Abstract
Purpose
We present a case of bilateral extensive peripapillary myelinated retinal nerve fibers (MRNF) in an individual with Crouzon syndrome, an inherited form of craniosynostosis caused by overactivation of fibroblast growth factor receptor 2. As a secondary aim, we examine the utility of optical coherence tomography (OCT) angiography for visualization of peripapillary vasculature obscured by myelination on other imaging modalities.
Methods
A 24-year-old woman with Crouzon syndrome was evaluated for suspected optic neuritis in the right eye.
Results
Funduscopic examination and photography revealed the incidental finding of bilateral extensive peripapillary MRNF. OCT angiography provided excellent visualization of peripapillary retinal vessels, which were partially obscured by myelination on other imaging modalities.
Conclusions
This association of Crouzon syndrome with bilateral peripapillary MRNF may lend insight into the developmental control of optic nerve myelination, the pathogenesis of MRNF, and the potential role of growth factors in these processes. Further, OCT angiography allowed for excellent blood vessel visualization in this case of MRNF.
Keywords: Myelin, Myelinated retinal nerve fibers, Crouzon syndrome, Fibroblast growth factor receptor, Optical coherence tomography angiography
Introduction
Myelinated retinal nerve fibers (MRNF) appear as grey or white striated patches with feathered borders, and are estimated to occur in 1% of the population.1 MRNF are typically congenital, and therefore likely represent anomalies of myelination control in utero.2 They are most commonly unilateral, with only 7.7% of cases estimated to occur bilaterally.1 We present a case of bilateral extensive peripapillary MRNF surrounding the majority of the optic disc circumference in a patient with Crouzon syndrome, an autosomal dominant craniosynostosis disorder with high penetrance and variable expressivity. Crouzon syndrome is caused by mutation of the fibroblast growth factor receptor 2 (FGFR2) gene, leading to overactivation that promotes premature fusion of cranial sutures.3 This can result in craniofacial and orbital structural anomalies, and has been associated with various ophthalmic manifestations including myopia, proptosis, hypertelorism, exotropia, globe subluxation, coloboma, and optic atrophy.4 To our knowledge, MRNF have not been previously described in association with Crouzon syndrome. We examine the potential common pathogenic mechanism for both the MRNF and craniosynostosis in Crouzon syndrome, and the role of FGFR2 in the developmental control of optic nerve myelination.
MRNF are typically asymptomatic and found incidentally on ophthalmic examination. However, because they are located superficially, MRNF can obstruct the view of underlying retinal vessels. Adequate visualization of retinal vasculature in cases of MRNF is important, as the abnormal structure and thickening of the nerve fiber layer may play a role in the development of significant retinal vascular disorders.5, 6, 7, 8 As a secondary aim of this report, we evaluate the use of optical coherence tomography (OCT) angiography for visualization of peripapillary vessels obscured by myelination on other imaging modalities.
Case report
A 24-year-old Caucasian woman was referred to neuro-ophthalmology clinic by her general ophthalmologist for suspicion of optic neuritis in the right eye. She had become aware of a scotoma in the superonasal visual field of her right eye over the prior six months. Additionally, she reported restricted ocular motility with binocular horizontal diplopia on left lateral gaze. She denied pain with eye movement, but described mild right-sided retrobulbar pain. The scotoma disappeared and the retrobulbar pain subsided while she was on a prior 5-day course of intravenous steroids. However, these two symptoms returned after completing the course. She denied loss of color vision and any neurologic abnormalities. She had no other past ocular history.
The patient was diagnosed with Crouzon syndrome as a child by genetic testing, which revealed mutation of the FGFR2 gene. She previously had multiple facial and nasal corrective surgeries but no prior ocular or orbital surgeries. Past medical history also included depression and anxiety, and her only medication was escitalopram. Laboratory studies, including complete blood count, erythrocyte sedimentation rate, lupus anticoagulant panel, vitamin B12, folate, aquaporin-4 antibody, rheumatoid factor, and Lyme antibody titers performed 4 months prior were within normal limits. An outside report of a gadolinium-enhanced MRI of the brain and orbits revealed no significant abnormality of the optic nerves or other orbital structures. The patient was adopted and her family history was unknown.
