Abstract
Metastatic tumours of the duodenum are relatively rare. Here we present a case of a 64-year-old Caucasian male who presented with a 3-week history of postprandial vomiting, weight-loss and epigastric discomfort. Imaging and biopsy revealed that the patient had a primary lung tumour in his right upper lung lobe as well as a duodenal metastasis leading to gastric outlet obstruction (GOO). The patient was stabilised and subsequently underwent a laparoscopic gastric bypass to palliate the gastric outlet obstruction. Appropriate management of metastatic GOO involves accurate diagnosis and treatment with either enteral stenting or laparoscopic gastric bypass. It is suggested that the decision whether to stent or surgically bypass the obstruction can be based on the patient's life expectancy and performance status. Regardless of the approach, palliating metastatic GOO can improve the quality of life of carefully chosen symptomatic patients. We describe a technique of laparoscopic palliative gastric bypass which has not been reported previously in the literature.
Background
Gastric outlet obstruction (GOO) is a clinical syndrome characterised by abdominal pain, early satiety and postprandial vomiting due to mechanical obstruction of the distal stomach or duodenum.1 This mechanical obstruction can be caused by benign or malignant processes that occurs intraluminally or extraluminally.1 Historically, it is most commonly attributable to benign intraluminal processes such as peptic ulcer disease; the advent of proton pump inhibitors makes malignancy the most common current cause of GOO accounting for 50–80% of all cases.2 In Western Europe and North America malignant GOO occurs most commonly as a result of extraluminal obstruction of the duodenum by periampullary cancer, particularly in the head of the pancreas, whereas in Asian countries the cause of malignant GOO is most commonly primary gastric cancer.3 Other less common causes of malignant GOO include lymphomas, as well as metastatic disease to the stomach or duodenum.3 Data regarding the prevalence of metastatic GOO are relatively limited.4 Most patients with malignant GOO are heavily pretreated and are best managed by palliative care teams using wide bore NG drainage and venting gastrostomy. Current management for fit, newly diagnosed patients with malignant GOO includes palliative stenting or laparoscopic gastric bypass. Here we present a case of GOO caused by metastasis from primary lung carcinoma, and the subsequent management of this patient with a palliative laparoscopic gastric bypass.
Case presentation
A 64-year-old Caucasian male smoker presented to a community hospital with a 3-week history of postprandial vomiting, weight-loss and epigastric discomfort. The patient was subsequently admitted and resuscitated with intravenous fluids, antiemetics, intravenous proton pump inhibitor and a nasogastric tube was placed.
Investigations
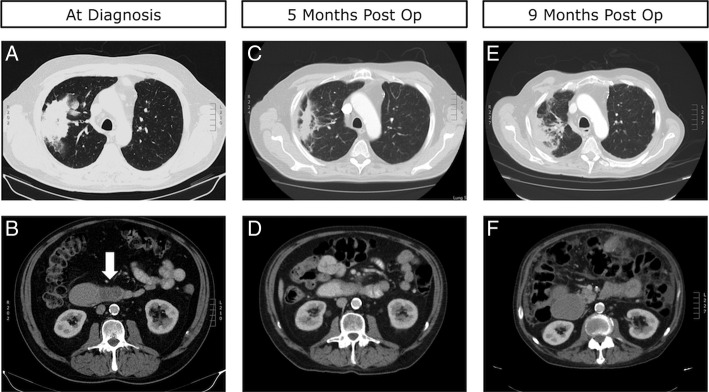
Further workup including CT scan revealed a large mass in the upper lobe of the right lung as well as an obstructing mass in the third part of the duodenum (figure 1A, B). Subsequent transbronchial biopsy of the lung mass revealed moderately differentiated adenocarcinoma, consistent with non-small cell lung adenocarcinoma. A duodenal biopsy revealed identical histological features including strong thyroid transcription factor-1 staining confirming the obstructing duodenal mass to be a metastasis from the primary lung cancer.
Figure 1.
(A) Preoperative CT scan of the chest demonstrating a large primary lesion in the patient's right upper lobe. (B) Preoperative CT scan of the abdomen demonstrating a hypodense, fully obstructive metastatic lesion in D3, identified by the white arrow. (C) Five-month postoperative CT scan of the chest demonstrating reduction in the size of the primary lung tumour. (D) Five-month postoperative CT scan of the abdomen demonstrating limited interval change in the metastatic lesion in D3. (E) Nine-month postoperative CT scan of the chest demonstrating moderate progression of the primary lung lesion. (F) Nine-month postoperative CT scan of the abdomen demonstrating distension of D2.
