Abstract
Primary adrenal lymphoma is an extremely rare condition. We describe a case of bilateral adrenal lymphoma in a man aged 55 years who was admitted to our hospital. He had a 3-month history of left flank pain, nausea and vomiting with weight loss. A CT scan at a private hospital revealed bilateral large adrenal masses; the patient was referred to our centre based on these findings. He was evaluated for pheochromocytoma by an endocrinology team; however, all findings were negative. In addition, a cosyntropin stimulation test indicated adrenal insufficiency. A Trucut biopsy of the adrenal gland revealed diffuse large B-cell lymphoma of the adrenal glands, and the patient responded extremely well to chemotherapy.
Background
Fewer than 200 cases of primary adrenal lymphoma (PAL) have been reported to date, and PAL accounts for <1% of all cases of non-Hodgkin's lymphoma.1–3 PAL tends to affect elderly men and is bilateral in ∼70% of cases. The two most common PAL subtypes according to 2008 WHO definitions are diffuse large B-cell lymphoma (DLBCL) (78%) and peripheral T-cell lymphoma (7%).4 Adrenal insufficiency is frequently observed in patients with PAL.5
Case presentation
Our patient was a man aged 55 years with an 18-year history of diabetes mellitus with diabetic complications, such as retinopathy and neuropathy. He was diagnosed with hypertension 5 years before presenting with the symptoms described in this report; his hypertension was controlled with amlodipine. The patient was evaluated at a private hospital for a 3-month history of persistent left flank pain, nausea and vomiting. He had lost 10 kg during this 3-month period but experienced no fevers or night sweats. A non-contrast CT scan of the abdomen and pelvis revealed large bilateral adrenal masses. The patient was referred to our centre for further evaluation.
Investigations
A physical examination indicated that the patient was a pale man with a blood pressure of 110/80 mm Hg and a pulse rate of 80 bpm with significant postural hypotension, as indicated by a blood pressure of 85/60 mm Hg and a pulse rate of 95 bpm in a standing position. No evidence of lymphadenopathy was detected. An abdominal examination revealed palpable bilateral abdominal masses in the lumbar areas with left flank tenderness.
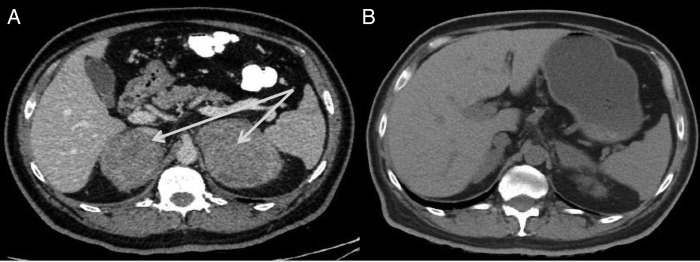
The patient was diagnosed with primary adrenal insufficiency, and steroid replacement was initiated. A CT scan of the abdomen with contrast (figure 1A) revealed large, bilateral, heterogeneously enhanced suprarenal masses. The right and left masses measured 6×7×6 and 10.6×6.3×8.7 cm3, respectively, with no observed calcification. An MRI (figure 2) of the abdomen revealed large, bilateral, well-defined, heterogeneous mass lesions with rather lobulated outlines at both adrenal glands. The right and left lesions measured ∼7.5×5.5 and 8.5×7 cm2, respectively. These lesions exhibited intermediate to low signal intensity on T1WI and high signal intensity on T2WI, with fluid restriction on diffusion-weighted images. After pheochromocytoma was excluded by biochemical tests, ultrasound-guided fine needle aspiration of the left adrenal gland was performed. In addition, a cosyntropin stimulation test indicated adrenal insufficiency (table 1).
Figure 1.
CT scans of the abdomen. (A) Large, bilateral, heterogeneously enhanced suprarenal masses. The right and left masses measured 6×7×6 and 10.6×6.3×8.7 cm3, respectively, with no observed calcification. (B) A postchemotherapy CT scan revealed significant interval regression in the sizes of the previously observed bilateral adrenal masses, with restoration of their configurations.
Figure 2.
