Osteoarthritis (OA) can no longer be labeled simply as a “degenerative” joint disease, implying a wear-and-tear process driven almost solely by mechanical events. A growing body of evidence supports the notion of an important role of both local and systemic inflammation in promoting structural damage in OA joints, as well as pain and reduced physical function (1). Numerous cytokines, chemokines, and other proinflammatory factors have been identified in OA synovial fluid. These mediators can be produced by activated synovial macrophages, which increase in number in OA, and also by joint tissue cells including synovial fibroblasts, chondrocytes, and cells in the subchondral bone. In the knee joint, the menisci and infrapatellar fat pad are additional sources of proinflammatory mediators, including adiposederived adipokines from the fat pad. The local production of inflammatory mediators in OA joints is thought to be the result of a cellular stress response to joint and tissue injury, further promoted by the response to the release of damaged proteins including matrix fragments produced by proteolytic enzymes.
Perhaps with the exception of obesity and metabolic syndrome (2), it has been more difficult to explain an association of OA with systemic inflammation, as clinical signs and symptoms typical of a systemic immune response are absent. However, a number of systemically measured candidate markers of inflammation have been demonstrated to be associated with symptoms or presence of disease in OA populations, though the data on their ability to predict progression are mixed (3). Among them, serum levels of C-reactive protein and hyaluronic acid have been the most studied (3–5).
In a report in this issue of Arthritis & Rheumatology, Attur and colleagues provide evidence that systemic markers of inflammation may serve as prognostic and diagnostic biomarkers to identify patients with symptomatic knee OA who are at greater risk of radiographic progression (6). Their results using peripheral blood leukocyte (PBL) transcription patterns and plasma eicosanoid levels to distinguish symptomatic knee OA cases from controls, and to predict joint space narrowing (JSN) over 2 years, build on their previous finding of an association between PBL interleukin-1β (IL-1β) transcript levels and progression of radiographic OA in a single cohort (7). In the current study, the authors again demonstrated a relationship between PBL IL-1β expression and JSN, in an expanded cohort from the one reported previously. They then used adjusted analysis of receiver operating characteristic curves to show the predictive value of IL-1β for the most rapid progressors (defined as JSN >0.5 mm in 2 years). More importantly, their previous analysis was expanded to include PBL tumor necrosis factor (TNF), cyclooxygenase 2 (COX-2), and prostaglandin E synthase (PGES) expression levels, which revealed even stronger predictive value for PBL COX-2 expression and JSN, regardless of the rate of progression. Addition of IL-1β levels to multivariate analyses did not add significantly more predictive value above that offered by PBL COX-2 levels.
COX-2 is the inducible form of the cyclooxygenase enzyme, an important target of nonsteroidal anti-inflammatory drugs. COX-2 is significantly up-regulated in leukocytes after an inflammatory stimulus, and acts on arachidonic acid to produce eicosanoid lipid mediators, specifically prostaglandins, prostacyclins, and thromboxanes. In addition, arachidonic acid is metabolized by lipoxygenases, leading to production of intermediates, including 15-hydroxyeicosatetraenoic acid (15-HETE), that are precursors for other eicosanoids, the leuko-trienes. Together, eicosanoid metabolites of arachidonic acid have a variety of effects on cells, including promotion of inflammatory signaling, cytokine production, and leukocyte migration and stimulation. Eicosanoids are important mediators of inflammatory pain.
In the current study by Attur et al (6), the results regarding PBL COX-2 transcript levels were strengthened by further analysis of PBL expression of PGES transcripts, and plasma levels of the eicosanoids PGE2 and 15-HETE, in 3 separate symptomatic knee OA cohorts. Mean PBL expression levels of PGES enzyme isoforms, measured by microarray analysis in 2 cohorts, were higher in patients with symptomatic knee OA from both cohorts compared to nonarthritic controls. Elevated mean plasma PGE2 levels were demonstrated in all 3 cohorts of OA patients compared to controls, and when PBLs were cultured ex vivo, OA patient PBLs produced higher levels of PGE2 than PBLs from controls. Finally, plasma levels of 15-HETE were measured in 2 cohorts and in both, were higher in cases than controls.
