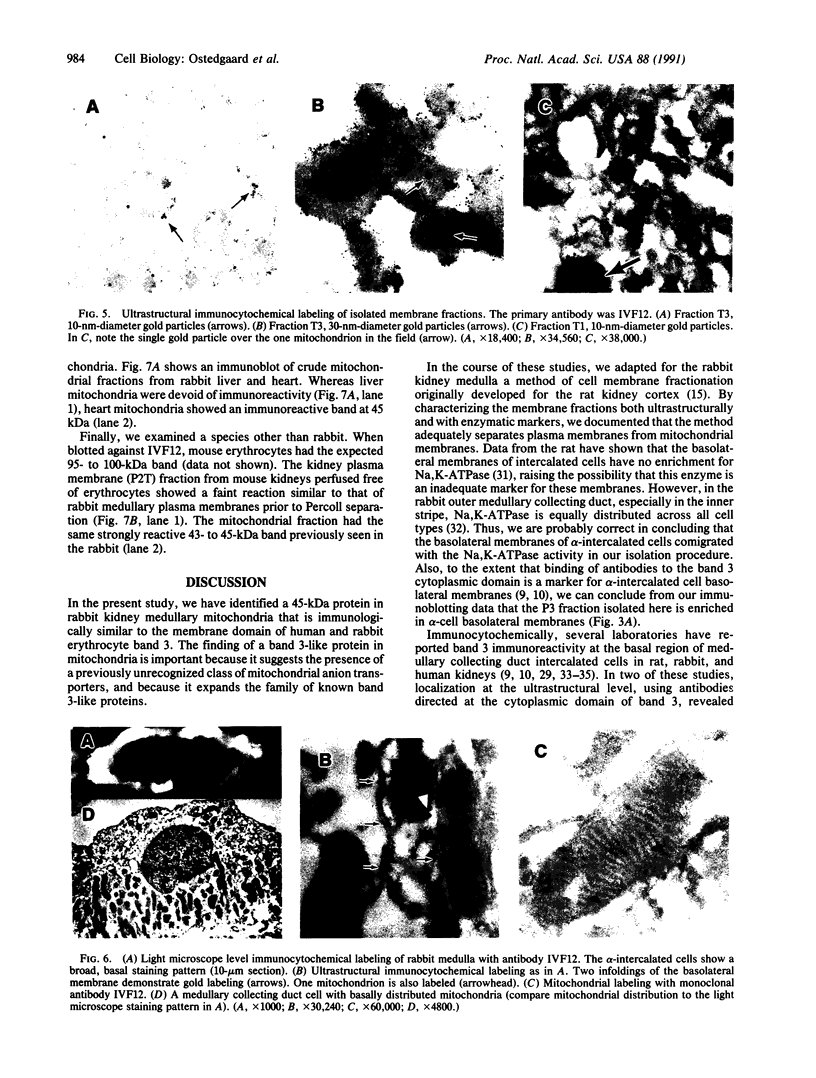
Abstract
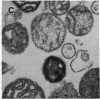
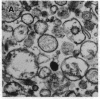
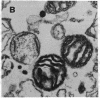
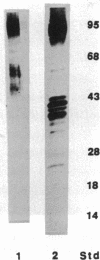
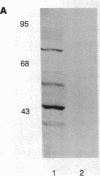
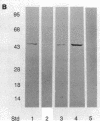
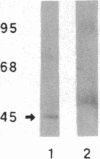
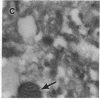
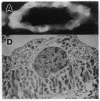
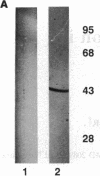
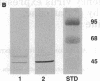
Anion exchange similar to that catalyzed by erythrocyte band 3 occurs across many nonerythroid cell membranes. To identify anion-exchange proteins structurally related to band 3, we immunoblotted rabbit kidney medullary membrane fractions with anti-band 3 antibodies. Immunoblots using antibodies to the cytoplasmic domain of band 3 revealed cross-reactive proteins in the plasma membrane fraction only. In contrast, two monoclonal antibodies against band 3 membrane domain labeled a 45-kDa protein; further immunoblotting and immunogold studies of membrane fractions and kidney sections using one of the anti-membrane domain antibodies showed that labeling was strongest in mitochondria of H(+)-secreting collecting duct cells. Tissue-to-tissue expression of the 45-kDa mitochondrial protein was variable: kidney medulla greater than heart greater than kidney cortex much greater than liver. We conclude that a 45-kDa protein with immunological cross-reactivity to the erythrocyte band 3 membrane domain is expressed in mitochondria in a highly cell-specific fashion and speculate that the protein may play a role in mitochondrial anion transport.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper S. L., Natale J., Gluck S., Lodish H. F., Brown D. Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5429–5433. doi: 10.1073/pnas.86.14.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquila H., Link T. A., Klingenberg M. Solute carriers involved in energy transfer of mitochondria form a homologous protein family. FEBS Lett. 1987 Feb 9;212(1):1–9. doi: 10.1016/0014-5793(87)81546-6. [DOI] [PubMed] [Google Scholar]
- Beavis A. D., Powers M. F. On the regulation of the mitochondrial inner membrane anion channel by magnesium and protons. J Biol Chem. 1989 Oct 15;264(29):17148–17155. [PubMed] [Google Scholar]
- Bergeron J. J., Ehrenreich J. H., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. II. Biochemical characterization. J Cell Biol. 1973 Oct;59(1):73–88. doi: 10.1083/jcb.59.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius F. C., 3rd, Alper S. L., Garcia A. M., Lodish H. F. The major kidney band 3 gene transcript predicts an amino-terminal truncated band 3 polypeptide. J Biol Chem. 1989 May 15;264(14):7784–7787. [PubMed] [Google Scholar]
- Butcher R. G. Studies on succinate oxidation. I. The use of intact tissue sections. Exp Cell Res. 1970 Apr;60(1):54–60. doi: 10.1016/0014-4827(70)90488-x. [DOI] [PubMed] [Google Scholar]
- Cox J. V., Lazarides E. Alternative primary structures in the transmembrane domain of the chicken erythroid anion transporter. Mol Cell Biol. 1988 Mar;8(3):1327–1335. doi: 10.1128/mcb.8.3.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Demuth D. R., Showe L. C., Ballantine M., Palumbo A., Fraser P. J., Cioe L., Rovera G., Curtis P. J. Cloning and structural characterization of a human non-erythroid band 3-like protein. EMBO J. 1986 Jun;5(6):1205–1214. doi: 10.1002/j.1460-2075.1986.tb04348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn D., Merte C. Restriction of the human kidney band 3-like anion exchanger to specialized subdomains of the basolateral plasma membrane of intercalated cells. Eur J Cell Biol. 1987 Dec;45(1):107–115. [PubMed] [Google Scholar]
