Summary
Pituitary adenomas are a common intracranial neoplasm, usually demonstrating a benign phenotype. They can be classified according to pathological, radiological or clinical behaviour as typical, atypical or carcinomas, invasive or noninvasive, and aggressive or nonaggressive. Prolactinomas account for 40–60% of all pituitary adenomas, with dopamine agonists representing the first-line treatment and surgery/radiotherapy reserved for drug intolerance/resistance or in neuro-ophthalmological emergencies. We present the case of a 62-year-old man with an apparently indolent prolactin-secreting macroadenoma managed with partial resection and initially showing a biochemical response to cabergoline. Five years later, the tumour became resistant to cabergoline, despite a substantial increase in dosage, showing rapid growth and causing worsening of vision. The patient then underwent two further transsphenoidal operations and continued on high-dose cabergoline; despite these interventions, the tumour continued enlarging and prolactin increased to 107 269 U/L. Histology of the third surgical specimen demonstrated features of aggressive behaviour (atypical adenoma with a high cell proliferation index) not present in the tumour removed at the first operation. Subsequently, he was referred for radiotherapy aiming to control tumour growth.
Learning points:
The development of secondary resistance to dopamine agonists (DAs) is a serious sign as it may be associated with de-differentiation of the prolactinoma and thus of aggressive or malignant transformation.
Significant de-differentiation of the adenoma documented on consecutive histologies suggests a possible transition to malignancy.
A combination of histological ‘alarm’ features associated with persistent growth and escape from DAs treatment in recurrent adenomas should alert clinicians and demands close follow-up.
A multidisciplinary approach by pathologists, endocrinologists and neurosurgeons is essential.
Background
Pituitary tumours are a relatively common intracranial neoplasm. Approximately 10–20% of the normal population may harbour these lesions according to autopsy and/or pituitary magnetic resonance imaging (MRI) series: most of these tumours are clinically insignificant with less than 5 mm in diameter and grow slowly over many years without local invasion or remain static. Prolactinomas account for 40–60% of all pituitary adenomas and up to 80% are present as microadenomas (1). Pituitary adenomas can be classified according to pathological, radiological or clinical behaviour as typical, atypical and carcinomas, invasive or noninvasive, and aggressive or nonaggressive: using the WHO 2004 classification (currently under revision), the majority are typical adenomas. They are slow-growing, well-demarcated, noninvasive adenomas, showing no major cellular and nuclear pleomorphism, few mitotic figures and a Ki-67 nuclear index <3%. Atypical adenomas are tumours that demonstrate a Ki-67 nuclear index >3%, elevated mitotic activity and excessive p53 immunoreactivity (2). Pituitary carcinomas can only be diagnosed if cerebrospinal and/or systemic metastases are documented.
First-line therapy for prolactinomas are dopamine agonists (DAs): these agents are effective in controlling clinical symptoms, normalising serum prolactin (PRL) and reducing tumour volume, while being well tolerated. Surgery and radiotherapy are reserved for drug-resistant tumours or intolerance to DAs or neuro-ophthalmological emergencies.
We present a patient with a macroprolactinoma who showed an unexpected and severe escape from cabergoline treatment. The tumour was originally a typical adenoma sensitive to DAs therapy, but later it became resistant to the DA and transformed into an atypical adenoma with aggressive features.
Case presentation
A 62-year-old man presented with collapse in August 2008. His admission was preceded by a one-year history of general lethargy/malaise, weight loss, deteriorating vision and loss of chest and pubic hair. His medical history included a pelvic sarcoma excised in 2001 followed by radiotherapy, and a fluctuating PSA on surveillance (biopsy in 2011 revealed no evidence of malignancy).
Investigations
On presentation, he had a serum PRL of 55 287 U/L (reference range 45–375 U/L) without significant macroprolactin identified, LH 0.1 IU/L (1.5–9.3), FSH 0.4 IU/L (2–20), testosterone <0.4 nmoL/L (8.4–28.7), IGF-1 6.6 nmoL/L (7.2–27.6), TSH 1.34 U/L (0.35–5.4), FT4 6.6 pmol/L (10.5–20) and cortisol 111 nmol/L at 0900 h and normal serum and urinary osmolalities. MRI revealed a 2.1 × 2.4 × 2.5 cm pituitary mass with marked suprasellar extension and compression of the optic chiasm, with areas of high T1-weighted signal suggesting possible apoplexy. Visual field testing was consistent with bitemporal hemianopia.
