Abstract
Background:
Ferrous sulfate is the most used supplement for treating anemia, but it can result in unfavorable side effects. Nowadays, nanotechnology is used as a way to increase bioavailability and decrease the side effects of drugs and nutrients. This study investigates the effects of nanoparticles containing iron on blood and inflammatory markers in comparison to ferrous sulfate in anemic rats.
Methods:
To induce the model of hemolytic anemia, 50 mg/kg bw phenylhydrazine was injected intraperitoneally in rats on the 1st day and 25 mg/kg bw for the four following days. Then, rats were randomly divided into five groups. No material was added to the nipple of the Group 1 (control). Group 2 received 0.4 mg/day nanoparticles of iron; Group 3 received 0.4 mg/day ferrous sulfate, and Groups 4 and 5 received double dose of iron nanoparticle and ferrous sulfate, respectively for ten days.
Results:
Hemoglobin and red blood cell (RBC) in Group 2 were significantly higher than Group 3 (P < 0.05). In addition, hemoglobin and RBC in Group 4 and 5 were significantly higher than Group 3 (P < 0.05). The average level of serum iron in Groups 2 and 4 was remarkably more than the groups received ferrous sulfate with similar doses (P < 0.05). C-reactive protein in Group 3 was more than Group 2 and in Group 5 was more compare to all other groups.
Conclusions:
Single dose of nanoparticles had more bioavailability compare to ferrous sulfate, but this did not occur for the double dose. Furthermore, both doses of nanoparticles caused lower inflammation than ferrous sulfate.
Keywords: Anemia, ferrous sulfate, nanoparticle
INTRODUCTION
Anemia is one of the most prevalent nutritional deficiencies in both developed and developing countries.[1] About 1.62 billion people or 24.8% of the world population suffer from iron deficiency.[2] According to the World Health Organization statistics, more than half of the anemia in the world is due to iron deficiency.[3] Research in South of Iran reported that 30% of children, 24% of women, and 7% of men suffer from anemia. In addition, 13.6% of pregnant women were diagnosed with anemia.[4] Since iron acts as a part of hemoglobin, myoglobin and some of enzymes, iron deficiency can lead to weakness, learning dysfunction, and increase the risk of infectious diseases.[5,6,7] Furthermore, iron deficiency is associated with increased risk of preterm delivery and low Birth Weight and imposes large costs on the health system.[8,9]
Phenylhydrazine and its derivatives are one of the useful compounds in experimental models studying anemia due to their toxic effects on red blood cells (RBCs). Phenylhydrazine leads to RBC hemolysis and induces hemolytic anemia.[10] Hemolytic anemia is a form of anemia which may result from either intravascular or extravascular RBC reduction.[11]
Not only iron bioavailability is estimated very low (about 14%–18% for mixed diets and 5%–12% for vegetable diets) but also inhibitors of iron absorption such as phytate, tanenes, and oxalate can worsen this rate.[12]
Ferrous sulfate is the common supplement prescribed for anemia shows acceptable absorption, but recent findings revealed that this supplement can cause unfavorable changes in colon bacteria and increase systemic infections and inflammatory signals of epithelium.[13,14,15] Oral consumption of drugs with low bioavailability needs high dose of the drug to absorb the required amount, but the unabsorbed amount can cause undesirable gastrointestinal complications.[16] When iron is received through the mouth, the lower part of it is absorbed in the upper gastrointestinal tract and the larger part goes through the colon which can react with superoxide and hydrogen peroxide and produce free radicals through Fenton reaction.[13] Therefore, the remaining of free iron can stimulate the intestine, and the created discontent makes the situation difficult for the patient to take the medicine regularly.[17]
Nanotechnology in one of the novel techniques that recently is using to increase nutrients bioavailability. Researches showed that when some of the materials are prepared in nanometer size, their bioavailability increases.[18,19,20] When nanocarriers were used for oral intake of iron, iron absorption increased 1.35 times compare to the reference ferrous sulfate.[21] In addition, iron absorption from nanoparticles containing iron was 13.42% more than ferrous sulfate in vitro.[22] Therefore, it is expected that with size reduction of iron to nanometer size, its bioavailability increases, and lower doses of the drug would be needed to meet desirable result, which consequently decreases unfavorable effects in gastrointestinal system and encourages the patient to continue the medication.