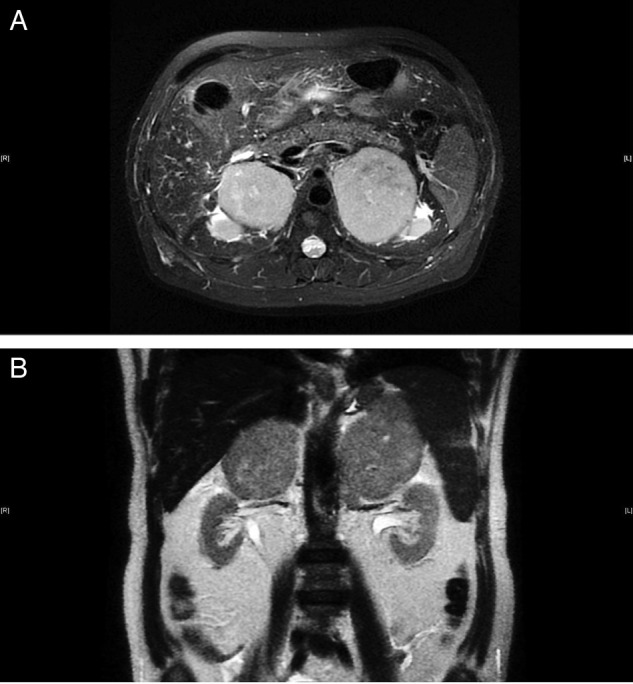
(A and B) MRI scan: bilateral large well-defined heterogeneous mass lesion seen at the region of both adrenal glands (red arrows) with rather lobulated outline, measuring about 7.5×5.5 cm2, in the right side and about 8.5×7 cm2, in the left side. The lesion displayed intermediate to low signal intensity on T1WI images and high signal on T2WI images with fluid restriction on diffusion-weighted images (no intravenous contrast was given).
Table 1.
Biochemical investigations
| Analyte | Result | Normal range |
|---|---|---|
| Haemoglobin (g/L) | 89 | 130–180 |
| MCV (fL) | 54 | 80–94 |
| White cell counts (cell/L) | 7.7×109 | 4–11×109 |
| ESR (mm/hour) | 70 | 0–17 |
| LDH (U/L) | 1324 | 85–227 |
| Serum Na+ (mmol/L) | 139 | 135–145 |
| Serum K+ (mmol/L) | 4.1 | 3.5–4.1 |
| 24-hour urine metanephrine (µmol/day) | 0.27 | 0–1.9 |
| 24-hour urine normetanephrine (µmol/day) | 3.3 | 0–4.5 |
| 24-hour urine free cortisol (nmol/day) | 15 | 160–365 |
| Aldosterone/renin ratio | Normal | |
| Basal ACTH (pmol/L) | 22 | 1.6–13.9 |
| Cosyntropin stimulation test | ||
| Basal cortisol (µg/dL) | 10.33 | 6.162–19.2 |
| 30 min cortisol (µg/dL) | 11.23 | |
| 60 min cortisol (µg/dL) | 11.598 | |
A Trucut biopsy of the left adrenal mass revealed DLBCL originating from non-germinal centre (activated) B cells. Immunohistochemical stains indicated that neoplastic cells were positive for CD20, Bcl2, Bcl6 and MUM-1 but negative for inhibin, synaptophysin, chromogranin, CK7, CK20, PanCK, cyclin D1, CD10, CD5 and CD23. Reactive T-lymphocytes were positive for CD3. The Ki67 proliferative index was ∼90%.
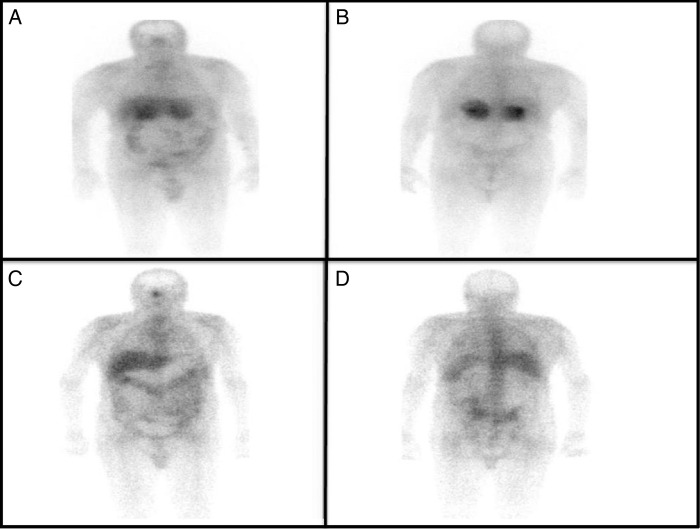
A bone marrow biopsy was negative for lymphoma. A gallium-67 (figure 3A, B) whole-body scan revealed positive uptake in both adrenal glands. A whole-body CT scan was negative for any other lymphadenopathy.
