Abstract
Sodium hypochlorite is a clear yellowish solution with a characteristic odour of chlorine and is commonly used as a disinfectant and a bleaching agent. It is used in various healthcare settings for its fast-acting and broad-spectrum antimicrobial activity. It is a known irritant and there are some reports that it can also cause allergic contact dermatitis of type IV hypersensitivity. We report a case of work-related type I hypersensitivity to sodium hypochlorite, presenting with recurrent urticarial rash and a positive prick test reaction to this chemical. He was subsequently excused from further exposure with no further recurrences of the urticarial rash. To the best of our knowledge, this is the first such reported case due to work in the healthcare setting.
Case report
The patient is a man aged 22 years, who works as an operating theatre technician. He gave a history of recurrent itchy, non-tender urticarial rash over the face, upper limb and trunk (figure 1). His rashes typically occurred ∼5–10 min after he started cleaning with 0.1% sodium hypochlorite cleansing solution in the operating theatres1 and usually last <24 hours. He did not have any symptoms of angioedema, periorbital oedema or shortness of breath. The rashes subsided within 24 hours of taking antihistamines. He had no rashes when he did cleaning work with other cleansers or outside his working environment. He also did not have a medical history of similar rashes.
Figure 1.
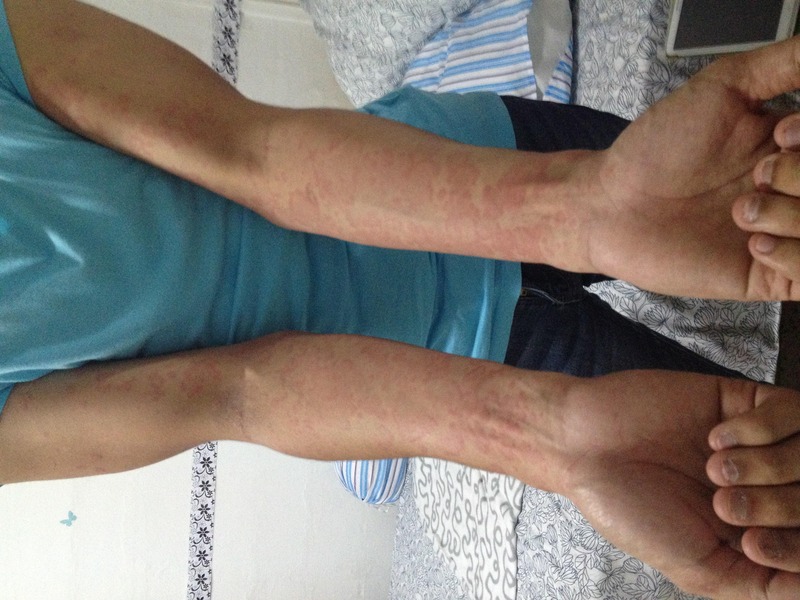
Photo of upper limbs showing urticarial plaques and papules.
He has a history of atopic dermatitis as a child. He also gave a history of lip swelling and itchy rashes after taking shrimps. He did not have a history of asthma or allergic rhinitis. There was no history of recent infection, drug use or physical triggers for his urticaria. He also did not have any associated symptoms of thyroid or autoimmune disorders.
Work exposure
He worked as an operating theatre technician for the last 6 months. His work entailed the cleaning of the operating theatre environment, assisting in positioning the patients postoperatively and preparing the surgical instruments. Sodium hypochlorite (confirmed in the material safety data sheet provided) was used only as a cleaning solution at the end of every infectious disease case listed for operation. The sodium hypochlorite solution was prepared by adding five tablets of 2.5 g of Actichlor tablets into 750 mL of water. Actichlor tablets are a mixture of sodium dichloro-1,3 S-triazinetrione and adipic acid. Sodium dichloro-1,3 S-triazinetrione dissolves in water to form sodium cyanurate and hypochlorous acid. This diluted solution of 0.1% concentration was then transferred into a spray bottle. He then sprayed the sodium hypochlorite solution onto the equipment that needed to be cleaned. Personal protective equipments, such as a yellow gown, latex gloves, surgical cap, face mask, face shield and boots, were worn during such cleaning which usually took ∼1.5 hours to complete.
His other colleagues did not have any rashes.