METHODS
Chemical materials used in this research including 7H2O ferrous sulfate and phenylhydrazine was purchased from Merck Co.
Complete blood count (CBC) was calculated by flow cytometry method using cell counter Sysmex 800i. Erythrocyte sedimentation rate (ESR) was measured by Westergren method. Ferritin and high-sensitivity C-reactive protein (hs-CRP) were measured by ELISA method using rat ferritin and hs-CRP kit of ZellBio Co., Germany. Transferrin saturation and serum iron were calculated by Pars Azmoon kits, Iran.
Preparation and treatment of animals
Thirty-five 2-month-old male Wistar albino rats weighing about 190 ± 30 g were obtained from the Faculty of Medicine, Tehran University of Medical Sciences. During the experiment, animals were kept in standard condition (21°C ± 1°C, humidity 45%, and 12-h-light/12-h-dark cycle), each rat in a separate cage with free access to water and standard chow. Hemolytic anemia was induced by intraperitoneal injection of phenyl hydrazine for 5 days: 50 mg/kg bw in the 1st day and 25 mg/kg bw in the 4 following days. Phenylhydrazine was sterilized by poly (vinylidene fluoride) 0.22 μm filter after dilution by sterile distilled water to avoid contamination and probable infections following intraperitoneal injection. This solution was kept in sterile vials away from light. Then, animals were randomly divided into five groups of seven rats each: N (control group), ND (received single dose of nanoparticles containing iron), FD (received single dose of ferrous sulfate), NDD (received double dose of nanoparticles containing iron), and FDD (received double dose of ferrous sulfate).
Iron supplementation was started one day after hemolytic anemia induction, and it was lasted for 10 consecutive days. The calculated amount of each supplement was added to the drinking water. The amount of iron supplementation in groups received single dose was 0.4 mg/day and in groups received double dose was 0.8 mg/day. To prepare nanoparticles of iron, iron particle size was reduced to nanometer size and then, it was emulsion in aqueous solution by emulsifiers. Rats were weighed at the beginning, in the middle, and at the end of the experiment to follow weight changes during the experiment. The amount of water remaining in each cage was calculated every day.
Rats were sacrificed after 10 days of iron supplementation. Blood was collected immediately after anesthetization from rat hearts to measure CBC and ESR. In additon, serum of rats was separated through centrifugation of blood at 2500 rpm, 15°C for 15 min for further analysis.
Statistical analysis
Statistical significance between means was analyzed by one-way ANOVA using SPSS 16.0 while P < 0.05 was considered statistically as significant. Tukey test was used for post hoc analysis.
RESULTS
Malvern test was used to determine particle size and showed that 96% of the nanoparticles had the mean size of 84 ± 17 nm. Figure 1 depicts the size distribution of synthesized nanoparticles of iron.
Figure 1.
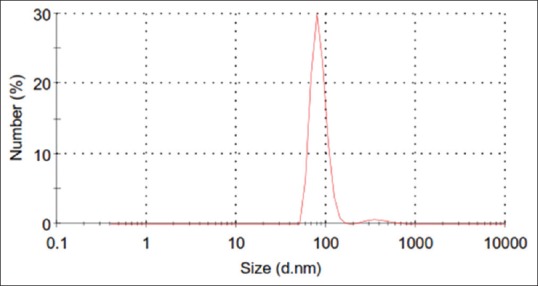
Size distribution by number of synthesized nanoparticles contacting iron
As shown in Table 1, administration of different doses of nanoparticles containing iron and ferrous sulfate did not change significantly ESR, total iron-binding capacity (TIBC), ferritin, and transferrin saturation in studied groups.
Table 1.
