U, Kumar R, Nagore E, Mayordomo JI, Rafnar T, Olafsson JH, Stefansson K. Germline sequence variants in TGM3 and RGS22 confer risk of basal cell carcinoma. Hum Mol Genet. 2014;23:3045–3053. doi: 10.1093/hmg/ddt671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Z, Huang X, Jiang H, Zhang Y, Liu H, Qin C, Eisner GM, Jose PA, Rudolph L, Ju Z. Short telomeres and prognosis of hypertension in a chinese population. Hypertension. 2009;53:639–645. doi: 10.1161/HYPERTENSIONAHA.108.123752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bojesen SE, Pooley KA, Johnatty SE, Beesley J, Michailidou K, Tyrer JP, Edwards SL, Pickett HA, Shen HC, Smart CE, Hillman KM, Mai PL, Lawrenson K, Stutz MD, Lu Y, Karevan R, Woods N, Johnston RL, French JD, Chen X, Weischer M, Nielsen SF, Maranian MJ, Ghoussaini M, Ahmed S, Baynes C, Bolla MK, Wang Q, Dennis J, McGuffog L, Barrowdale D, Lee A, Healey S, Lush M, Tessier DC, Vincent D, Bacot F, Vergote I, Lambrechts S, Despierre E, Risch HA, González-Neira A, Rossing MA, Pita G, Doherty JA, Alvarez N, Larson MC, Fridley BL, Schoof N, Chang-Claude J, Cicek MS, Peto J, Kalli KR, Broeks A, Armasu SM, Schmidt MK, Braaf LM, Winterhoff B, Nevanlinna H, Konecny GE, Lambrechts D, Rogmann L, Guénel P, Teoman A, Milne RL, Garcia JJ, Cox A, Shridhar V, Burwinkel B, Marme F, Hein R, Sawyer EJ, Haiman CA, Wang-Gohrke S, Andrulis IL, Moysich KB, Hopper JL, Odunsi K, Lindblom A, Giles GG, Brenner H, Simard J, Lurie G, Fasching PA, Carney ME, Radice P, Wilkens LR, Swerdlow A, Goodman MT, Brauch H, Garcia-Closas M, Hillemanns P, Winqvist R, Dürst M, Devilee P, Runnebaum I, Jakubowska A, Lubinski J, Mannermaa A, Butzow R, Bogdanova NV, Dörk T, Pelttari LM, Zheng W, Leminen A, Anton-Culver H, Bunker CH, Kristensen V, Ness RB, Muir K, Edwards R, Meindl A, Heitz F, Matsuo K, du Bois A, Wu AH, Harter P, Teo SH, Schwaab I, Shu XO, Blot W, Hosono S, Kang D, Nakanishi T, Hartman M, Yatabe Y, Hamann U, Karlan BY, Sangrajrang S, Kjaer SK, Gaborieau V, Jensen A, Eccles D, Høgdall E, Shen CY, Brown J, Woo YL, Shah M, Azmi MA, Luben R, Omar SZ, Czene K, Vierkant RA, Nordestgaard BG, Flyger H, Vachon C, Olson JE, Wang X, Levine DA, Rudolph A, Weber RP, Flesch-Janys D, Iversen E, Nickels S, Schildkraut JM, Silva Idos S, Cramer DW, Gibson L, Terry KL, Fletcher O, Vitonis AF, van der Schoot CE, Poole EM, Hogervorst FB, Tworoger SS, Liu J, Bandera EV, Li J, Olson SH, Humphreys K, Orlow I, Blomqvist C, Rodriguez-Rodriguez L, Aittomäki K, Salvesen HB, Muranen TA, Wik E, Brouwers B, Krakstad C, Wauters E, Halle MK, Wildiers H, Kiemeney LA, Mulot C, Aben KK, Laurent-Puig P, Altena AM, Truong T, Massuger LF, Benitez J, Pejovic T, Perez JI, Hoatlin M, Zamora MP, Cook LS, Balasubramanian SP, Kelemen LE, Schneeweiss A, Le ND, Sohn C, Brooks-Wilson A, Tomlinson I, Kerin MJ, Miller N, Cybulski C, Henderson BE, Menkiszak J, Schumacher F, Wentzensen N, Le Marchand L, Yang HP, Mulligan AM, Glendon G, Engelholm SA, Knight JA, Høgdall CK, Apicella C, Gore M, Tsimiklis H, Song H, Southey MC, Jager A, den Ouweland AM, Brown R, Martens JW, Flanagan JM, Kriege M, Paul J, Margolin S, Siddiqui N, Severi G, Whittemore AS, Baglietto L, McGuire V, Stegmaier C, Sieh W, Müller H, Arndt V, Labrèche F, Gao YT, Goldberg MS, Yang G, Dumont M, McLaughlin JR, Hartmann A, Ekici AB, Beckmann MW, Phelan CM, Lux MP, Permuth-Wey J, Peissel B, Sellers TA, Ficarazzi F, Barile M, Ziogas A, Ashworth A, Gentry-Maharaj A, Jones M, Ramus SJ, Orr N, Menon U, Pearce CL, Brüning T, Pike MC, Ko YD, Lissowska J, Figueroa J, Kupryjanczyk J, Chanock SJ, Dansonka-Mieszkowska A, Jukkola-Vuorinen A, Rzepecka IK, Pylkäs K, Bidzinski M, Kauppila S, Hollestelle A, Seynaeve C, Tollenaar RA, Durda K, Jaworska K, Hartikainen JM, Kosma VM, Kataja V, Antonenkova NN, Long J, Shrubsole M, Deming-Halverson S, Lophatananon A, Siriwanarangsan P, Stewart-Brown S, Ditsch N, Lichtner P, Schmutzler RK, Ito H, Iwata H, Tajima K, Tseng CC, Stram DO, van den Berg D, Yip CH, Ikram MK, Teh