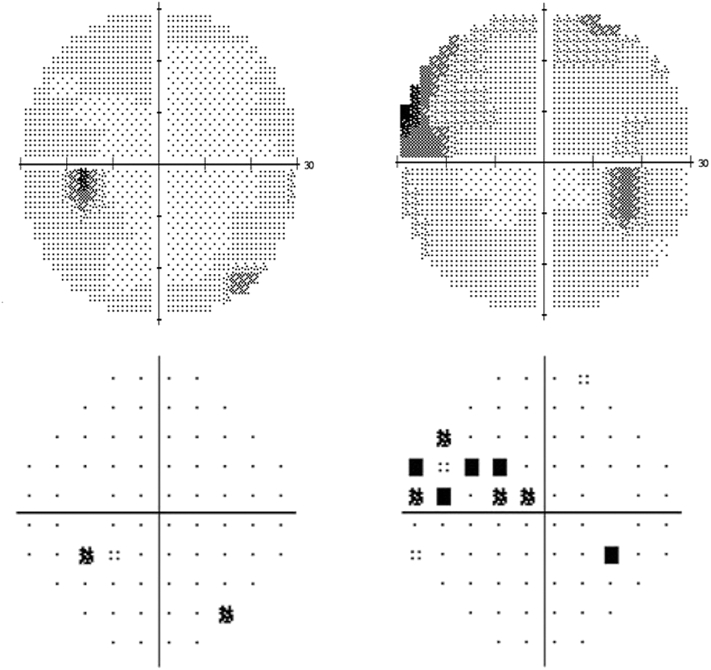
On examination, the patient had craniofacial structural abnormalities consistent with Crouzon syndrome. There was no proptosis of either eye. Uncorrected distance visual acuity was 20/25 in the right eye, with pinhole improvement to 20/20, and 20/20 in the left eye. Ishihara color vision testing showed correct identification of 14 out of 14 plates in both eyes. Pupils were reactive to light, with a trace afferent pupillary defect in the right eye. Intraocular pressures were 21 mmHg in both eyes. The patient demonstrated slow adduction of the right eye. 30-2 Humphrey visual field testing (HVF, Fig. 1) revealed a superonasal visual field defect with mean deviation (MD) of −4.64 dB and foveal threshold of 35 dB in the right eye. HVF testing of the left eye was normal with MD of −1.56 dB and foveal threshold of 39 dB.
Fig. 1.
30-2 Humphrey visual field (HVF). Grey scale of the patient's left eye (top left) was essentially normal with mean deviation (MD) of −1.56 dB and foveal threshold of 39 dB. Grey scale of the right eye (top right) demonstrates a superonasal visual field defect with MD of −4.64 dB and foveal threshold of 35 dB. Pattern deviation is normal in the left eye (bottom left) and shows loss superonasally in the right eye (bottom right).
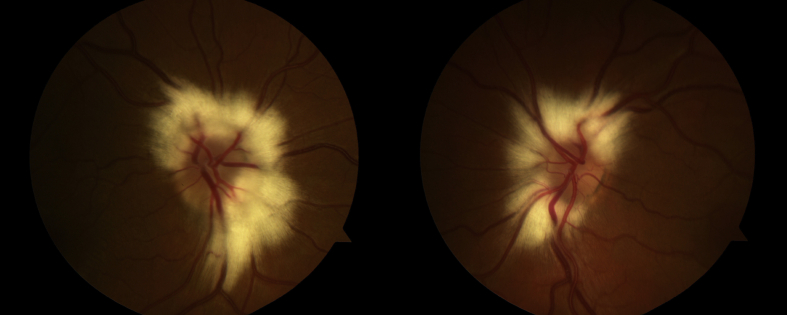
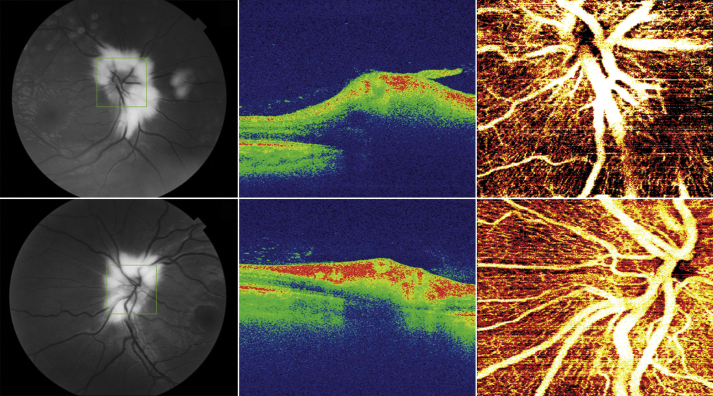
Dilated funduscopic examination revealed extensive whitish peripapillary MRNF with irregular, feathered borders in both eyes. This finding was further visualized with color and red-free fundus photography (Fig. 2, Fig. 3). Myelinated fibers spanned roughly three-quarters of the circumference of the optic disc (9/12 clock hours) in both eyes. The inferotemporal sector of each disc was largely unaffected, and these visible disc margins appeared crisp and with normal contour. However, most portions of the peripapillary retinal vasculature and optic disc margins underlying the myelinated fibers were not readily visible. Swept-source OCT of both optic nerve heads demonstrated a substantially thickened retinal nerve fiber layer (Fig. 3). Swept-source OCT angiography (DRI OCT Triton, Topcon Corp., Tokyo, Japan) of the optic discs revealed normal blood vessel anatomy, with no tortuosity or other structural anomalies in either eye (Fig. 3). Upon review of the previous gadolinium-enhanced MRI of the orbits, enhancement of the right posterior orbit was evident involving the medial and lateral rectus muscles and posterior intraorbital optic nerve.
Fig. 2.
Color photographs of the patient's right fundus (left image) and left fundus (right image) demonstrating extensive distribution of peripapillary myelinated retinal nerve fibers with feathered borders in both eyes. Peripapillary retinal vasculature and disc margins underlying the myelinated retinal nerve fibers are partially obscured.
