Abstract
Although selective metabolic and receptor-based molecular agents will surely be included in the future of prostate cancer diagnosis and therapy, currently available inorganic compounds—such as 18F-NaF for the diagnosis of bony disease and 223RaCl2 for the therapy of bone metastases—were recently shown to be superior to standard 99mTc-phosphonates for diagnosis and 153Sm-ethylenediaminetetramethylene phosphonate or 89SrCl2 for therapy. The advantages of 18F-NaF include improved lesion detection and, when used in combination with CT, improved diagnostic confidence and specificity. In addition to being the first approved α-emitter, 223RaCl2 is the first radiopharmaceutical to show an increase in overall survival, a decrease in skeletal events, palliation of bone pain, and a low profile of adverse reactions (which are mild and manageable). The management of metastatic bone disease with 223RaCl2 is uniquely satisfying, as patients can be monitored directly during their monthly treatment visits.
Keywords: PET/CT, PET, prostate, cancer, bone, radium
The American Cancer Society has estimated that there will be 180,890 new prostate cancer (PC) patients and 28,170 deaths in 2016 (1). Prostate cancer ranges from asymptomatic to a progressive systemic malignancy. Lymph nodes and bones are the most common sites of metastases. Serum prostate-specific antigen (PSA) levels, Gleason score, and clinical stage at presentation are used to assess pretreatment risk and the probability of recurrence or metastases. On the basis of these criteria, patients are characterized as having low risk (bone metastases unlikely) or high risk (bone metastases likely). Skeletal metastases correlate with a poor prognosis. 99mTc-methylene diphosphonate (99mTc-MDP) scintigraphy has been the imaging method of choice for the evaluation of predominantly sclerotic skeletal PC metastases. However, the growing availability of PET imaging systems, the establishment of commercial regional distribution centers for PET radiotracers, and the relatively recent concerns with shortages of 99mTc-labeled radiotracers have prompted much interest in the use of PET in this clinical setting (2).
18F-NAF PET IN PC
18F-NaF is a positron-emitting radiopharmaceutical that was used for skeletal scintigraphy in the 1970s. Clinical use was limited then because of logistic difficulties in delivering tracers with a half-life of 110 min and the absence of PET technology. 18F-NaF was replaced in the late 1970s by 99mTc-labeled phosphonates, which had improved γ-camera compatibilities (3,4). As an analog of the hydroxyl group found in hydroxyapatite bone crystals, 18F-NaF is an avid bone seeker. Ion exchange is its mechanism of uptake, and blood flow is the rate-limiting step in the transfer of fluoride ions from blood to bone (4,5). As with 99mTc-phosphonates, which adhere to bone by chemisorption, fluorine is directly incorporated into the bone matrix, converting hydroxyapatite to fluoroapatite (6). 18F-NaF is rapidly cleared from plasma, with only 10% of 18F-NaF remaining in plasma at 1 h (7,8). This desirable characteristic of rapid blood clearance in association with fast and high bone uptake leads to high-quality (high target-to-background uptake ratio) skeletal images in less than 1 h after intravenous 18F-NaF administration (Fig. 1). Nonuniform uptake of 18F-NaF in normal skeleton reflects differential regional blood flow and bone crystal surface nonuniformity. Regional high uptake in abnormal scans corresponds to processes that increase blood flow to the exposed bone crystal surface.
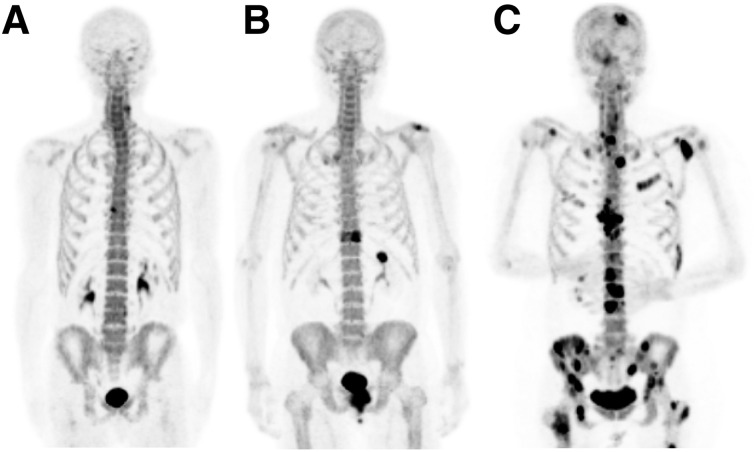
FIGURE 1.