Investigations
We performed a prick test for our patient using the substances he was exposed to at work. A prick test was performed to evaluate the presence of IgE-mediated type I hypersensitivity response to a suspected allergen.2
A prick test (figure 2) was performed over the left forearm with 0.1% sodium hypochlorite solution (the concentration used at work) with positive and negative controls at our outpatient clinic. Prick tests to his gown, powdered and non-powdered gloves were also performed (figure 3). The test showed a positive urticarial reaction to 0.1% sodium hypochlorite solution, confirming a type I hypersensitivity reaction. There was no reaction to the yellow gown, powdered and non-powdered gloves (figure 2). A positive skin prick test result is defined as weal >3 mm in diameter comparable with the positive control.2
Figure 2.
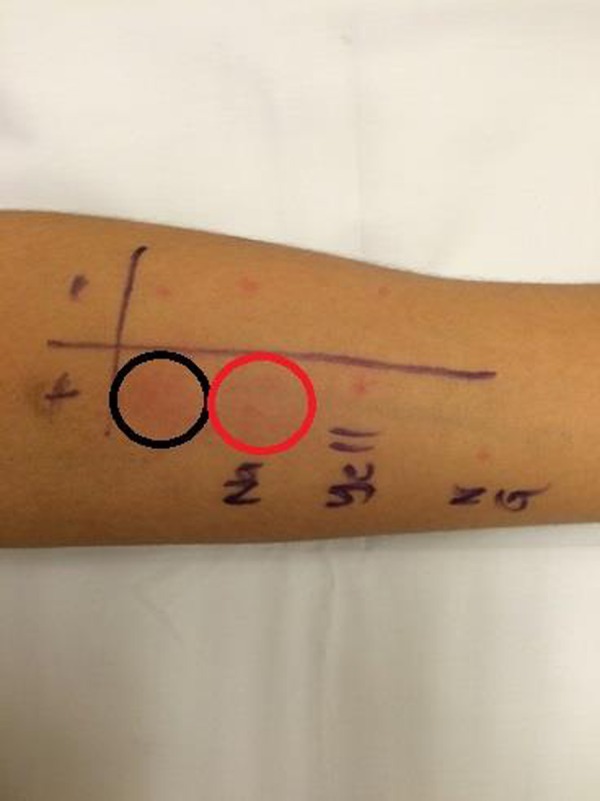
Photo of the prick test performed over the left forearm which showed positive urticarial reaction to sodium hypochlorite solution (marked with red circle) and the positive control (marked with black circle).
Figure 3.
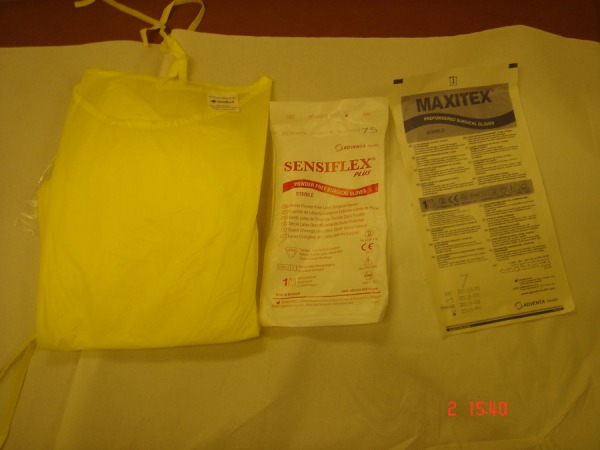
Photo of allergens tested: yellow gown, powdered and non-powdered gloves.
Within 10 min of the prick test, the patient started reporting of itch with an onset of weals starting from the ears, the face, neck and then the trunk (figure 4). He was given fexofenadine 180 mg once daily and the rashes subsequently subsided.
Figure 4.