Fig. 3.
Optic disc imaging findings. Red-free fundus photographs of the right eye (top left) and left eye (bottom left) highlight the white frayed appearance of the peripapillary myelinated retinal nerve fibers. Each green box outlines the sample area of the corresponding optical coherence tomography (OCT) imaging, which is centered on the optic discs. False color OCT B-scans of the optic nerve head in the right eye (top middle) and left eye (bottom middle) reveal a substantially thickened retinal nerve fiber layer. En face OCT angiogram images of the right eye (top right) and left eye (bottom right) provide detailed visualization of the dense vascular network surrounding each disc.
A diagnosis of idiopathic orbital inflammatory syndrome (IOIS) was made, in accordance with the patient's mild limitation in ocular motility and non-specific orbital inflammation on MRI. The patient's recent-onset visual field defect, while not typical of IOIS, was nonetheless determined to have occurred as a consequence of optic nerve inflammation. The bilateral extensive MRNF were determined to be an incidental finding associated with the patient's Crouzon syndrome, though not contributory to the patient's visual symptoms.
Discussion
This case documents a new association between bilateral extensive MRNF and Crouzon syndrome. The precise etiology of MRNF is not known, however the pathogenesis is believed to involve defects of the lamina cribrosa,9, 10, 11 or the presence of intraretinal oligodendrocytes irrespective of lamina cribrosa function.12 Most occurrences are congenital and sporadic,2, 12 though some cases show familial inheritance.13 Rarely, MRNF may be acquired in childhood in association with optic nerve structural abnormalities that can cause changes to the lamina cribrosa, such as optic nerve gliomas or optic nerve sheath fenestration.14 Elbaz et al have demonstrated an association between MRNF and a history of stroke, and suggest that such cases of acquired MRNF may result from stroke-related structural changes in the lamina cribrosa, or from upregulation of growth factors and production of new myelin by oligodendrocytes after an ischemic event.15 Of note, the patient described in the present case had no history of cerebrovascular disease.
Myelination of the central nervous system (CNS) during development is highly regulated. Proper proliferation, migration, and differentiation of oligodendrocytes and their progenitor cells depend on signaling from multiple growth factors between neurons and glia, including platelet derived growth factor, insulin-like growth factor 1, and fibroblast growth factors, among others.16 Disruptions to the precise and coordinated temporal and spatial expression of signaling molecules or their receptors can result in aberrant patterns of myelination.