18F-NaF PET/CT maximum-intensity-projection images illustrating appearance of anterior osteophyte (A), solitary metastasis (B), and multiple metastases (C) in 3 different PC patients.
18F-NAF PET/CT VS. 99MTC-MDP BONE SCINTIGRAPHY
18F-NaF PET/CT is currently under evaluation for the detection of malignant skeletal lesions. In December 2015, the Centers for Medicare & Medicaid Services issued a decision memorandum regarding the use of 18F-NaF PET for the detection of bony metastasis, concluding that available outcome evidence was not sufficient to allow for 18F-NaF PET reimbursement (9). The Centers for Medicare & Medicaid Services recommended continuation of coverage with evidence development for another 2 y, as before, through the National Oncologic PET Registry for 18F-NaF (9). 99mTc-MDP bone scintigraphy has been the method of choice for the evaluation of osseous metastases, as it enables a relatively low-cost whole-skeleton survey. Applications of skeletal scintigraphy include initial staging, monitoring the response to therapy, and detection of areas at risk for pathologic fracture. Although 99mTc-MDP scintigraphy is sensitive for the detection of advanced skeletal metastatic lesions, early involvement may be missed because this technique relies on identification of the osteoblastic reaction of the involved bone rather than detection of the tumor itself. The technique relies significantly on regional blood flow to bone. Spatial resolution limitations imposed by planar scintigraphy and SPECT also affect bone scintigraphy sensitivity for osseous metastases (10). In a study by Even-Sapir (11), patients with high-risk PC underwent 99mTc-MDP bone scintigraphy with SPECT and 18F-NaF PET/CT. In a patient-based analysis, the sensitivities of 99mTc-MDP planar scintigraphy, SPECT, and 18F-NaF PET/CT were 70%, 92%, and 100%, respectively. The specificities of 99mTc-MDP planar scintigraphy, SPECT, 18F-NaF PET, and 18F-NaF PET/CT were 57%, 82%, 62%, and 100%, respectively. Another study involving 42 PC patients demonstrated a sensitivity of 91%, a specificity of 89%, and an accuracy of 90% for 18F-NaF PET/CT in the detection of bone metastases (12). Cook et al. compared qualitative bone scintigraphy with semiquantitative 18F-NaF PET for evaluation of the response to bone metastasis treatment with 223RaCl2 (13) and concluded that 18F-NaF PET was more accurate than 99mTc-MDP (Fig. 2).
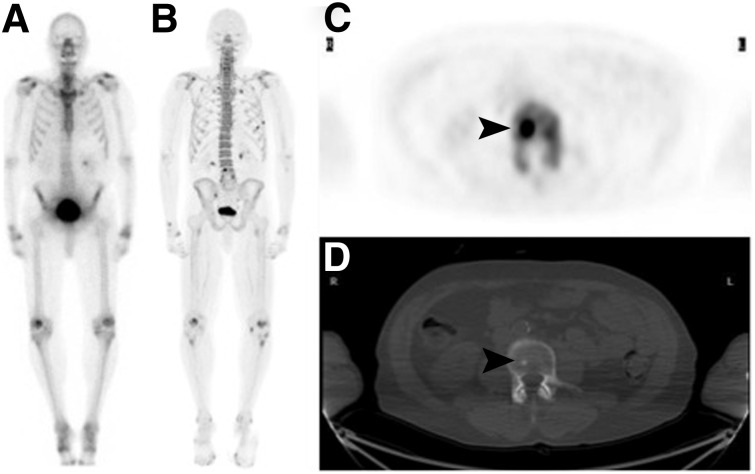
FIGURE 2.
73-y-old man with PC. (A and B) 99mTc-MDP bone scan was negative (A), whereas 18F-NaF PET/CT revealed single L4 metastasis (B). (C and D) Axial PET (C) and CT (D) revealed focal uptake in small sclerotic lesion (arrowhead).