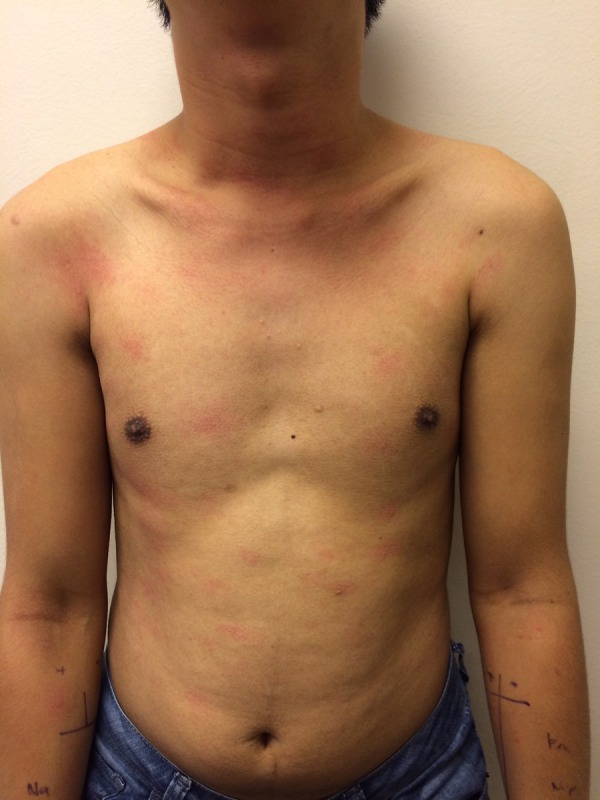
Photo showing urticarial plaques and papules over the upper limbs and trunk.
Dermographism was tested negative, suggesting that the reaction observed during the prick test was not confounded by the presence of concomitant physical urticaria.
Patch testing was not carried out as the patient did not present with the pruritic, eczematous lesions characteristic of allergic contact dermatitis of the type IV hypersensitivity.
Discussion
The inflammatory and irritant effects of sodium hypochlorite on the skin are well documented. Exposure can occur via inhalation, ingestion, dermal or ocular contact, leaving behind inflamed tissues with burning pain and even blisters.3 4 Sodium hypochlorite has also been reported to cause allergic skin reactions.4–6
Our patient presented with urticarial rash, atypical for sodium hypochlorite exposure. His rashes occurred despite him donning full personal protective equipment leaving only a small area of his neck exposed. There was no direct contact of sodium hypochlorite with his skin during the preparation of the diluted solution. We postulate that the hypersensitivity reaction is likely due to exposure to chlorine gas that is produced as a result of dissolving Actichlor tablets in water. The chlorine gas can form hypochlorous acid or hydrochloric acid, which are both irritants, in the atmosphere. In addition, the mist generated from the spray bottle may come into contact with his exposed skin over the neck area, resulting in contact urticaria with secondary generalisation.
He experienced an immediate onset of urticarial rash following the skin prick test with 0.1% sodium hypochlorite. These findings together with the good resolution of his rash following the intake of antihistamine confirmed the type I hypersensitivity reaction. We searched the electronic databases of PubMed and The Cochrane library for relevant systemic reviews, case series and case reports using the medical subject headings (MeSH) of ‘Type I hypersensitivity’, ‘angioedema,’ ‘anaphylaxis’ or ‘urticarial’ together with ‘sodium hypochlorite’ or ‘bleach’. Literature documenting sodium hypochlorite-mediated type I reaction was scarce and was limited to case reports. Caliskan et al7 had reported a case of a female aged 32 years who developed angioedema and respiratory distress after being exposed to sodium hypochlorite irrigation during the endodontic procedure, whereas Neering8 described the occurrence of intermittent contact urticaria after exposure to chlorinated pools and cleansing agents containing sodium hypochlorite. A scratch test performed using chlorinated water was strongly positive for both cases.7 8 Despite the presence of these case reports, the material safety data sheet provided by the manufacturer did not reflect the possibility of type I hypersensitivity reaction as a potential hazard.
The prick and patch tests are commonly used to diagnose type I and type IV hypersensitivity responses, respectively. However, performing these tests to differentiate between the two hypersensitivity reactions in the back drop of a corrosive substance is difficult as results may be confounded by the irritative physical and chemical properties of the substance. Proper preparation and dilution of the irritant is essential for the accuracy of the tests. Literature search using key words like ‘prick test’ and ‘sodium hypochlorite’ returned no study that has evaluated the recommended non-irritant concentration of sodium hypochlorite for use in a diagnostic prick test. However, a study performed in 1990 showed that the recommended non-irritant concentration of sodium hypochlorite for use in a diagnostic patch test was 1%.9 The concentration of sodium hypochlorite used by our patient was 0.1%, which is below the 1% cut-off recommended by the study above, suggesting that the reaction observed in the prick test was unlikely to be confounded by the irritant nature of sodium hypochlorite.
Patients with allergic reaction to sodium hypochlorite solution should be aware that sodium hypochlorite is commonly present in household bleach and is also used in a number of industrial processes such as commercial laundering, manufacture of paper and pulp, industrial chemical synthesis and disinfection of swimming pools.10 As such, patients with allergic reactions should be advised to avoid such exposures.