That the pattern of myelination in this case was bilateral serves as an initial suggestion of a non-coincidental association between the patient's developmental anomalies and intraretinal myelination. Further, the extensive peripapillary distribution of MRNF surrounding the majority of the optic disc in this case is striking, as most cases of MRNF are localized to an individual sector adjacent to the disc5 and typically involve less than half the disc circumference.17
The findings in this case may lend insight into the developmental control of optic nerve myelination, and the potential role of FGFR2 in this process. Several mutations in the FGFR2 gene have been reported to cause Crouzon syndrome; these mutations appear to overactivate signaling by the FGFR2 protein, which promotes premature fusion of bones in the skull.3 FGFR2-IIIc, for example, is one of the isoforms involved in Crouzon syndrome18 and is also expressed by astrocytes in humans,19 though its potential role in CNS myelination in humans has not been elucidated. In mice, however, CNS myelination by oligodendrocytes requires FGFR2,20 and in vitro analysis has demonstrated that FGFR2 helps regulate oligodendrocyte process growth.21 Expression of FGFR2 and its ligands has also been detected in the human and mouse optic nerve and retina.22, 23, 24 This molecular link between developmental anomalies in Crouzon syndrome and myelination in animal and in vitro models may indicate that an overactivation of FGFR2 signaling might be associated with abnormal regulation of myelination.
The development of MRNF in this case of Crouzon syndrome may also be structural in origin. In humans, myelination of retinal ganglion cell axons begins at the lateral geniculate nucleus in the fetus and ends at the lamina cribrosa at birth or shortly thereafter. The lamina cribrosa – a complex network of astrocyte processes, neurons, and connective tissue – is believed to block migration of oligodendrocytes and/or their precursors into the retina.10, 11 Structural abnormalities of the LC may therefore permit oligodendrocyte access to the retina, leading to intraretinal myelination.9 Khan et al postulate that FGFR2 overactivity in Crouzon syndrome may be associated with accumulation of abnormal fibrous tissue at the LC.22 This might lend a further explanation for the potential link between Crouzon syndrome and MRNF. Abnormal fibrous tissue may alter the integrity of the LC, thereby allowing oligodendrocytes and/or their precursors to enter the retina. Additionally, such structural compromise of the LC may further disrupt signaling that is necessary for cessation of myelination at the LC.
A developmental link between Crouzon syndrome and MRNF may therefore involve multiple mechanisms. Dysregulation of FGFR2 might lead to perturbations in oligodendrocyte signaling pathways and altered integrity of the lamina cribrosa. However, as this is, to our knowledge, the only documented case of MRNF in Crouzon syndrome, this potential developmental link remains hypothetical. Nonetheless, the association between MRNF and Crouzon syndrome in this case may provide insight into the regulation of myelination of visual pathways and the potential role of FGFR2 in this process, and lends credence to existing theories on the pathogenesis of MRNF.
OCT angiography is a new imaging modality that provides non-invasive high resolution images of the optic nerve and retinal vessels.25 The influence of MRNF on retinal vessel density measurement with OCT angiography has been previously reported in two cases of focal MRNF.26 Visualization of retinal vasculature is essential in all comprehensive ophthalmologic examinations, and may be of particular importance in cases of MRNF. The opaque appearance of MRNF can obscure underlying retinal vessels,27 and therefore can present diagnostic and monitoring difficulties for clinicians. In addition, although MRNF are typically a benign finding, they have been associated with – and may be involved in the development of – various retinal vascular disorders including telangiectatic vessels, branch artery and vein occlusion, microaneurysms, neovascularization, and recurrent vitreous hemorrhage.5, 6, 7, 8 This is hypothesized to be a consequence of the abnormal structure and increased thickness of the overlying retinal nerve fiber layer,7 which may promote retinal ischemia,6, 8 mechanical disturbance of retinal vessels,6 or disordered glial regulation of vascular tight junctions.8 Additionally, vascular abnormalities may develop independently in regions of MRNF, unrelated to the overlying myelination and structural anomalies of the retinal nerve fiber layer. Monitoring of underlying vasculature is therefore critical in patients with MRNF.