Taken together, the findings of increased PBL expression of messenger RNA (mRNA) for 2 enzymes involved in arachidonic acid metabolism (COX-2 and PGES), along with elevated peripheral levels of eicosanoid products of that metabolism (PGE2 and 15-HETE), suggest that leukocyte arachidonic acid metabolism is increased in OA. Using receiver operating characteristic curve analysis, Attur et al went on to demonstrate that the PGE2 and 15-HETE lipid markers may have diagnostic value in distinguishing patients with symptomatic knee OA from controls. Additional investigations comparing symptomatic knee OA to other conditions causing knee pain will need to be performed before any clinical utility of this measurement can be assumed. Future studies focusing on early-stage symptomatic knee OA would be most helpful, given that distinguishing OA as a cause of knee pain is particularly difficult before obvious radiographic changes are evident.
With regard to predictive markers of OA progression, Attur et al used multivariate modeling to investigate which variables were most strongly predictive of JSN progression in one of their cohorts, which was followed up radiographically for 2 years. This revealed that plasma 15-HETE levels and PBL COX-2 mRNA levels correlated with JSN most strongly, and PBL COX-2 expression added significant predictive value after controlling for age, sex, body mass index (BMI), and Kellgren/Lawrence grade (8). These results are intriguing, and add to the list of potential predictive biomarkers in OA. Joint alignment at the knee, an important risk factor for progression of OA, was not accounted for in this analysis, so whether these measurements add additional predictive value over measures of alignment cannot yet be determined. Additionally, it cannot be determined from Attur and colleagues’ analysis whether these measurements will perform similarly in other OA cohorts (i.e., early-stage or preradiographic disease) or can be applied to OA at joints other than the knee. Therefore, the assessments need to be repeated in other populations.
The focus on measures of PBL activity, by transcriptome profiling and ex vivo stimulation of PBLs, in concert with plasma levels of metabolites of this activity is intriguing. Although plasma levels of PGE2 and 15-HETE determined in the current study may be confounded by sources other than activated PBLs, the authors demonstrate relationships of similar magnitude between JSN and plasma lipid mediators, PBL micro-somal PGES transcript levels, and ex vivo PBL PGE2 production, suggesting that the source of the measured lipids may be activated PBLs. Another particularly interesting finding is the differential ex vivo production of cytokines by PBLs from patients with OA. When ex vivo production of IL-1β was categorized as above or below the median, patients with higher-than-median levels of IL-1β mRNA had higher rates of JSN over 2 years. Differential activity of peripheral blood cells has also been reported by other investigators (9,10). Lower production of IL-1β, IL-6, and IL-1 receptor antagonist by whole blood samples stimulated with lipopolysaccharide was associated with absence of OA (evaluated in multiple joint sites) in an aged population (10), while higher TNF and IL-10 production by peripheral blood cells was associated with radiographic progression of knee OA (9). The authors of those reports attributed their findings to genetic differences in innate production of cytokines by peripheral leukocytes.
Attur and colleagues suggest another possible explanation for differential PBL activity in OA patients. They speculate that PBL cytokine production profiles may be a reflection of local inflammation at joint sites where leukocytes are exposed repeatedly to inflammatory stimuli, prior to trafficking back to the peripheral circulation where the consequences of local stimulation can then be detected. Pools of activated precursors in the bone marrow are also hypothesized to contribute.