The effect of nanoparticles containing iron and ferrous sulfate administration on blood and inflammatory markers in anemic rats
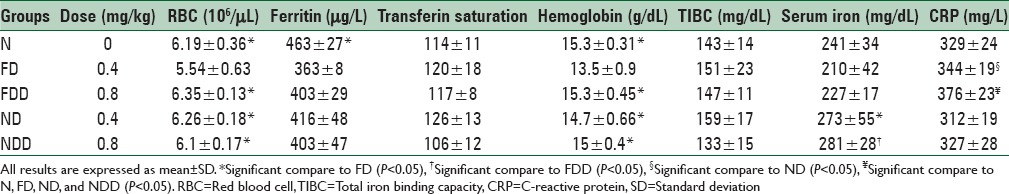
Our results showed that hemoglobin and RBC in group ND were significantly higher than group FD (P < 0.05). In addition, NDD and FDD groups had higher hemoglobin, and RBC compare to group FD (P < 0.05). Hemoglobin and RBC of rats in group N were significantly more than group FD (P < 0.05).
The highest TIBC was observed in group ND, though the difference between groups was not statistically significant.
Serum ferritin of group N was significantly higher than group FD (P < 0.05).
We also observed that serum iron of group ND was higher than group FD significantly (P < 0.05).
Mean CRP was significantly higher in group FDD compare to groups N, FD, ND, and NDD and in group, FD compares to group ND (P < 0.05).
There were no remarkable changes in transferrin saturation and rats’ weight between studied groups.
DISCUSSION
In this research, we observed that hemoglobin, RBC, and serum iron were increased significantly in rats received single dose of nanoparticles containing iron compare to rats received single dose of ferrous sulfate which shows more bioavailability of iron in the form of nanoparticles rather than the form of ferrous sulfate. Different researches have been conducted on different iron compounds, and it was observed in all of them that reduction of particle size increases bioavailability.[23,24,25] For example, one study used solid lipid nanoparticle (SLN) made from stearic acid and chitozan and reported that absorption of iron from SLN and SLN-chi increased 13.42 and 24.9%, respectively compare to the reference ferrous sulfate.[22] Wegmüller et al. assessed the effect of size reduction and encapsulation of iron pyrophosphate on hemoglobin retention in anemic rats and reported that bioavailability of iron pyrophosphate with mean size of 2.5 μm was 43% and with the size of 0.5 μm was 95% compare to ferrous sulfate.[23]
Other studies conducted on nanotechnology mostly studied hemoglobin, but we also measured other blood factors. We observed that mean serum ferritin in group ND was higher than group FD, but this difference was not statistically significant. Since phenylhydrazine injection induces inflammation and ferritin is a positive phase protein, it is probable that inflammation induced by phenylhydrazine injection has been acted as a confounding factor. In addition, rats received usual diet with adequate iron content during injection and supplementation time that could prevent iron stores from being emptied despite RBC lyse and increasing blood cell synthesis.[25]
The previous studies have shown that ferrous sulfate supplementation for anemia treatment leads to some noticeable undesirable effects for instance unfavorable changes in colon bacteria, increase in systemic infection, inflammatory signal of gastrointestinal tract, and free radicals production through fenton reaction which is made by the remaining free iron unabsorbed from ferrous sulfate. Carrier et al. declared that ferrous sulfate supplementation in rats suffering from colon inflammation, increased plasma and colon lipid peroxidation, and generally activity of the disease.[13] We also observed that groups received ferrous sulfate had higher CRP compare to groups received nanoparticles with the same dose, and CRP in group FDD was 13% more than group NDD. Groups ND and NDD had the lowest CRP concentrations in the experiment. The studies on other nanoparticles such as nanoparticles of adipate tartrate iron hydroxide and ferrihydrite nanoparticles showed that nanoparticle supplementation reduces side effects and inflammation induced by iron supplementation via reduction of free radicals production, and colon bacteria changes which are completely in consistence with our findings.[26,27]
Limitations
As we were not able to provide iron deficient diet to induce anemia in rats, we had to use phenylhydrazine compound which induces hemolytic anemia.
CONCLUSIONS
In this research, single dose of nanoparticles containing iron showed more bioavailability compare to single dose of ferrous sulfate and more efficiently restored hemoglobin, but it was not occurred for the double dose. Furthermore, inflammation measured in both groups received nanoparticles was lower than groups received ferrous sulfate at the end of the experiment.