In the present case, obscuration of peripapillary vessels was apparent on color and red-free fundus photography. However, OCT angiography provided extensive visualization of the underlying vasculature, revealing a dense peripapillary capillary network without signs of structural anomalies. OCT angiography may be a useful imaging modality in cases of MRNF for evaluation of retinal vessels.
Footnotes
The authors have no conflicts of interest. The authors have no disclosures. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Straatsma B.R., Foos R.Y., Heckenlively J.R., Taylor G.N. Myelinated retinal nerve fibers. Am J Ophthalmol. 1981;91(1):25–38. doi: 10.1016/0002-9394(81)90345-7. [DOI] [PubMed] [Google Scholar]
- 2.Shelton J.B., Digre K.B., Gilman J., Warner J.E., Katz B.J. Characteristics of myelinated retinal nerve fiber layer in ophthalmic imaging: findings on autofluorescence, fluorescein angiographic, infrared, optical coherence tomographic, and red-free images. JAMA Ophthalmol. 2013;131(1):107–109. doi: 10.1001/jamaophthalmol.2013.560. [DOI] [PubMed] [Google Scholar]
- 3.Galvin B.D., Hart K.C., Meyer A.N., Webster M.K., Donoghue D.J. Constitutive receptor activation by Crouzon syndrome mutations in fibroblast growth factor receptor (FGFR)2 and FGFR2/Neu chimeras. Proc Natl Acad Sci USA. 1996;93(15):7894–7899. doi: 10.1073/pnas.93.15.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreiborg S., Cohen M.M., Jr. Ocular manifestations of Apert and Crouzon syndromes: qualitative and quantitative findings. J Craniofac Surg. 2010;21(5):1354–1357. doi: 10.1097/SCS.0b013e3181ef2b53. [DOI] [PubMed] [Google Scholar]
- 5.Kodama T., Hayasaka S., Setogawa T. Myelinated retinal nerve fibers: prevalence, location and effect on visual acuity. Ophthalmologica. 1990;200(2):77–83. doi: 10.1159/000310082. [DOI] [PubMed] [Google Scholar]
- 6.Minning C.A., Davidorf F.H. Neovascularization associated with myelinated nerve fibers: a case report. Ann Ophthalmol. 1983;15(12):1142–1144. [PubMed] [Google Scholar]
- 7.Leys A.M., Leys M.J., Hooymans J.M. Myelinated nerve fibers and retinal vascular abnormalities. Retina. 1996;16(2):89–96. doi: 10.1097/00006982-199616020-00001. [DOI] [PubMed] [Google Scholar]
- 8.Silvestri G., Sehmi K., Hamilton P. Retinal vascular abnormalities. A rare complication of myelinated nerve fibers? Retina. 1996;16(3):214–218. [PubMed] [Google Scholar]
- 9.Williams T.D. Medullated retinal nerve fibers: speculations on their cause and presentation of cases. Am J Optom Physiol Opt. 1986;63(2):142–151. [PubMed] [Google Scholar]
- 10.Perry V.H., Lund R.D. Evidence that the lamina cribrosa prevents intraretinal myelination of retinal ganglion cell axons. J Neurocytol. 1990;19(2):265–272. doi: 10.1007/BF01217304. [DOI] [PubMed] [Google Scholar]
- 11.Ffrench-Constant C., Miller R.H., Burne J.F., Raff M.C. Evidence that migratory oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells are kept out of the rat retina by a barrier at the eye-end of the optic nerve. J Neurocytol. 1998;17(1):13–25. doi: 10.1007/BF01735374. [DOI] [PubMed] [Google Scholar]
- 12.Tarabishy A.B., Alexandrou T.J., Traboulsi E.I. Syndrome of myelinated retinal nerve fibers, myopia, and amblyopia: a review. Surv Ophthalmol. 2007;52(6):588–596. doi: 10.1016/j.survophthal.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Funnell C.L., George N.D., Pai V. Familial myelinated retinal nerve fibres. Eye (Lond) 2003;17(1):96–97. doi: 10.1038/sj.eye.6700266. [DOI] [PubMed] [Google Scholar]