Figure 3.
Gallium-67 whole-body scans. (A and B) Positive uptake in both adrenal glands. (C and D) A postchemotherapy gallium-67 whole-body scan was negative.
Differential diagnosis
The initial impression for bilateral adrenal masses was leading to the most likely causes: pheochromocytoma, metastatic malignancy and tuberculosis. In addition, other less likely causes of adrenal mass are given in table 2.6 Final diagnosis of PAL was confirmed by Trucut biopsy of adrenal tissues.
Table 2.
Differential diagnosis of adrenal mass6
| Unilateral adrenal mass | Bilateral adrenal mass |
|---|---|
| Non-functional adrenal adenoma | Metastatic diseases: lung, Renal and Melanoma |
| Functional adenoma: cortisol or aldosterone secretion | Lymphoma |
| Pheochromocytoma | Infection: tuberculosis, fungal |
| Metastasis | Pheochromocytoma |
| Adrenocortical carcinoma | ACTH-dependent Cushing's |
| Lymphoma | Congenital adrenal hyperplasia |
| Ganglioneuroma | Primary aldosteronism |
| Adrenal cyst | Bilateral macronodular adrenal hyperplasia |
| Haemorrhage | |
| Infiltrative disease: amyloidosis |
Treatment
The patient was administered R-CHOP (rituximab–cyclophosphamide, doxorubicin, vincristine and prednisone) chemotherapy. He was re-evaluated after three chemotherapy cycles, after which a non-contrast CT scan revealed significant interval regression in the sizes of the previously detected bilateral adrenal masses, with restoration of their configurations. The regions of maximum thickening on the left and right sides measured 7×3 and 5×2 cm2, respectively (figure 1B). A gallium-67 whole-body scan was negative (figure 3C, D).
Outcome and follow-up
The patient received three cycles of chemotherapy with a promising partial response to the therapy (reduction in the size of both adrenal masses) and negative gallium scan.
Discussion
Adrenal lesions are commonly detected during cross-sectional imaging examinations, and the majority of these lesions are incidental, benign adrenal adenomas. Diagnoses of PAL are relatively rare. Certain conditions associated with bilateral adrenal lesions with adrenal insufficiency are metastatic lung carcinoma, pheochromocytoma, tuberculosis and amyloidosis.6 PAL often presents with bilateral tumour masses (in 70% of cases).7 8 Elderly men are most commonly affected by PAL; in particular, a prior study observed that PAL patients ranged between 39 and 89 years of age with a mean age of 68 years.8 Primary adrenal non-Hodgkin's lymphoma is a rare extranodal involvement that accounts for <3% of PAL cases.9
The clinical presentation of patients with adrenal lymphoma is typically non-specific and can include abdominal pain, weight loss, fever, anaemia, nausea and vomiting.6 All of these symptoms can mask the adrenal insufficiency diagnosis and the patient could have presented with adrenal crisis if unrecognised early. In fact, 60–70% of adrenal lymphoma patients present with biochemical or clinical adrenal insufficiency9 irrespective of tumour size.