YC, Cai H, Lu W, Signorello LB, Cai Q, Noh DY, Yoo KY, Miao H, Iau PT, Teo YY, McKay J, Shapiro C, Ademuyiwa F, Fountzilas G, Hsiung CN, Yu JC, Hou MF, Healey CS, Luccarini C, Peock S, Stoppa-Lyonnet D, Peterlongo P, Rebbeck TR, Piedmonte M, Singer CF, Friedman E, Thomassen M, Offit K, Hansen TV, Neuhausen SL, Szabo CI, Blanco I, Garber J, Narod SA, Weitzel JN, Montagna M, Olah E, Godwin AK, Yannoukakos D, Goldgar DE, Caldes T, Imyanitov EN, Tihomirova L, Arun BK, Campbell I, Mensenkamp AR, van Asperen CJ, van Roozendaal KE, Meijers-Heijboer H, Collée JM, Oosterwijk JC, Hooning MJ, Rookus MA, van der, Os TA, Evans DG, Frost D, Fineberg E, Barwell J, Walker L, Kennedy MJ, Platte R, Davidson R, Ellis SD, Cole T, Bressac-de Paillerets B, Buecher B, Damiola F, Faivre L, Frenay M, Sinilnikova OM, Caron O, Giraud S, Mazoyer S, Bonadona V, Caux-Moncoutier V, Toloczko-Grabarek A, Gronwald J, Byrski T, Spurdle AB, Bonanni B, Zaffaroni D, Giannini G, Bernard L, Dolcetti R, Manoukian S, Arnold N, Engel C, Deissler H, Rhiem K, Niederacher D, Plendl H, Sutter C, Wappenschmidt B, Borg A, Melin B, Rantala J, Soller M, Nathanson KL, Domchek SM, Rodriguez GC, Salani R, Kaulich DG, Tea MK, Paluch SS, Laitman Y, Skytte AB, Kruse TA, Jensen UB, Robson M, Gerdes AM, Ejlertsen B, Foretova L, Savage SA, Lester J, Soucy P, Kuchenbaecker KB, Olswold C, Cunningham JM, Slager S, Pankratz VS, Dicks E, Lakhani SR, Couch FJ, Hall P, Monteiro AN, Gayther SA, Pharoah PD, Reddel RR, Goode EL, Greene MH, Easton DF, Berchuck A, Antoniou AC, Chenevix-Trench G, Dunning AM Australian Cancer Study; Australian Ovarian Cancer Study; Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab); Gene Environment Interaction and Breast Cancer (GENICA); Swedish Breast Cancer Study (SWE-BRCA); Hereditary Breast and Ovarian Cancer Research Group Netherlands (HEBON); Epidemiological study of BRCA1 & BRCA2 Mutation Carriers (EMBRACE); Genetic Modifiers of Cancer Risk in BRCA1/2 Mutation Carriers (GEMO) Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer. Nat Genet. 2013;45:371–384. 384e1–384e2. doi: 10.1038/ng.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bressler J, Franceschini N, Demerath EW, Mosley TH, Folsom AR, Boerwinkle E. Sequence variation in telomerase reverse transcriptase (TERT) as a determinant of risk of cardiovascular disease: the Atherosclerosis Risk in Communities (ARIC) study. BMC Med Genet. 2015;16:52. doi: 10.1186/s12881-015-0194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soerensen M, Thinggaard M, Nygaard M, Dato S, Tan Q, Hjelmborg J, Andersen-Ranberg K, Stevnsner T, Bohr VA, Kimura M, Aviv A, Christensen K, Christiansen L. Genetic variation in TERT and TERC and human leukocyte telomere length and longevity: a cross-sectional and longitudinal analysis. Aging Cell. 2012;11:223–227. doi: 10.1111/j.1474-9726.2011.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardikar S, Song X, Risques RA, Montine TJ, Duggan C, Blount PL, Reid BJ, Anderson GL, Kratz M, White E, Vaughan TL. Obesity and inflammation markers in relation to leukocyte telomere length in a cross-sectional study of persons with Barrett’s esophagus. BMC Obes. 2015;2:32. doi: 10.1186/s40608-015-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atzmon G, Cho M, Cawthon RM, Budagov T, Katz M, Yang X, Siegel G, Bergman A, Huffman DM, Schechter CB, Wright WE, Shay JW, Barzilai N, Govindaraju DR, Suh Y. Evolution in health and medicine Sackler colloquium: Genetic variation in human telomerase is associated with telomere length in Ashkenazi centenarians. Proc Natl Acad Sci U S A. 2010;107(Suppl 1):1710–1717. doi: 10.1073/pnas.0906191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 34.Pavanello S, Hoxha M, Dioni L, Bertazzi PA, Snenghi R, Nalesso A, Ferrara SD, Montisci M, Baccarelli A. Shortened telomeres in individuals with abuse in alcohol consumption. Int J Cancer. 2011;129:983–992. doi: 10.1002/ijc.25999. [DOI] [PMC free article] [PubMed] [Google Scholar]