A recent study comparing 18F-NaF PET with 99mTc-MDP scintigraphy demonstrated that 18F-NaF PET/CT was more accurate than conventional bone scintigraphy for localizing and characterizing malignant bone lesions (14). High-quality imaging, increased clinical accuracy, and greater patient and referring physician convenience support 18F-NaF PET/CT in comparison with planar bone scintigraphy, SPECT, SPECT/CT, and whole-body MRI, including diffusion-weighted imaging, for the evaluation of skeletal malignancy (15,16). Despite the high performance of 18F-NaF PET/CT, clinical use remains low because of limited access and nonuniform reimbursement. The exact PSA threshold for using 18F-NaF PET/CT also remains to be established, although the threshold is probably lower than that for 99mTc-MDP scintigraphy (typically a serum PSA level of >20 ng/mL).
Our results indicated the feasibility and utility of the combined administration of 18F-NaF and 18F-FDG in a single PET/CT examination for the detection of malignancy, including PC (17–20).
TARGETED RADIONUCLIDE THERAPY FOR BONE METASTASES
Initial and subsequent treatment of PC is complex and may involve surgery, radiation therapy, hormonal therapy, chemotherapy, bisphosphonates, pain medication, or a combination of these. Typically, the initial phase of hormone dependence gives way to hormone independence or castration resistance, at which time the cancer tends to metastasize more readily, especially to bone. About 90% of patients with advanced castration-resistant PC (CRPC) will develop bone metastases (21). Bone lesions may cause pain, disability, deterioration of quality of life, and skeletal events (e.g., pathologic fractures or spinal cord compression) that may require treatment, with associated costs and potential complications. Metastatic CRPC (mCRPC) is associated with significant morbidity and mortality. Successful mCRPC treatment alleviates bone pain, reduces skeletal events, reduces disease biomarkers (such as alkaline phosphatase and PSA levels), and improves survival.
Prostate cancer cell proliferation generally induces an osteoblastic reaction in bone. One successful strategy for improving clinical outcomes in PC patients with bone metastases is the use of radiopharmaceutical agents that preferentially accumulate at sites of increased osteoblastic activity and provide a local antitumor effect (21,22). Targeted radionuclide therapy is used to deliver a specific amount of radioactivity to specific tissues in a certain area of the body to destroy cancer lesions (Table 1).
TABLE 1.
Bone-Targeted Radiopharmaceuticals
| Radiopharmaceutical | Physical half-life (d) | Particle type | Usual administered activity | Typical response time (d) | Typical response duration | Retreatment interval |
| 89SrCl2 (Metastron; GE Healthcare Ltd.) | 50.5 | β | 148 MBq | 14–28 | 12–26 wk | >3 mo |
| 153Sm-EDTMP (Quadramet; IBA-Molecular) | 1.9 | β | 37 MBq/kg | 2–7 | 8 wk | >2 mo |
| 223RaCl2 (Xofigo; Bayer Healthcare) | 11.4 | α | 50 kBq/kg × 6 every 4 wk | <10 | Unknown | Unknown |
89SRCL2 AND 153SM-ETHYLENEDIAMINETETRAMETHYLENE PHOSPHONATE (153SM-EDTMP)
Historically, the most common radiopharmaceuticals for bone pain palliation in the United States were the β-emitting radiopharmaceuticals 89SrCl2 and 153Sm-EDTMP. 89SrCl2 was approved by the U.S. Food and Drug Administration (FDA) in 1993 and has a physical half-life of 50.5 d, a maximum β-energy of 1.463 MeV (100%), and a tissue range of 8 mm. The typical dose is 148 MBq (4 mCi) through a slow intravenous push. Doses can be repeated at 3-mo intervals. Pain relief usually begins in 10–20 d and lasts up to 6 mo, resulting in reduced management expenditures (23). Although the tumor-to-marrow absorbed dose is approximately 10:1, to avoid complications from marrow suppression, a pretreatment platelet count of greater than 60,000/mm3 and a white blood cell count of greater than 2,400/mm3 are suggested. With specific instructions, effective doses to bystanders are acceptable (24). Kurosaka et al. showed that 89SrCl2 was effective in reducing pain, including a subset of patients who became pain-free, with resultant improvement in quality of life (25).