Conclusion
This is the first such reported case of type I hypersensitivity to sodium hypochlorite due to work in the healthcare setting. A careful clinical history alerted us to sodium hypochlorite as a possible allergen, enabling us to establish the diagnosis and the offending agent in this case. Early recognition and exposure avoidance are necessary in management of type I hypersensitivity. The patient was subsequently excused from exposure to sodium hypochlorite solution permanently with no further recurrences of the urticaria.
Patient's perspective.
I am currently well and have not experienced anymore allergic attacks after being transferred out of major operating theatre. Currently, I do not experience any redness or itchy sensation as I no longer contact sodium hypochlorite solution in my current working environment.
Learning points.
Irritants like sodium hypochlorite solution can trigger a type I hypersensitivity reaction in susceptible patients.
A careful clinical history is key in establishing the diagnosis and offending agent in patients with type I hypersensitivity reactions. The rapid onset of symptoms in an individual with a history of allergy should raise suspicions of this.
Early recognition and avoidance of the culprit agent is important in the management of patients with type I hypersensitivity reaction.
Acknowledgments
The authors thank Dr Lim Mee Ling for referring this patient for investigation at the joint occupational dermatoses clinic and Nurse Clinician Moarie Grace Tan for allowing us access to his workplace, providing information on the material safety data sheet on the cleaning agents used.
Footnotes
Contributors: GCSZ was the primary author of this research article. He was overall responsible for the conception, design and analysis of data in this research article. He was also overall in charge of the initial drafting of this article and revising it critically for important intellectual content. AG assisted in the conception and design of this research article. He also assisted in the drafting and revision of the research article. YTF is an occupational physician by training. She is involved in the conception and design of this research article. She also assisted in the drafting and the final revision of this research article. HYL is a dermatologist by training. He provided assistance in the conception and design of this research article. He also provided critical input on the analysis and interpretation of data in this article as well as aiding in the drafting and revision of the research article. SFH is an occupational physician by training. She provided the overall guidance on the conception, design and analysis of data in this research article. She also provided much assistance and direction on the drafting and revision of the research article. The final version published is approved by all five authors. There is an agreement among all five authors to be accountable for the article and to ensure that all questions regarding the accuracy or integrity of the article are investigated and resolved.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Rutala WA, Weber DJ. Uses of inorganic hypochlorite (bleach) in health-care facilities. Clin Microbiol Rev 1997;10:597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinzerling L, Mari A, Bergmann KC et al. The skin prick test—European standards. Clin Transl Allergy 2013;3:3 10.1186/2045-7022-3-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agency for Toxic Substances and Disease Registry. Medical Management Guidelines for Calcium Hypochlorite. Secondary Medical Management Guidelines for Calcium Hypochlorite 21 October 2014 2002.
- 4.Public Health England. Sodium hypochlorite toxicological overview. UK: Public Health England, 2015. [Google Scholar]
- 5.Habets JM, Geursen-Reitsma AM, Stolz E et al. Sensitization to sodium hypochlorite causing hand dermatitis. Contact Derm 1986;15:140–2. 10.1111/j.1600-0536.1986.tb01314.x [DOI] [PubMed] [Google Scholar]
- 6.Ng SK, Goh CL. Contact allergy to sodium hypochlorite in Eusol. Contact Derm 1989;21:281 10.1111/j.1600-0536.1989.tb03218.x [DOI] [PubMed] [Google Scholar]
- 7.Caliskan MK, Turkun M, Alper S. Allergy to sodium hypochlorite during root canal therapy: a case report. Int Endod J 1994;27:163–7. 10.1111/j.1365-2591.1994.tb00247.x [DOI] [PubMed] [Google Scholar]
- 8.Neering H. Contact urticaria from chlorinated swimming pool water. Contact Derm 1977;3:279 10.1111/j.1600-0536.1977.tb03679.x [DOI] [PubMed] [Google Scholar]
- 9.Hostynek JJ, Wilhelm KP, Cua AB et al. Irritation factors of sodium hypochlorite solutions in human skin. Contact Derm 1990;23:316–24. 10.1111/j.1600-0536.1990.tb05165.x [DOI] [PubMed] [Google Scholar]
- 10.Public Health England. Sodium hypochlorite. General Information. PHE publications gateway number: 2014790. Published: October 2016. [Google Scholar]