Treatment
The patient underwent transsphenoidal adenomectomy (TSA) on August 2008 with an uneventful post-operative course and resolution of his visual field defect. Pathology identified features of a pituitary adenoma with rare mitotic figures, overall Ki-67 nuclear index ~3%, only weak expression of p53 and no other features of atypia. Immunohistochemistry showed expression of PRL by the majority of cells (Fig. 1).
Figure 1.
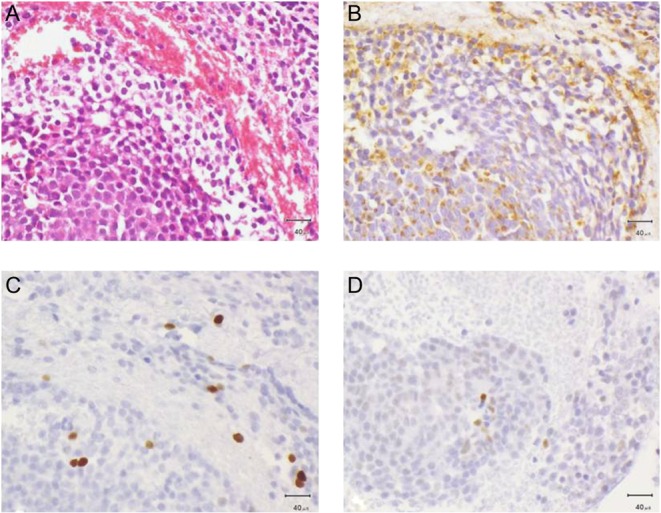
Histologic slides of first biopsy. (A) Haematoxylin and eosin, (B) PRL expression, (C) Ki-67 expresstion, (D) p53 expression.
Outcome and follow-up
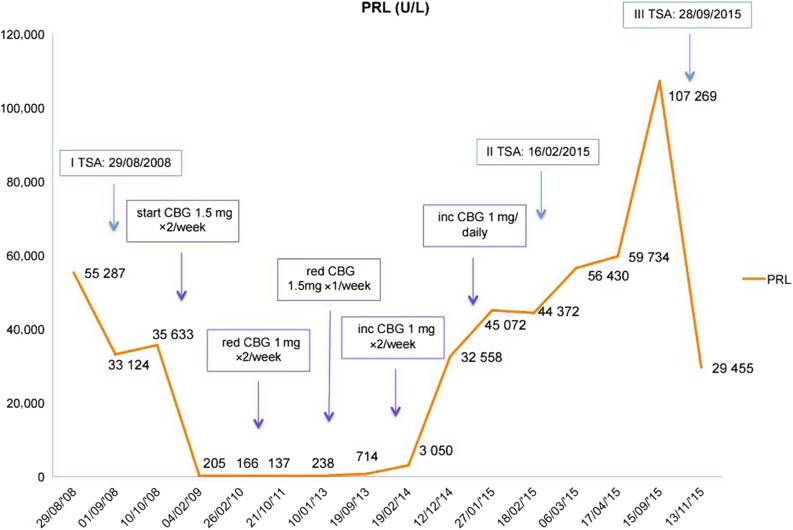
Post-operatively, serum PRL decreased, although it remained significantly elevated (Fig. 2) but normalised later on cabergoline (Fig. 2). Appropriate hormone replacement was initiated (hydrocortisone, l-thyroxine and testosterone gel). An MRI performed one year after surgery demonstrated a 5 mm rim of soft tissue along the floor of the pituitary fossa without compression of the optic chiasm.
Figure 2.
PRL level according to surgery and variations in cabergoline dose. inc, increased; red, reduced.
The patient remained stable over the next 5 years (Fig. 2 shows PRL levels in relation to surgery and changes in cabergoline dose). However, in February 2014, serum PRL started increasing, and in December 2014, the patient reported deterioration of his vision, confirmed by visual field examination. At this stage, his MRI demonstrated tumour growth (mass of 21 mm within the expanded sella, which slightly elevated the optic chiasm), and the patient underwent a second TSA for debulking: the tumour was found to be soft and semi-liquid. Histological examination identified a tiny tissue fragment showing PRL expression, consistent with the original pathology. After surgery, the patient had slight improvement in his vision and continued on cabergoline. In the following months, despite good compliance with the DAs, he developed further visual deterioration with bitemporal hemianopia. The MRI revealed progressive enlargement of the large macroadenoma (27 mm) with increased superior elevation and distortion of the optic chiasm. The patient underwent a further debulking, and histology showed strong diffuse positivity for PRL and weak focal staining for LH, but with features of an atypical pituitary adenoma (mammotroph/prolactinoma) with a very high mitotic index (9–10 per 10 HP fields) and a Ki-67 index of 20–30%. Most tumour cell nuclei showed moderate-to-strong nuclear staining for p53 (Fig. 3). PRL after surgery was 29 455 U/L, and the patient is currently undergoing external beam radiotherapy, 45 Gy in 25 fractions.