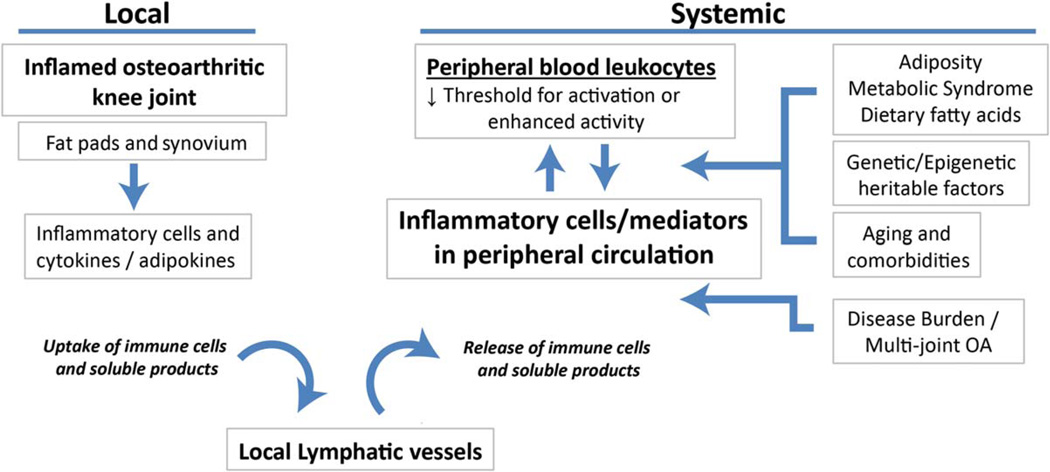
Results of other studies support the idea that local inflammation in OA joints may be reflected in systemically measured markers (i.e., C-reactive protein [11], plasma arachidonic acid [12], soluble CD163 [13]). However, the possibility that patients with rapid progression of JSN may be characterized by PBL populations that have a lower threshold for activation, through other mechanisms not directly related to local inflammation, also needs to be considered. Potential explanations for Attur and colleagues’ results include the impact of genetic and epigenetic influences on cellular activation. Additionally, the potential for a systemic influence on PBL inflammatory activity, for example in the setting of metabolic syndrome (2), needs to be determined. Although the authors did control for BMI in their study, other measures of adiposity may convey more information regarding risk of metabolic derangement (14). Dietary intake of fatty acids, another variable that could influence inflammatory profiles of PBLs, was also not assessed. How much of the influence on PBL activity in OA is local, and how much is systemic, remains to be further investigated in order to gain full understanding of the relationship between these systemic measures of inflammation and disease progression (Figure 1).
Figure 1.
Local and systemic factors that could contribute to systemic measures of inflammation in knee osteoarthritis (OA). Inflammatory cells infiltrate the synovial tissues and fat pads of the osteoarthritic joint to a variable extent. Infiltrating cells become activated locally by inflammatory cytokines and adipokines within the joint. These cells, as well as soluble products such as cytokines, can be taken up by local lymphatic vessels within the joint, where they eventually may traffic back to the peripheral circulation and be detected peripherally. A number of systemic factors may have an effect on the behavior of the peripheral blood leukocyte pool. These include adiposity/metabolic syndrome through the effects of soluble adipokines and lipid mediators, genetic or epigenetic heritable effects on cellular activation, and biologically active molecules released from the joint that become systemic from increased disease burden in patients with multiple joints affected by arthritis. Additionally, the impact of aging and comorbidities, as well as dietary fatty acids, on peripheral blood leukocyte activity should be considered.
The potential for measurement of plasma PGE2 and 15-HETE to distinguish symptomatic knee OA cases and controls, and for measurement of PBL COX-2 or cytokine production to predict radiographic progression, adds to the list of biomarkers of interest in OA (15). As with other systemic inflammatory markers, specificity for OA should not be implied from the findings reported by Attur et al since there was considerable overlap in plasma lipid levels between patient and control groups. The microarray technique used for the transcriptomic analysis in this study has the advantage of high-throughput capability, but was not validated by more accurate measurements such as quantitative polymerase chain reaction. However, the authors thoughtfully combined the microarray COX-2 expression with measurement of plasma eicosanoid levels to strengthen their findings. Still, these results need to be replicated in other populations. A measure of burden of disease, or number of joints affected, was not accounted for in this population, and could influence systemically assessed markers. Additionally, how comorbid conditions that are common in older OA patients may influence PBL activity needs to be determined.
Despite many remaining questions, the current report strengthens the increasingly expanding evidence challenging the historical categorization of OA as a strictly noninflammatory disease. It highlights the importance of inflammatory mediators and pathways in OA pathogenesis. Measurement of the inflammatory markers chosen in this study substantially expands the list of biomarkers that may be useful in future clinical trials. That these markers can be detected in peripheral blood significantly increases their practical utility to help identify more rapid progressors, and potentially to categorize patients into subgroups with a more inflammatory component to their disease, who might be expected to respond more readily to antiinflammatory treatment approaches.