Differential diagnosis
The differential diagnosis at presentation post-CT scan and prebiopsy included a primary gastrointestinal (GI) malignancy with lung metastasis, two synchronous cancers; lung and GI and disseminated tuberculosis.
Treatment
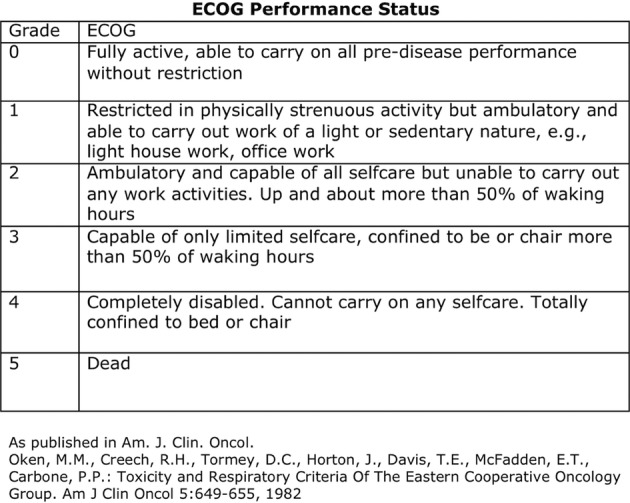
Three weeks prior to presentation the patient’s Eastern Cooperative Oncology Group (ECOG) status was 1, while on presentation his ECOG status was 3 (figure 2). Based on his rapid and potentially reversible decline and his chemo-naive cancer it was determined by the multidisciplinary team that the most appropriate management would be a laparoscopic gastric bypass to palliate this metastatic GOO.
Figure 2.
ECOG Chart. ECOG, Eastern Cooperative Oncology Group.
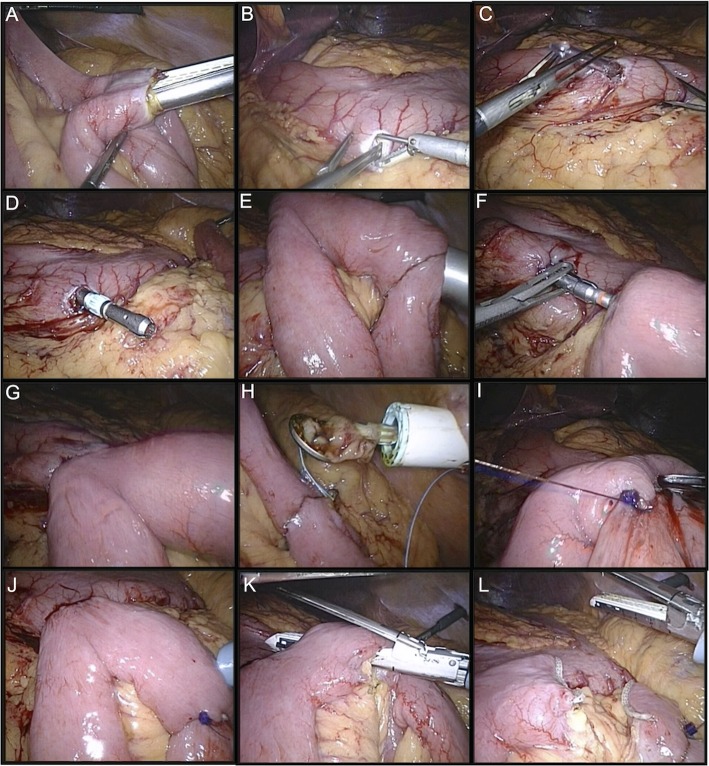
On establishing pneuomperitoneum a formal laparoscopy was performed to confirm the suspected diagnosis and the location of the distal obstruction was identified. The duodenaljejunal flexure was then identified and a 50 cm loop of jejunum was brought cephalad anterior to the transverse colon and anastomosed in a side-to-side fashion using a linear stapler, thus creating a closed loop jejunojejunal anastamosis with an open enterotomy (figure 3A).
Figure 3.
(A) Fashioning of Jejunojejunal anastomosis. (B–D) Gastric enterotomy and passing the 22 mm anvil through this enterotomy. (E–H) Formation of circular stapled anastomosis. (I–J) Sewing and of jejunojejunal and testing for leakage. (K–L) Creation of Roux and afferent limbs.
Next, an enterotomy was made in the gastric antrum and a silastic tube with a 22 mm anvil attached is passed per orum. The anvil was then retracted through this enterotomy in the gastric antrum and the silastic tubing was then detached leaving the anvil free. A purse-string suture is then placed around the anvil to ensue close apposition of the stomach wall enterotomy (figure 3B–D).