- Drenckhahn D., Schlüter K., Allen D. P., Bennett V. Colocalization of band 3 with ankyrin and spectrin at the basal membrane of intercalated cells in the rat kidney. Science. 1985 Dec 13;230(4731):1287–1289. doi: 10.1126/science.2933809. [DOI] [PubMed] [Google Scholar]
- ENGSTROM L. Studies on calf-intestinal alkaline phosphatase. I. Chromatographic purification, microheterogeneity and some other properties of the purified enzyme. Biochim Biophys Acta. 1961 Sep 2;52:36–48. doi: 10.1016/0006-3002(61)90901-5. [DOI] [PubMed] [Google Scholar]
- Forbush B., 3rd Assay of Na,K-ATPase in plasma membrane preparations: increasing the permeability of membrane vesicles using sodium dodecyl sulfate buffered with bovine serum albumin. Anal Biochem. 1983 Jan;128(1):159–163. doi: 10.1016/0003-2697(83)90356-1. [DOI] [PubMed] [Google Scholar]
- Jennings M. L., Anderson M. P., Monaghan R. Monoclonal antibodies against human erythrocyte band 3 protein. Localization of proteolytic cleavage sites and stilbenedisulfonate-binding lysine residues. J Biol Chem. 1986 Jul 5;261(19):9002–9010. [PubMed] [Google Scholar]
- Jennings M. L. Structure and function of the red blood cell anion transport protein. Annu Rev Biophys Biophys Chem. 1989;18:397–430. doi: 10.1146/annurev.bb.18.060189.002145. [DOI] [PubMed] [Google Scholar]
- Karniski L. P., Aronson P. S. Anion exchange pathways for Cl- transport in rabbit renal microvillus membranes. Am J Physiol. 1987 Sep;253(3 Pt 2):F513–F521. doi: 10.1152/ajprenal.1987.253.3.F513. [DOI] [PubMed] [Google Scholar]
- Kashgarian M., Biemesderfer D., Caplan M., Forbush B., 3rd Monoclonal antibody to Na,K-ATPase: immunocytochemical localization along nephron segments. Kidney Int. 1985 Dec;28(6):899–913. doi: 10.1038/ki.1985.216. [DOI] [PubMed] [Google Scholar]
- Kellokumpu S., Neff L., Jämsä-Kellokumpu S., Kopito R., Baron R. A 115-kD polypeptide immunologically related to erythrocyte band 3 is present in Golgi membranes. Science. 1988 Dec 2;242(4883):1308–1311. doi: 10.1126/science.2461589. [DOI] [PubMed] [Google Scholar]
- Kim H. R., Yew N. S., Ansorge W., Voss H., Schwager C., Vennström B., Zenke M., Engel J. D. Two different mRNAs are transcribed from a single genomic locus encoding the chicken erythrocyte anion transport proteins (band 3). Mol Cell Biol. 1988 Oct;8(10):4416–4424. doi: 10.1128/mcb.8.10.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopito R. R., Lee B. S., Simmons D. M., Lindsey A. E., Morgans C. W., Schneider K. Regulation of intracellular pH by a neuronal homolog of the erythrocyte anion exchanger. Cell. 1989 Dec 1;59(5):927–937. doi: 10.1016/0092-8674(89)90615-6. [DOI] [PubMed] [Google Scholar]
- Kopito R. R., Lodish H. F. Primary structure and transmembrane orientation of the murine anion exchange protein. Nature. 1985 Jul 18;316(6025):234–238. doi: 10.1038/316234a0. [DOI] [PubMed] [Google Scholar]