Financial support and sponsorship
Financial support was provided by Tehran University of Medical Sciences, Tehran, Iran.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Scholl TO, Hediger ML. Anemia and iron-deficiency anemia: Compilation of data on pregnancy outcome. Am J Clin Nutr. 1994;59(2 Suppl):492S–500S. doi: 10.1093/ajcn/59.2.492S. [DOI] [PubMed] [Google Scholar]
- 2.De Benoist B, McLean E, Egli I, Cogswell M. WHO global database on anaemia. Geneva: WHO; 1993. pp. 2005–8. [Google Scholar]
- 3.Verster A. Regional Office for the Eastern Mediterranean. Fortification of Flour with Iron in Countries of the Eastern Mediterranean, Middle East and North Africa. WHO Regional Office for the Eastern Mediterranean. 1998 [Google Scholar]
- 4.Esmat B, Mohammad R, Behnam S, Shahrzad M, Soodabeh T, Minoo A, et al. Prevalence of iron deficiency anemia among Iranian pregnant women; a systematic review and meta-analysis. J Reprod Infertil. 2010;11:17–24. [PMC free article] [PubMed] [Google Scholar]
- 5.Weatherall DJ, Kwiatkowski D. Hematologic disorders of children in developing countries. Pediatr Clin North Am. 2002;49:1149–64. doi: 10.1016/s0031-3955(02)00087-1. [DOI] [PubMed] [Google Scholar]
- 6.Sadighi J, Mohammad K, Sheikholeslam R, Torabi P, Salehi F, Abdolahi Z, et al. Flour fortification with iron and folic acid in Bushehr and Golestan Provinces, Iran: Program evaluation. J Sch Public Health Inst Public Health Res. 2010;7:11–24. [Google Scholar]
- 7.Low M, Farrell A, Biggs BA, Pasricha SR. Effects of daily iron supplementation in primary-school-aged children: Systematic review and meta-analysis of randomized controlled trials. CMAJ. 2013;185:E791–802. doi: 10.1503/cmaj.130628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Earl RO, Woteki CE, Calloway DH, et al. Institute of Medicine; Committee on the Prevention D, Management of Iron Deficiency Anemia among USC, Women of Childbearing A. Children and Women of Childbearing Age. Washington, DC: National Academy Press; 1993. Iron Deficiency Anemia: Recommended Guidelines for the Prevention, Detection, and Management among U.S. [PubMed] [Google Scholar]
- 9.Tontisirin K, Nantel G, Bhattacharjee L. Food-based strategies to meet the challenges of micronutrient malnutrition in the developing world. Proc Nutr Soc. 2002;61:243–50. doi: 10.1079/PNS2002155. [DOI] [PubMed] [Google Scholar]
- 10.Ashour TH. Phenylhydrazine-induced hemolytic anemia in rats. Res J Med Sci. 2014;8:67–72. [Google Scholar]
- 11.Hoffman R, Hoffman R, Benz EJ, Silberstein LE, Heslop HE, Weitz JI, et al. Hematology: basic principles and practice.Sixth edition. Philadelphia, PA: Saunders/Elsevier; 2013. [Google Scholar]
- 12.Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr. 2010;91:1461S–7S. doi: 10.3945/ajcn.2010.28674F. [DOI] [PubMed] [Google Scholar]
- 13.Carrier J, Aghdassi E, Cullen J, Allard JP. Iron supplementation increases disease activity and Vitamin E ameliorates the effect in rats with dextran sulfate sodium-induced colitis. J Nutr. 2002;132:3146–50. doi: 10.1093/jn/131.10.3146. [DOI] [PubMed] [Google Scholar]