A 22 mm circular stapler was passed through the anterior abdominal wall, through the jejunojejunal enterotomy and up through the proximal jejeunal limb for ∼7 cm.
The spike of the gun was then deployed through the jejeunal wall and connected to the anvil. A circular stapled anastomosis was then fashioned and the circular stapler withdrawn through the abdominal wall. The jejunojejunal enterotomy was then sutured using a single layer of 2–0 vicryl continuous suture (figure 3E–I).
Both anastomoses were checked for leakage and patency using methylene blue under pressure. The shorter limb of jejunum between the two anastomsoses was then divided using a linear stapler, leaving an afferent limb roughly 30 cm in length and a roux limb roughly 50 cm in length. All port sites were closed under direct vision (figure 3J–L). A video highlighting the key procedural steps is available online (video 1).
Outcome and follow-up
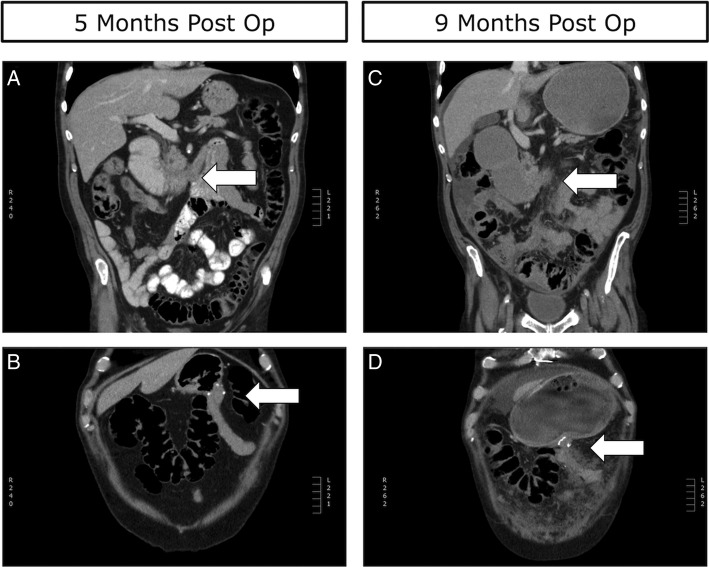
The patient's postoperative course was uncomplicated and he was reintroduced to oral intake on day two postoperatively and subsequently discharged 8 days after undergoing the procedure. Three weeks after discharge he presented again to our service with symptoms of nausea, vomiting and possible obstruction, but after conservative management was successfully discharged. The patient received palliative radiotherapy to his lung lesion while he recovered from his gastric bypass and about 2 months postgastric bypass he began treatment with carboplatin and pemetrexed chemotherapy. The patient tolerated this therapy well and CT scans at 5 months postgastric bypass revealed a patent Roux-En-Y limb with a decompressed stomach (figure 4A, B). Additionally, a significant decrease in the size the primary lung tumour and limited progression of his abdominal disease was also noted (figure 1C, D). He was switched to maintenance pemetrexed at this time, and his disease remained stable until 9 months postsurgery when he presented again to our service with signs and symptoms consistent with GOO of his Roux-En-Y limb. This was confirmed by CT scans (figure 1E, F) and (figure 4C, D). After stabilisation and discussion with the patient and his family, it was decided that he would be discharged home under the supervision with palliative care team support. The patient died peacefully at his home 11 months postgastric bypass.
Figure 4.
(A) Five-month postoperative coronal CT scan demonstrating the obstructive D3 lesion identified by the white arrow. (B) Five-month postoperative coronal CT scan demonstrating the patent Roux-En-Y bypass identified by the white arrow and a decompressed stomach. (C) Nine-month postoperative coronal CT scan demonstrating the obstructive D3 lesion, identified by white arrow, as well as extensive peritoneal disease. (D) Nine-month postoperative coronal CT scan demonstrating an obstructed Roux-En-Y limb as well as a grossly distended stomach.
Video 1.
Video of the surgical procedure.