Figure 3.
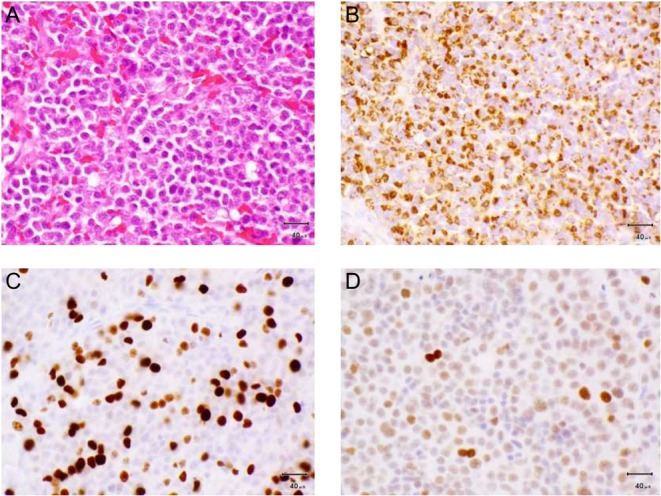
Histologic slides of third biospy. (A) Haematoxylin and eosin, (B) PRL expression, (C) Ki-67 expression, (D) p53 expression.
Discussion
Our case shows a combination of features indicative of a non-benign clinical course, such as persistent growth requiring multiple operations, secondary resistance to DA therapy and transformation to an atypical histology over 5 years.
A variety of definitions of DA resistance have been proposed. The most commonly used definition includes failure to achieve normoprolactinaemia (biochemical response) and/or failure to achieve at least 50% shrinkage (tumour response) with maximal conventional doses of medication (bromocriptine 7.5 mg/day or cabergoline 2.0 mg/week) (3). When non-compliance is ruled out, as seems likely in this case, escape from initial control may be associated with de-differentiation of the tumour and thus the potential for malignant transformation. Indeed, tumours very sensitive to DAs are more likely to show persistent remission of hyperprolactinaemia after treatment withdrawal (4), whereas tumours less responsive to medical therapy, as here, often express histological markers of increased cell proliferation and may show a turbulent clinical course.
Recently, questions regarding the legitimacy and utility of the WHO 2004 classification for pituitary adenomas have invoked some controversy following its initial description. Lately, a German group further specifed certain histomorphological and immunohistochemical parameters for the diagnosis of an atypical adenoma (APA) using the German pituitary adenoma registry. They defined a cutoff value for the number of mitotic figures as ≥2 mitoses per 10 high power fields (HPFs) and a p53 ≥2% in APA cases. Moreover, according to this study, the best marker for differentiating typical pituitary adenomas and APAs is a Ki-67 nuclear index >4% (5). Others reported that the presence of p53 in combination with increased Ki-67% is probably superior to either method alone (6).
Others have also pointed out that the 2004 WHO classification does not take into account the invasive status of the tumour and that its numerous ultrastructural subtypes are confusing (7). A French multicentric collaborative study recently published a clinicopathological classification of pituitary adenomas, which relies on proliferation markers, invasion into the cavernous and sphenoid sinus and tumour size (7). To confirm invasiveness, the authors suggested histological and/or radiological signs of cavernous or sphenoid sinus invasion. For the assessment of cell proliferation, they used the presence of at least two markers with cutoff values of 3% for the Ki-67 index (with formol fixative and 1% for Bouin-Hollande fixative), mitoses >2 per 10 HPFs and positivity for p53 in at least 10 nuclei per 10 HPFs (7). These questions are being addressed in the latest WHO assessment, where the current classification is likely to be modified.