- 14.Dandekar P, Dhumal R, Jain R, Tiwari D, Vanage G, Patravale V. Toxicological evaluation of pH-sensitive nanoparticles of curcumin: Acute, sub-acute and genotoxicity studies. Food Chem Toxicol. 2010;48:2073–89. doi: 10.1016/j.fct.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Werner T, Wagner SJ, Martínez I, Walter J, Chang JS, Clavel T, et al. Depletion of luminal iron alters the gut microbiota and prevents Crohn's disease-like ileitis. Gut. 2011;60:325–33. doi: 10.1136/gut.2010.216929. [DOI] [PubMed] [Google Scholar]
- 16.Hetal TB, Sneha T. A review on techniques for oral bioavailability enhancement of drugs. Int J Pharm Sci Rev Res. 2010;4:203–23. [Google Scholar]
- 17.Schümann K, Ettle T, Szegner B, Elsenhans B, Solomons NW. On risks and benefits of iron supplementation recommendations for iron intake revisited. J Trace Elem Med Biol. 2007;21:147–68. doi: 10.1016/j.jtemb.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Mozafari MR, Johnson C, Hatziantoniou S, Demetzos C. Nanoliposomes and their applications in food nanotechnology. J Liposome Res. 2008;18:309–27. doi: 10.1080/08982100802465941. [DOI] [PubMed] [Google Scholar]
- 19.Shudo J, Pongpeerapat A, Wanawongthai C, Moribe K, Yamamoto K. In vivo assessment of oral administration of probucol nanoparticles in rats. Biol Pharm Bull. 2008;31:321–5. doi: 10.1248/bpb.31.321. [DOI] [PubMed] [Google Scholar]
- 20.Kumar R, Chen MH, Parmar VS, Samuelson LA, Kumar J, Nicolosi R, et al. Supramolecular assemblies based on copolymers of PEG600 and functionalized aromatic diesters for drug delivery applications. J Am Chem Soc. 2004;126:10640–4. doi: 10.1021/ja039651w. [DOI] [PubMed] [Google Scholar]
- 21.Gao H, Chen H, Chen W, Tao F, Zheng Y, Jiang Y, et al. Effect of nanometer pearl powder on calcium absorption and utilization in rats. Food Chem. 2008;109:493–8. [Google Scholar]
- 22.Zariwala MG, Elsaid N, Jackson TL, Corral López F, Farnaud S, Somavarapu S, et al. A novel approach to oral iron delivery using ferrous sulphate loaded solid lipid nanoparticles. Int J Pharm. 2013;456:400–7. doi: 10.1016/j.ijpharm.2013.08.070. [DOI] [PubMed] [Google Scholar]
- 23.Wegmüller R, Zimmermann MB, Moretti D, Arnold M, Langhans W, Hurrell RF. Particle size reduction and encapsulation affect the bioavailability of ferric pyrophosphate in rats. J Nutr. 2004;134:3301–4. doi: 10.1093/jn/134.12.3301. [DOI] [PubMed] [Google Scholar]
- 24.Hilty FM, Arnold M, Hilbe M, Teleki A, Knijnenburg JT, Ehrensperger F, et al. Iron from nanocompounds containing iron and zinc is highly bioavailable in rats without tissue accumulation. Nat Nanotechnol. 2010;5:374–80. doi: 10.1038/nnano.2010.79. [DOI] [PubMed] [Google Scholar]
- 25.Criswell KA, Sulkanen AP, Hochbaum AF, Bleavins MR. Effects of phenylhydrazine or phlebotomy on peripheral blood, bone marrow and erythropoietin in Wistar rats. J Appl Toxicol. 2000;20:25–34. doi: 10.1002/(sici)1099-1263(200001/02)20:1<25::aid-jat624>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Pereira DI, Bruggraber SF, Faria N, Poots LK, Tagmount MA, Aslam MF, et al. Nanoparticulate iron(III) oxo-hydroxide delivers safe iron that is well absorbed and utilised in humans. Nanomedicine. 2014;10:1877–86. doi: 10.1016/j.nano.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powell JJ, Bruggraber SF, Faria N, Poots LK, Hondow N, Pennycook TJ, et al. A nano-disperse ferritin-core mimetic that efficiently corrects anemia without luminal iron redox activity. Nanomedicine. 2014;10:1529–38. doi: 10.1016/j.nano.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