Acknowledgments
Supported by the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases grants K08-AR-057859 to Dr. Scanzello and P60-AR-064166 to Dr. Loeser).
Footnotes
AUTHOR CONTRIBUTIONS
Drs. Scanzello and Loeser drafted the article, revised it critically for important intellectual content, and approved the final version to be published.
REFERENCES
- 1.Orlowsky EW, Kraus VB. The role of innate immunity in osteoarthritis: when our first line of defense goes on the offensive. J Rheumatol. 2015;42:363–371. doi: 10.3899/jrheum.140382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Courties A, Gualillo O, Berenbaum F, Sellam J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthritis Cartilage. 2015 doi: 10.1016/j.joca.2015.05.016. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Van Spil WE, Welsing PM, Bierma-Zeinstra SM, Bijlsma JW, Roorda LD, Cats HA, et al. The ability of systemic biochemical markers to reflect presence, incidence, and progression of early-stage radiographic knee and hip osteoarthritis: data from CHECK. Osteoarthritis Cartilage. 2015;23:1388–1397. doi: 10.1016/j.joca.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Jin X, Beguerie JR, Zhang W, Blizzard L, Otahal P, Jones G, et al. Circulating C reactive protein in osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:703–710. doi: 10.1136/annrheumdis-2013-204494. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki E, Tsuda E, Yamamoto Y, Iwasaki K, Inoue R, Takahashi I, et al. Serum hyaluronan levels increase with the total number of osteoarthritic joints and are strongly associated with the presence of knee and finger osteoarthritis. Int Orthop. 2013;37:925–930. doi: 10.1007/s00264-013-1849-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attur M, Krasnokutsky S, Statnikov A, Samuels J, Li Z, Friese O, et al. Low-grade inflammation in symptomatic knee osteoarthritis: prognostic value of inflammatory plasma lipids and peripheral blood leukocyte biomarkers. Arthritis Rheumatol. 2015;67:2905–2915. doi: 10.1002/art.39279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attur M, Belitskaya-Levy I, Oh C, Krasnokutsky S, Greenberg J, Samuels J, et al. Increased interleukin-1β gene expression in peripheral blood leukocytes is associated with increased pain and predicts risk for progression of symptomatic knee osteoarthritis. Arthritis Rheum. 2011;63:1908–1917. doi: 10.1002/art.30360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Botha-Scheepers S, Watt I, Slagboom E, de Craen AJ, Meulenbelt I, Rosendaal FR, et al. Innate production of tumour necrosis factor α and interleukin 10 is associated with radiological progression of knee osteoarthritis. Ann Rheum Dis. 2008;67:1165–1169. doi: 10.1136/ard.2007.084657. [DOI] [PubMed] [Google Scholar]
- 10.Goekoop RJ, Kloppenburg M, Kroon HM, Frolich M, Huizinga TW, Westendorp RG, et al. Low innate production of interleukin-1β and interleukin-6 is associated with the absence of osteoarthritis in old age. Osteoarthritis Cartilage. 2010;18:942–947. doi: 10.1016/j.joca.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Pearle AD, Scanzello CR, George S, Mandl LA, DiCarlo EF, Peterson M, et al. Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthritis Cartilage. 2007;15:516–523. doi: 10.1016/j.joca.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Baker KR, Matthan NR, Lichtenstein AH, Niu J, Guermazi A, Roemer F, et al. Association of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: the MOST study. Osteoarthritis Cartilage. 2012;20:382–387. doi: 10.1016/j.joca.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daghestani HN, Pieper CF, Kraus VB. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol. 2015;67:956–965. doi: 10.1002/art.39006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol. 2006;35:83–92. doi: 10.1093/ije/dyi253. [DOI] [PubMed] [Google Scholar]
- 15.Hunter DJ, Nevitt M, Losina E, Kraus V. Biomarkers for osteoarthritis: current position and steps towards further validation. Best Pract Res Clin Rheumatol. 2014;28:61–71. doi: 10.1016/j.berh.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]