Discussion
Malignant GOO caused by metastatic disease to the stomach or duodenum is a relatively rare presentation.4 Breast cancer, lung cancer, oesophageal cancer and melanoma are believed to be the most common primary sites leading to gastric and duodenal metastasis.4 Postmortem examinations have demonstrated that GI metastasis are not uncommon in lung cancer, however, these metastatic deposits are rarely discovered as they are most often asymptomatic.5 6 When they do occur GI metastasis can lead to abdominal pain, GI bleeding, obstruction and perforation.6 7 A large single-centre retrospective study of 4114 patients with lung cancer revealed seven cases (0.17%) of symptomatic small bowel metastasis.8 Of these seven cases, five patients presented with perforation and two presented with obstruction. Only one of the seven patients had a duodenal metastasis and presented with obstruction.8
Presently, the median survival and 5-year survival for stage IV non-small cell lung cancer treated with chemotherapy is 18 months and 2%, respectively.9 However, as the efficacy of palliative chemotherapy and immunotherapy improves it is probable that more cases of gastric and small bowel metastases will be encountered.8 Furthermore, with the advent and use of improved diagnostic methods such as positron emission tomography CT it is also likely that more cases of occult metastasis will be discovered.10 This changing framework makes accurate diagnosis and evidence-based treatment of gastric or small bowel metastasis and subsequent malignant GOO clinically relevant.
The first step in managing malignant GOO is a correct diagnosis. History and physical examination are key to proper diagnosis and postprandial nausea and vomiting should raise the suspicion of GOO.11 Examination may reveal a succussion splash, although the sensitivity of this test has been reported to be only 48%.12 Medical imaging is the main tool for diagnosis of GOO and plain films, contrast studies and CT-Scans, can all reveal hallmark signs of GOO including gastric distention and/or distal obstruction. Upper GI endoscopy is often needed to establish a diagnosis, particularly in the context of malignant GOO where biopsy of the obstructing segment can reveal the tissue of origin. It should be noted that determining a specific tissue diagnosis of metastatic GI cancer can be difficult in some situations, thus clinical features combined with specific immunohistological stains and CT imaging can all contribute to a diagnosis of metastatic GI cancer.
The management of GOO regardless of aetiology should initially be: adequate fluid and electrolyte replacement, and gastric decompression. Parenteral proton pump inhibitors as well as antiemetics should also be started regardless of aetiology and parenteral nutrition should be considered if definitive therapy will be delayed.1 3 Once the diagnosis of malignant GOO has been confirmed, patients should be considered for either enteral stenting or surgical bypass through a gastrojejunostomy. The published data on enteral stenting and surgical bypass for malignant GOO are mainly based on observational studies and small randomised trials. A systematic review published by (Jeurnink et al, 2007) on enteral stenting (1046 patients) versus surgical gastrojejunostomy (297 patients) revealed that enteral stenting and surgical bypass have similar technical success rates (96 vs 100%), early complications (7 vs 6%), late major complications (18 vs 17%) and persisting symptoms (8 vs 9%).13 Furthermore, initial clinical success was higher with stent placement (89 vs 72%); however, recurrent obstructive symptoms were more common after stenting (18 vs 1%).13 Thus, it has been suggested that patients considered for stenting would have a short life expectancy (<2–6 months), whereas surgical gastrojejunostomy should be considered for patients with longer life expectancies.13 The ECOG performance status has been used to examine the estimation of survival length in malignant GOO (figure 1). It has been suggested that patients with an ECOG performance status of 0–2 should be considered for surgical bypass or stenting, patients with a performance status of 3–4 should be considered for stenting or a less invasive forms of palliation such as venting gastrostomy.14 Despite these guidelines individual decisions are more complex and need to include the patient and disease specific factors. In the case of our patient, the decision for choosing a laparoscopic surgical bypass despite an ECOG status of three was based on the patient's excellent recent premorbid condition (ECOG status 1), low tumour burden (ie, no liver or brain metastasis) and the chemo-naïve disease. The success of open versus laparoscopic surgical gastrojejunostomy has not been compared directly in the literature; however it has been suggested that laparoscopic gastrojejunostomy is a superior operative approach in the setting of malignant GOO.13 Our technique, which has not been described previously in the literature, involves a single sutured enterotomy. In a situation where there is partial obstruction of the stomach and an increased likelihood of peritoneal contamination we feel that the use of a circular stapler/Orvil technique reduces the likelihood of causing contamination when compared to the linear stapler approach which requires a larger gastric enterotomy. Furthermore, we have found the circular stapler to be more technically reliable in an oedematous stomach and reduces the amount of potential operator error. We believe this is the least risky surgical approach for this high-risk condition. We also believe that the use of a Roux-En-Y gastric bypass, as we described here, is preferred to a gastrojejunostomy (Billroth-II) in patients with excellent premorbid performance status as it reduces bile reflux and in experienced hands is a very safe procedure.
Learning points.