89SrCl2 may also be effective in combination with other drugs. In a study by Tu et al., 36 patients who had CRPC and were randomly assigned to receive 89SrCl2 and doxorubicin had a median survival time of 27.7 mo; in contrast, for 36 patients who received doxorubicin alone, the median survival time was 16.8 mo (P = 0.0014) (26). However, such an outcome benefit with combined therapy was not demonstrated in patients with advanced castration-sensitive prostate cancer (27).
153Sm-EDTMP was approved by the FDA in 1997, emits both medium-energy β-particles and a γ-photon (which allows for imaging), and has a physical half-life of 46.3 h (Fig. 3). The recommended dose of 153Sm-EDTMP is 37 MBq/kg (1.0 mCi/kg), administered intravenously. The patient should ingest or receive fluids and should void often to minimize bladder radiation. 153Sm-EDTMP has a desirable combination of high bone uptake, low nonosseous uptake, and rapid blood clearance, making it useful in treating bone metastases. The highest doses are present in bone and the urinary bladder wall (28). 99mTc-MDP and 153Sm-EDTMP concentrate in metastatic skeletal lesions through the same mechanisms (29). In a phase 1 study of patients with skeletal metastases, prospective radiation dosimetry calculation permitted an accurate 153Sm-EDTMP dosage on the basis of total red marrow exposure, starting at 100 cGy and escalating to 280 cGy, to define dose-limiting myelotoxicity (30). Pain was relieved in 22 of 34 patients (65%) for periods ranging from 4 to 35 wk (30). Other, larger studies confirmed similar response rates in both breast cancer and prostate cancer (31–33). Transient myelosuppression with delayed thrombocytopenia was noted in some patients (30). Bayouth et al. reported that radiation estimates permitted the identification of at-risk patients before myelotoxicity was reached (34).
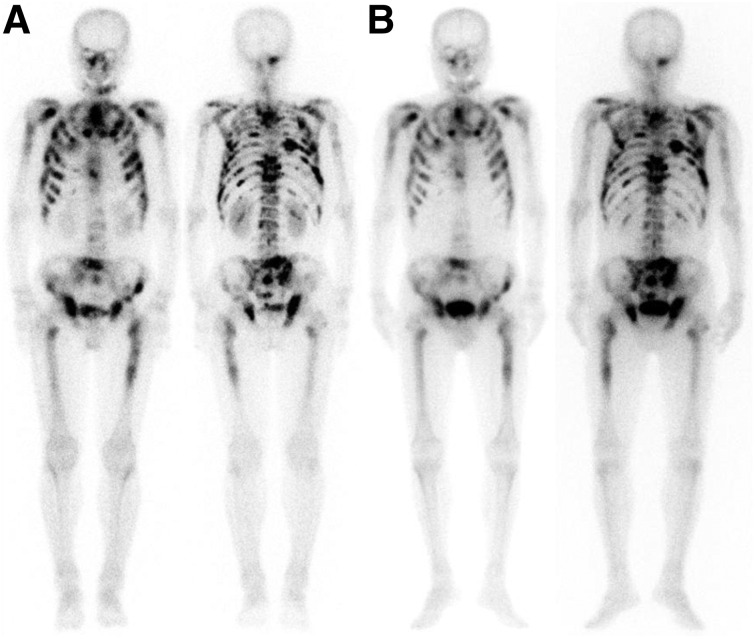
FIGURE 3.
72-y-old man who had PC and was referred for 153Sm-EDTMP treatment of painful bone metastases. 99mTc-MDP bone scan (A) and 153Sm-EDTMP (B) revealed extensive metastases.
In 55 patients with painful bone metastases, Sartor et al. reported that repeat dosing with 153Sm-EDTMP at 37 MBq/kg (1.0 mCi/kg) was safe and effective, and Heron et al. found that multiple treatments with 153Sm-EDTMP had no effect on the severity of myelotoxicity (35,36). This treatment option is reasonable in patients who have adequate hematologic function and bone pain that recurs after an initial successful dose.