- Kudrycki K. E., Shull G. E. Primary structure of the rat kidney band 3 anion exchange protein deduced from a cDNA. J Biol Chem. 1989 May 15;264(14):8185–8192. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Low P. S., Westfall M. A., Allen D. P., Appell K. C. Characterization of the reversible conformational equilibrium of the cytoplasmic domain of erythrocyte membrane band 3. J Biol Chem. 1984 Nov 10;259(21):13070–13076. [PubMed] [Google Scholar]
- Lowe A. G., Lambert A. Chloride-bicarbonate exchange and related transport processes. Biochim Biophys Acta. 1982 Dec;694(4):353–374. doi: 10.1016/0304-4157(82)90002-8. [DOI] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Kopito R. R., Lodish H. F. Cloning and characterization of band 3, the human erythrocyte anion-exchange protein (AE1). Proc Natl Acad Sci U S A. 1989 Dec;86(23):9089–9093. doi: 10.1073/pnas.86.23.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Ridderstrale Y., Kashgarian M., Koeppen B., Giebisch G., Stetson D., Ardito T., Stanton B. Morphological heterogeneity of the rabbit collecting duct. Kidney Int. 1988 Nov;34(5):655–670. doi: 10.1038/ki.1988.230. [DOI] [PubMed] [Google Scholar]
- Scalera V., Huang Y. K., Hildmann B., Murer H. A simple isolation method for basal-lateral plasma membranes from rat kidney cortex. Membr Biochem. 1981;4(1):49–61. doi: 10.3109/09687688109065422. [DOI] [PubMed] [Google Scholar]
- Schuster V. L., Bonsib S. M., Jennings M. L. Two types of collecting duct mitochondria-rich (intercalated) cells: lectin and band 3 cytochemistry. Am J Physiol. 1986 Sep;251(3 Pt 1):C347–C355. doi: 10.1152/ajpcell.1986.251.3.C347. [DOI] [PubMed] [Google Scholar]
- Simpson D. P. Dissociation of acid-base effects on substrate accumulation and on delta pH in dog mitochondria. Am J Physiol. 1988 Jun;254(6 Pt 2):F863–F870. doi: 10.1152/ajprenal.1988.254.6.F863. [DOI] [PubMed] [Google Scholar]
- Störkel S., Pannen B., Thoenes W., Steart P. V., Wagner S., Drenckhahn D. Intercalated cells as a probable source for the development of renal oncocytoma. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;56(3):185–189. doi: 10.1007/BF02890016. [DOI] [PubMed] [Google Scholar]
- Tanner M. J., Martin P. G., High S. The complete amino acid sequence of the human erythrocyte membrane anion-transport protein deduced from the cDNA sequence. Biochem J. 1988 Dec 15;256(3):703–712. doi: 10.1042/bj2560703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H. A., Machen T. E., Smolka A., Baron R., Kopito R. R. Identification of a 185-kDa band 3-related polypeptide in oxyntic cells. Am J Physiol. 1989 Sep;257(3 Pt 1):C537–C544. doi: 10.1152/ajpcell.1989.257.3.C537. [DOI] [PubMed] [Google Scholar]
- Timms B. G. Postembedding immunogold labeling for electron microscopy using "LR White" resin. Am J Anat. 1986 Feb-Mar;175(2-3):267–275. doi: 10.1002/aja.1001750211. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlander J. W., Madsen K. M., Low P. S., Allen D. P., Tisher C. C. Immunocytochemical localization of band 3 protein in the rat collecting duct. Am J Physiol. 1988 Jul;255(1 Pt 2):F115–F125. doi: 10.1152/ajprenal.1988.255.1.F115. [DOI] [PubMed] [Google Scholar]
- Wagner S., Vogel R., Lietzke R., Koob R., Drenckhahn D. Immunochemical characterization of a band 3-like anion exchanger in collecting duct of human kidney. Am J Physiol. 1987 Aug;253(2 Pt 2):F213–F221. doi: 10.1152/ajprenal.1987.253.2.F213. [DOI] [PubMed] [Google Scholar]