Adrenal gland involvement in malignancies is typically metastatic, induced by haematological spread from other organs such as the skin, lungs, breasts and colon. Such involvement is the most common type of adrenal malignancy. Lymphatic spread to the adrenal glands, generally from the retroperitoneal lymph nodes, is less common.10
The pathogenesis of primary bilateral adrenal gland non-Hodgkin's lymphoma is multifactorial and has not been fully elucidated. The proposed underlying pathogenic mechanisms include immune system dysfunction, the proliferation of haematopoietic tissue in both adrenal glands after resting owing to the effects of the Epstein-Barr and JC polyoma viruses, and mutations in the p53 and c-KIT genes.11 PAL most commonly involves DLBCL (85% of cases), followed by mixed large-cell and small-cell lymphoma, T-cell lymphoma and undifferentiated lymphoma.2 12
The clinical features of adrenal insufficiency manifest clinically (in the form of nausea, vomiting, postural hypotension and/or skin hyperpigmentation) after both adrenal gland tissues have been more than 90% destroyed due to the infiltration of neoplastic lymphoma cells and autoimmune adrenalitis.13 Patients may present with asymptomatic adrenal insufficiency like our patient revealed by an inadequate response to a cosyntropin stimulation test. Patients with PAL may present with various clinical manifestations other than the signs and symptoms of adrenal insufficiency, such as tumour-related symptoms (fevers, night sweats and weight loss) and abdominal pain related to mechanical pressure caused by a large tumour (which occurs in 67% of patients).1
PAL is sometimes diagnosed radiologically as an ‘incidentaloma’ during an examination for abdominal pain.14 The radiological features of PAL on CT scans are variable, and this disease should be differentiated from tuberculosis, non-functional adrenal adenoma, adrenal carcinoma, pheochromocytoma and secondary metastasis. Ninety per cent of adrenal incidentalomas >4 cm in size are malignant. In addition, other radiological features are predictors of malignancy including irregular borders, calcifications, a heterogeneous mass with cystic degeneration and an attenuation of >20 HU on non-enhanced CT. Conversely, an absolute contrast medium washout of >50% after 10 min is 100% sensitive and specific for a benign adrenal lesion.9 MRI findings for adrenal lymphoma are characterised by low signal intensity on T1 and high signal intensity on T2.9
PAL is difficult to diagnose from radiological images; therefore, such diagnoses should be histologically confirmed by image-guided biopsies after excluding adrenal insufficiency and disorders affecting the functional status of the adrenal mass, such as pheochromocytoma, Cushing's syndrome and hyperaldosteronism.15 16 An elevated lactate dehydrogenase (LDH) level is characteristic of lymphoma.
Management options for PAL include surgery, radiation and chemotherapy. Optimal management of PAL remains debatable because PAL is a rare disorder for which management decisions are supported by the few case series reported in the literature. Adrenalectomy is not an effective option. To date, the best treatment option with respect to patient outcomes is R-CHOP chemotherapy.10
The prognosis for PAL is generally poor. Overall 2-year survival is estimated to be 68.3% and progression-free survival is 51.1%. Similarly, overall survival rates have been observed for cases involving a single adrenal gland and cases involving both adrenal glands.16
Predictors of poor prognosis in PAL cases are older age, bilateral adrenal involvement, adrenal insufficiency on presentation, large tumour size, an elevated LDH level and non-germinal B-cell lymphoma.1 4
The patient described in this report exhibited an excellent response to the initial three cycles of chemotherapy, as shown by regression in the sizes of both adrenal glands. We believe that this case will complement the few other reported cases in the literature regarding PAL with adrenal insufficiency that showed a reasonable response. Although our patient had most of the predictors of poor prognosis and had a great response to therapy, we did not find any predictors of good response in his case. More cases of PAL with adrenal insufficiency that showed a good response to chemotherapy are needed to be studied to identify the predictors of good clinical and radiological response to therapy.
Learning points.
Primary adrenal lymphoma (PAL) is generally rare. More than 50% of the cases present with adrenal insufficiency if both adrenals are involved.
The clinical presentation is non-specific (adrenal insufficiency and lymphoma both have similar clinical presentation).
The patient with adrenal insufficiency in PAL may present without any symptoms and patient might develop adrenal crisis if it is missed. All patients with infiltrative pathology involving both adrenals should be evaluated for adrenal insufficiency.17
There are no clear guidelines on managing PAL and current available options are based on the outcomes of reported cases in the literature.
The prognosis of PAL is generally poor. Predictors of poor prognosis are older age, large tumour size, bilateral adrenal involvement, adrenal insufficiency on presentation, elevated LDL level and non-germinal B-cell lymphoma.
More cases and studies are needed in the future to identify the predictors of good clinical and radiological response to therapy.