Baczyk et al. compared 89SrCl2 and 153Sm-EDTMP in 60 patients with PC and 40 patients with breast cancer (37). Complete pain relief was found in 40% of women and men treated with 153Sm-EDTMP and in 25% of women and 33% of men treated with 89SrCl2. No relief occurred in 20% of patients. Greater pain reduction occurred with osteoblastic metastases than with mixed metastases. Statistically significant pain reduction, pain drug requirements, and Karnofsky scale improvement were found with both radionuclides (37). Montesano et al. reported on 27 patients who had bone metastases (16 prostate, 5 breast, and 6 lung cancers) and received 89SrCl2 (17 patients) or 153Sm-EDTMP (10 patients) (38). After therapy, there was no significant tumor marker or bone scan changes. 89SrCl2 and 153Sm-EDTMP enabled partial or total pain relief and improvement in quality of life. No tumoricidal effect was found. Hematologic toxicity was limited and reversible. Patients with PC seemed to have a higher response rate (38).
223RACL2
223RaCl2 (Xofigo; previously Alpharadin; Bayer Healthcare) is the newest radiopharmaceutical approved by the FDA (on May 15, 2013) for the treatment of painful osseous lesions from mCRPC (39). Compared with 89SrCl2 and 153Sm-EDTMP, 223RaCl2 represents a significant departure for 2 important reasons. First, 223RaCl2 is primarily an α-emitter rather than a β-emitter (decay scheme: 93.5% as α-particles, <3.6% as β-particles, and <1.1% as γ-radiation). Second, 223RaCl2 is the first radiopharmaceutical to show an increase in overall survival (OS), a decrease in skeletal events, palliation of bone pain, and low toxicity.
Until 2010, docetaxel was the only chemotherapeutic agent able to prolong survival after development of CRPC, and 89SrCl2 and 153Sm-EDTMP were used for bone pain palliation, along with pain medications (40). Since then, 5 new drugs have been proven to be efficacious in prolonging survival: sipuleucel-T, cabazitaxel, abiraterone, enzalutamide, and 223RaCl2 (41–44).
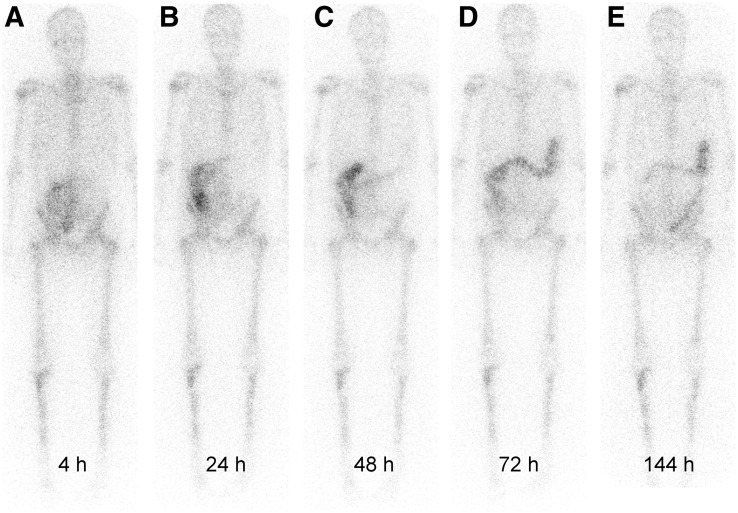
Because of their short range, α-particles deliver a large amount of radiation to target tissue while relatively sparing normal surrounding tissue. 223RaCl2 has a much higher energy and a shorter tissue range than either 89SrCl2 or 153Sm-EDTMP (45). The range of α-particles in human tissue is approximately 0.1 mm. The administration of 223RaCl2 is similar to that of 89SrCl2 and 153Sm-EDTMP (a slow intravenous push). Dosimetry was studied during a phase 1 study of 223RaCl2 for 3 cohorts, at 50, 100, and 200 kBq/kg. Dose rates were usually less than 2 μSv/h/MBq on contact and averaged 0.02 μSv/h/MBq at 1 m immediately after administration. Fecal excretion was the primary method of clearance, with resulting whole-body effective half-lives of 2.5–11.4 d (Fig. 4) (45–47).