However, our patient showed a change in tumour pathology over time: originally, it was a typical adenoma, with few atypical features, changing from rare mitotic figures to 9–10 per 10 HPFs, from a Ki-67 index of 3–5% to 20–30%, and from only weak expression of p53 to a moderate-to-strong nuclear staining for p53, suggesting a possible transition to a highly aggressive phenotype. This is in accord with the concept that pituitary carcinomas mainly arise from the transformation of initially benign adenomas, although de novo development cannot be excluded. In general, highly aggressive transformation, including that to a carcinoma, is rare, and mainly occurs in PRL- and ACTH-secreting tumours, possibly because these tumours are less likely to show markers of senescence compared with those of other adenoma types (8). If further progression occurs in our patient, we will consider the option of temozolomide (9, 10). In fact, various chemotherapy regimens have been proposed for highly aggressive pituitary tumours and carcinomas, and temozolomide, an orally administered alkylating agent, is becoming the mainstream choice. The outcome of treatment might depend on the expression of O(6)-methylguanine-DNA methyltransferase (MGMT), a DNA repair enzyme that potentially interferes with drug efficacy. Furthermore, some encouraging data with pasireotide, a somatostatin analogue that exhibit a high affinity for the majority of somatostatin receptors, have been reported (6, 9, 10).
Most importantly, in such complex cases, multidisciplinary collaboration between pathologists, endocrinologists, neurosurgeons, neuro-ophthalmologists and oncologists is essential offering a holistic approach and more optimal outcomes.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written informed consent was obtained from the patient for publication of this case report.
Author contribution statement
E S with the collaboration of G F and F A wrote the paper with the essential and constant supervision of Prof. A G, S C operated on the patient, O A analysed the surgical specimen and provided slides for the paper and N K and A G are involved in the management of the patient.
References
- 1.Fernandez A, Karavitaki N, Wass JA. 2010. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clinical Endocrinology 72 377–382. ( 10.1111/j.1365-2265.2009.03667.x) [DOI] [PubMed] [Google Scholar]
- 2.Al-Shraim M, Asa SL. 2006. The 2004 World Health Organization classification of pituitary tumors: what is new? Acta Neuropathologica 111 1–7. ( 10.1007/s00401-005-1093-6) [DOI] [PubMed] [Google Scholar]
- 3.Molitch ME. 2014. Management of medically refractory prolactinoma. Journal of Neuro-Oncology 117 421–428. ( 10.1007/s11060-013-1270-8) [DOI] [PubMed] [Google Scholar]
- 4.Delgrange E, Daems T, Verhelst J, Abs R, Maiter D. 2009. Characterization of resistance to the prolactin-lowering effects of cabergoline in macroprolactinomas: a study in 122 patients. European Journal of Endocrinology/European Federation of Endocrine Societies 160 747–752. ( 10.1530/EJE-09-0012) [DOI] [PubMed] [Google Scholar]
- 5.Miermeister CP, Petersenn S, Buchfelder M, Fahlbusch R, Ludecke DK, Holsken A, Bergmann M, Knappe HU, Hans VH, Flitsch J, et al. 2015. Histological criteria for atypical pituitary adenomas – data from the German pituitary adenoma registry suggests modifications. Acta Neuropathologica Communications 3 50 ( 10.1186/s40478-015-0229-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaltsas GA, Nomikos P, Kontogeorgos G, Buchfelder M, Grossman AB. 2005. Clinical review: diagnosis and management of pituitary carcinomas. Journal of Clinical Endocrinology and Metabolism 90 3089–3099. ( 10.1210/jc.2004-2231) [DOI] [PubMed] [Google Scholar]
- 7.Trouillas J, Roy P, Sturm N, Dantony E, Cortet-Rudelli C, Viennet G, Bonneville JF, Assaker R, Auger C, Brue T, et al. 2013. A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case-control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathologica 126 123–135. ( 10.1007/s00401-013-1084-y) [DOI] [PubMed] [Google Scholar]
- 8.Alexandraki KI, Munayem Khan M, Chahal HS, Dalantaeva NS, Trivellin G, Berney DM, Caron P, Popovic V, Pfeifer M, Jordan S, et al. 2012. Oncogene-induced senescence in pituitary adenomas and carcinomas. Hormones 11 297–307. ( 10.14310/horm.2002.1358) [DOI] [PubMed] [Google Scholar]
- 9.McCormack AI, Wass JA, Grossman AB. 2011. Aggressive pituitary tumours: the role of temozolomide and the assessment of MGMT status. European Journal of Clinical Investigation 41 1133–1148. ( 10.1111/j.1365-2362.2011.02520.x) [DOI] [PubMed] [Google Scholar]
- 10.Raverot G, Castinetti F, Jouanneau E, Morange I, Figarella-Branger D, Dufour H, Trouillas J, Brue T. 2012. Pituitary carcinomas and aggressive pituitary tumours: merits and pitfalls of temozolomide treatment. Clinical Endocrinology 76 769–775. ( 10.1111/j.1365-2265.2012.04381.x) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a