- 14.Prakalapakorn S.G., Buckley E.G. Acquired bilateral myelinated retinal nerve fibers after unilateral optic nerve sheath fenestration in a child with idiopathic intracranial hypertension. J Pediatr Ophthalmol Strabismus. 2012;16(6):534–538. doi: 10.1016/j.jaapos.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elbaz H., Peto T., Butsch C. Prevalence and associations of myelinated retinal nerve fibers: results from the population-based gutenberg health study. Retina. 2016 Jun 2 doi: 10.1097/IAE.0000000000001093. [Epub ahead of print], PMID: 27258670. [DOI] [PubMed] [Google Scholar]
- 16.McMorris F.A., McKinnon R.D. Regulation of oligodendrocyte development and CNS myelination by growth factors: prospects for therapy of demyelinating disease. Brain Pathol. 1996;6(3):313–329. doi: 10.1111/j.1750-3639.1996.tb00858.x. [DOI] [PubMed] [Google Scholar]
- 17.Hunter S.F., Leavitt J.A., Rodriguez M. Direct observation of myelination in vivo in the mature human central nervous system. Brain. 1997;120(Pt 11):2071–2082. doi: 10.1093/brain/120.11.2071. [DOI] [PubMed] [Google Scholar]
- 18.Meyers G.A., Day D., Goldberg R. FGFR2 exon IIIa and IIIc mutations in Crouzon, Jackson-Weiss, and Pfeiffer syndromes: evidence for missense changes, insertions, and a deletion due to alternative RNA splicing. Am J Hum Genet. 1996;58(3):491–498. [PMC free article] [PubMed] [Google Scholar]
- 19.Ohashi R., Matsuda Y., Ishiwata T., Naito Z. Downregulation of fibroblast growth factor receptor 2 and its isoforms correlates with a high proliferation rate and poor prognosis in high-grade glioma. Oncol Rep. 2014;32(3):1163–1169. doi: 10.3892/or.2014.3283. [DOI] [PubMed] [Google Scholar]
- 20.Furusho M., Dupree J.L., Nave K.A., Bansal R. Fibroblast growth factor receptor signaling in oligodendrocytes regulates myelin sheath thickness. J Neurosci. 2012;32(19):6631–6641. doi: 10.1523/JNEUROSCI.6005-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fortin D., Rom E., Sun H., Yayon A., Bansal R. Distinct fibroblast growth factor (FGF)/FGF receptor signaling pairs initiate diverse cellular responses in the oligodendrocyte lineage. J Neurosci. 2005;25(32):7470–7479. doi: 10.1523/JNEUROSCI.2120-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan S.H., Britto J.A., Evans R.D., Nischal K.K. Expression of FGFR-2 and FGFR-3 in the normal human fetal orbit. Br J Ophthalmol. 2005;89(12):1643–1645. doi: 10.1136/bjo.2005.075978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sapieha P.S., Peltier M., Rendahl K.G., Manning W.C., Di Polo A. Fibroblast growth factor-2 gene delivery stimulates axon growth by adult retinal ganglion cells after acute optic nerve injury. Mol Cell Neurosci. 2003;24(3):656–672. doi: 10.1016/s1044-7431(03)00228-8. [DOI] [PubMed] [Google Scholar]
- 24.Catalani E., Tomassini S., Dal Monte M., Bosco L., Casini G. Localization patterns of fibroblast growth factor 1 and its receptors FGFR1 and FGFR2 in postnatal mouse retina. Cell Tissue Res. 2009;336(3):423–438. doi: 10.1007/s00441-009-0787-9. [DOI] [PubMed] [Google Scholar]
- 25.Ghasemi Falavarjani K., Tian J.J., Akil H., Garcia G.A., Sadda S.R., Sadun A.A. Swept-source optical coherence tomography angiography of the optic disk in optic neuropathy. Retina. 2016 Aug 22 doi: 10.1097/IAE.0000000000001259. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Holló G. Influence of myelinated retinal nerve fibers on retinal vessel density measurement with AngioVue OCT angiography. Int Ophthalmol. 2016 Feb 27 doi: 10.1007/s10792-016-0207-6. [Epub ahead of print], PMID: 26922060. [DOI] [PubMed] [Google Scholar]
- 27.Gradle H.S. The blind spot III. The relation of the blind spot to medullated nerve fibers in the retina. JAMA. 1921;77(19):1483–1487. [Google Scholar]