FIGURE 4.
Biodistribution of 223RaCl2. (Adapted with permission of (47).)
α-particles require relatively minimal radiation safety precautions (48). For example, in the average patient receiving 3.5 MBq (95 μCi) of 223RaCl2, the dose measures 0.35 μSv/h (0.035 mrem/h) at 1 m. Thus, no contact precautions are associated with 223RaCl2. Similar to recommendations for 131I therapy, bathroom precautions are recommended for 1 wk to minimize radiation exposure to others; these precautions include multiple flushing, area cleanup, and hand washing.
An early phase 2 study (49) was done to investigate the dose–response relationship and pain-relieving effect of 223RaCl2. In that study, 100 mCRPC patients were randomized to receive a single 223RaCl2 dose of 5, 25, 50, or 100 kBq/kg. The pain index 2 wk after 223Ra administration was significantly related to the dose (P = 0.035). At week 8, there were 40%, 63%, 56%, and 71% pain responders in the groups receiving 5, 25, 50, and 100 kBq/kg, respectively. All doses were considered safe, and a decrease in the bone alkaline phosphatase level in the group receiving the highest dose was noted (P = 0.0067).
In another phase 2 study, the efficacy and safety of 223RaCl2 at 3 different doses were evaluated in mCRPC patients (50). In this phase 2 double-blind multicenter study, 122 patients were randomized to receive 3 injections of 223Ra at 6-wk intervals: 25 kBq/kg (n = 41), 50 kBq/kg (n = 39), and 80 kBq/kg (n = 42). The primary endpoint was reached, with a statistically significant dose–response relationship, for no patients (0%) in the group receiving 25 kBq/kg, for 2 patients (5%) in the group receiving 50 kBq/kg, and for 5 patients (12%) in the group receiving 80 kBq/kg (P = 0.0297). A decrease in the bone alkaline phosphatase level of greater than or equal to 50% was identified in 6 patients (16%), 24 patients (67%), and 25 patients (66%) in the groups receiving 25, 50, and 80 kBq/kg, respectively (P < 0.0001). The most common treatment-related adverse events occurring up to wk 24 across all dose groups were diarrhea (21%), nausea (16%), and anemia (14%). No difference in the incidences of hematologic events was seen among the dose groups. 223RaCl2 was well tolerated at all doses and had a dose-dependent effect on serum CRPC activity markers.
Final approval of 223RaCl2 followed the phase 3 ALSYMPCA (Alpharadin in Symptomatic PC) trial (51). In this randomized, double-blind, placebo-controlled study, 921 patients who had received, were not eligible to receive, or declined docetaxel (in a 2:1 ratio) were randomized to receive six injections of 223RaCl2 (50 kBq/kg) or matching placebo every 4 wk. The primary endpoint was OS. The main secondary efficacy endpoints included time to the first symptomatic skeletal event and various biochemical endpoints. A prespecified interim analysis conducted when 314 deaths had occurred assessed the effect of 223RaCl2 versus placebo on survival. An updated analysis conducted when 528 deaths had occurred was performed before the crossover from placebo to 223RaCl2.
At an interim analysis of 809 patients, compared with placebo, 223RaCl2 significantly improved OS (median, 14.0 vs. 11.2 mo; hazard ratio, 0.70; 95% confidence interval, 0.55–0.88; 2-sided P = 0.002). An updated analysis of 921 patients confirmed the 223RaCl2 survival benefit (median, 14.9 vs. 11.3 mo; hazard ratio, 0.70; 95% confidence interval, 0.58–0.83; P < 0.001). Assessments of efficacy endpoints also revealed a benefit of 223RaCl2 compared with placebo. 223RaCl2 was associated with low myelosuppression rates and fewer adverse events. The study was terminated for efficacy, as 223RaCl2 improved OS (51–53). The encouraging outcome of the ALSYMPCA clinical trial not only resulted in FDA approval but also led to the recent incorporation of 223RaCl2 into the updated National Comprehensive Cancer Network guideline (version 3.2013).