Footnotes
Contributors: AE recruited the patient, did the investigations and collected the data. She prepared the final manuscript. AM performed the literature review.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Rashidi A, Fisher SI. Primary adrenal lymphoma: a systematic review. Ann Hematol 2013;92:1583–93. 10.1007/s00277-013-1812-3 [DOI] [PubMed] [Google Scholar]
- 2.Ichikawa S, Fukuhara N, Inoue A et al. Clinicopathological analysis of primary adrenal diffuse large B-cell lymphoma: effectiveness of rituximab-containing chemotherapy including central nervous system prophylaxis. Exp Hematol Oncol 2013;2:19 10.1186/2162-3619-2-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erçolak V, Kara O, Günaldı M et al. Bilateral primary adrenal non-Hodgkin lymphoma. Turk J Haematol 2014;31:205–6. 10.4274/tjh.2013.0195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khurana A, Kaur P, Chauhan AK. Primary non Hodgkin's lymphoma of left adrenal gland—a rare presentation. J Clin Diagn Res 2015;9:XD01–3. 10.7860/JCDR/2015/8079.5745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanpitukpongse TP, Kamalian S, Punsoni M et al. Radiology–pathology conference: primary adrenal lymphoma. Clin Imaging 2012;36:156–9. 10.1016/j.clinimag.2011.08.022 [DOI] [PubMed] [Google Scholar]
- 6.Diamanti-Kandarakis E, Chatzismalis P, Economou F et al. Primary adrenal lymphoma presented with adrenal insufficiency. Hormones (Athens) 2004;3:68–73. 10.14310/horm.2002.11115 [DOI] [PubMed] [Google Scholar]
- 7.Holm J, Breum L, Stenfeldt K et al. Bilateral primary adrenal lymphoma presenting with adrenal insufficiency. Case Rep Endocrinol 2012;2012:1–3. 10.1155/2012/638298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouchikhi A, Tazi MF, Amiroune D et al. Primary bilateral non-Hodgkin's lymphoma of the adrenal gland: a case report. Case Rep Urol 2012;2012:1–3. 10.1155/2012/325675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson WG, Babbar P, Payne LF. Bilateral primary adrenal non-Hodgkin's lymphoma without adrenal insufficiency. Urol Ann 2015;7:259 10.4103/0974-7796.152942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aziz SA, Laway BA, Rangreze I et al. Primary adrenal lymphoma: differential involvement with varying adrenal function. Indian J Endocrinol Metab 2011;15:220 10.4103/2230-8210.83414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padhi S, Sahoo J. Primary adrenal non Hodgkin lymphoma: changing trends. Turk J Gastroenterol 2015;26:85–6. 10.5152/tjg.2015.4882 [DOI] [PubMed] [Google Scholar]
- 12.Kacem K, Zriba S, Lakhal RB et al. Primary adrenal lymphoma. Turk J Haematol 2014;31:188 10.4274/tjh.2012.0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spyroglou A, Schneider HJ, Mussack T et al. Primary adrenal lymphoma: 3 case reports with different outcomes. Exp Clin Endocrinol Diabetes 2011;119:208–13. 10.1055/s-0031-1271629 [DOI] [PubMed] [Google Scholar]
- 14.Schreiber CS, Sakon JR, Simião FP et al. Primary adrenal lymphoma: a case series study. Ann Hematol 2008;87:859–61. 10.1007/s00277-008-0492-x [DOI] [PubMed] [Google Scholar]
- 15.Malik S, Chapman C, Drew O. A case of primary adrenal diffuse large B-cell lymphoma in HIV. Int J STD AIDS 2016;27:687–9. 10.1177/0956462415593000 [DOI] [PubMed] [Google Scholar]
- 16.Kim Y, Kim J, Min Y et al. Prognostic factors in primary diffuse large B-cell lymphoma of adrenal gland treated with rituximab-CHOP chemotherapy from the Consortium for Improving Survival of Lymphoma (CISL). J Hematol Oncol 2012;5:49 10.1186/1756-8722-5-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma V, Sharma S, Aggarwal A et al. Adrenal insufficiency in primary adrenal lymphoma: innocuous presentation of a rare sinister illness. Niger J Clin Pract 2011;14:115 10.4103/1119-3077.79251 [DOI] [PubMed] [Google Scholar]