Gastric outlet obstruction (GOO) presenting in an adult should raise the suspicion of malignant gastric outlet obstruction.
Malignant GOO can be caused by primary tumours of small bowel, stomach or pancreas as well as metastatic tumours to the small bowel.
Initial management of GOO involves intravenous fluids, nasogastric tube decompression as well as appropriate diagnostic workup.
The management of malignant GOO should be guided by the patient's performance status (Eastern Cooperative Oncology Group (ECOG)) and well as patient and disease specific factors. Patients with ECOG scores 0–2 should be considered for stenting or surgical bypass and those with ECOG score 3 or higher should be treated with an enteral stent or other less invasive forms of palliation.
Laparoscopic gastric bypass is a well-tolerated surgical option for the treatment of malignant GOO and can contribute to an increased quality of life.
The use of a circular stapler—single sutured enterotomy—Roux-En-Y bypass as we describe may be more appropriate in patients with GOO where the risk of peritoneal contamination in high.
Acknowledgments
The authors wish to thank the patient and his family for allowing the publication of this case report.
Footnotes
Contributors: CJO'B performed the surgery. BRHB was the oncological lead. All authors contributed equally to writing the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Johnson CD. Gastric outlet obstruction malignant until proved otherwise. Am J Gastroenterol 1995;90:1740. [PubMed] [Google Scholar]
- 2.Shone DN, Nikoomanesh P, Smith-Meek MM et al. Malignancy is the most common cause of gastric outlet obstruction in the era of H2 blockers. Am J Gastroenterol 1995;90:1769–70. [PubMed] [Google Scholar]
- 3.Frederickson D, Gan SI. Gastric outlet obstruction in adults. In: UpToDate, Post TW. ed. UpToDate Waltham, MA: (accessed 14 Jun 2015). [Google Scholar]
- 4.Oda, Kondo H, Yamao T et al. Metastatic tumors to the stomach: analysis of 54 patients diagnosed at endoscopy and 347 autopsy cases. Endoscopy 2001;33:507–10. http://dx.doi.org/10.1055/s-2001-14960 [DOI] [PubMed] [Google Scholar]
- 5.McNeill PM, Wagman LD, Neifeld JP. Small bowel metastases from primary carcinoma of the lung. Cancer 1987;59:1486–9. [DOI] [PubMed] [Google Scholar]
- 6.Berger A, Cellier C, Daniel C et al. Small bowel metastases from primary carcinoma of the lung: clinical findings and outcome. Am J Gastroenterol 1999;94:1884–7. 10.1111/j.1572-0241.1999.01224.x [DOI] [PubMed] [Google Scholar]
- 7.Garwood RA, Sawyer MD, Ledesma EJ et al. A case and review of bowel perforation secondary to metastatic lung cancer. Am Surg 2005;71:110–16. [PubMed] [Google Scholar]
- 8.Nishizawa Y, Kobayashi A, Saito N et al. Surgical management of small bowel metastases from primary carcinoma of the lung. Surg Today 2012;42:233–7. 10.1007/s00595-011-0005-8 [DOI] [PubMed] [Google Scholar]
- 9.Goldstraw P, Crowley J, Chansky K et al. The IASCL lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (Seventh) edition of the TNM classification of malignant tumours. J ThoracOncol 2007;2:706–14. [DOI] [PubMed] [Google Scholar]
- 10.Subedi N, Scarsbrook A, Darby M et al. The clinical impact of integrated FDG PET-CT on the management decision in patients with lung cancer. Lung Cancer 2009;64:301–7. 10.1016/j.lungcan.2008.09.006 [DOI] [PubMed] [Google Scholar]
- 11.Chowdhury A, Dhali GK, Banerjee PK. Etiology of gastric outlet obstruction. Am J Gastroenterol 1996;91:1679. [PubMed] [Google Scholar]
- 12.Lau JY, Chung SC, Sung JJ et al. Through-the-scope balloon dilation for pyloric stenosis: long-term results. Gastrointest Endosc 1996;43(2 Pt 1):98–101. [DOI] [PubMed] [Google Scholar]
- 13.Jeurnink SM, van Eijck CH, Steyerberg EW et al. Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: a systematic review. BMC Gastroenterol 2007;7:18 10.1186/1471-230X-7-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.vanHooft JE, Dijkgraaf MG, Timmer R et al. Independent predictors of survival in patients with incurable malignant gastric outlet obstruction: a multicenter prospective observational study. Scand J Gastroenterol 2010;45:1217–22. 10.3109/00365521.2010.487916 [DOI] [PubMed] [Google Scholar]