Although there may be a decrease in the PSA or alkaline phosphatase level similar to that seen with 89SrCl2 or 153Sm-EDTMP, some patients either did not have a decrease in PSA or had an increase in PSA after 223RaCl2 therapy. Thus, PSA can fail to accurately indicate the response of mCRPC to a variety of therapies (54). Both patients and physicians should be aware of this potential unlinking of OS and PSA in mCRPC so that therapy is not unnecessarily stopped prematurely.
On the basis of phase 2 and 3 trials, the most common (>10%) adverse reactions in patients receiving 223RaCl2 were nausea, diarrhea, vomiting, and peripheral edema. The most common hematologic abnormalities were anemia, lymphocytopenia, leukopenia, thrombocytopenia, and neutropenia (52). One study reviewed the 1-y clinical experience with 223RaCl2 in 25 mCRPC patients (55). About one-quarter of the cohort completed the entire 6-dose treatment cycle. Advancing soft-tissue disease was the primary reason for the cessation of therapy. Adverse events were mild and manageable. A decline in the serum alkaline phosphatase level was more common than a decline in the PSA level. Another retrospective investigation reviewed the clinical experience after 532 cycles of 223RaCl2 administration in 110 patients (56). That study showed that there were significant reductions in the serum alkaline phosphatase level and pain score and that progression risk was associated with a decrease in the serum PSA doubling time. Simultaneous external-beam radiation therapy was associated with an increased risk of bone marrow failure.
The effectiveness of 223RaCl2 therapy was recently evaluated in relation to skeletal tumor burden on whole-body 18F-NaF PET/CT (57). In 42 patients, skeletal tumor burden indices derived from PET/CT at baseline were highly correlated and were significant independent predictors of OS. In an earlier study, an SUVmax threshold of 10 was shown to exclude nearly all normal bone activity from volumetric calculations (58).
CONCLUSION
18F-NaF PET provides superior diagnostic performance compared with 99mTc-MDP bone scintigraphy for the evaluation of patients with PC. Radionuclide therapy for painful bone metastases is undergoing a significant resurgence because of the introduction of 223Ra and the use of radiopharmaceuticals in combination with standard chemotherapy. These developments will contribute significantly to the care of patients with PC in the era of personalized and precision medicine (59).
DISCLOSURE
Hossein Jadvar was supported in part by National Institutes of Health grants R01-CA111613, R21-CA142426, R21-EB017568, and P30-CA014089. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Picchio M, Giovannini E, Messa C. The role of PET/computed tomography scan in the management of prostate cancer. Curr Opin Urol. 2011;21:230–236. [DOI] [PubMed] [Google Scholar]
- 3.Jadvar H, Desai B, Conti PS. Sodium 18F-fluoride PET/CT of bone, joint, and other disorders. Semin Nucl Med. 2015;45:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czernin J, Satyamurthy N, Schiepers C. Molecular mechanisms of bone 18F-NaF deposition. J Nucl Med. 2010;51:1826–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant FD, Fahey FH, Packard AB, Davis RT, Alavi A, Treves ST. Skeletal PET with 18F-fluoride: applying new technology to an old tracer. J Nucl Med. 2008;49:68–78. [DOI] [PubMed] [Google Scholar]
- 6.Blau M, Ganatra R, Bender MA. 18F-fluoride for bone imaging. Semin Nucl Med. 1972;2:31–37. [DOI] [PubMed] [Google Scholar]
- 7.Bridges RL, Wiley CR, Christian JC, Strohm AP. An introduction to Na18F bone scintigraphy: basic principles, advanced imaging concepts, and case examples. J Nucl Med Technol. 2007;35:64–76. [DOI] [PubMed] [Google Scholar]
- 8.Cook GJR. PET and PET/CT imaging of skeletal metastases. Cancer Imaging. 2010;10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Medicare & Medicaid Services. Decision memo for positron emission tomography (NaF-18) to identify bone metastasis of cancer (CAG-00065R2). https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=279. Accessed July 22, 2016.
- 10.Savelli G, Maffioli L, Maccauro M, De Deckere E, Bombardieri E. Bone scintigraphy and the added value of SPECT (single photon emission tomography) in detecting skeletal lesions. Q J Nucl Med. 2001;45:27–37. [PubMed] [Google Scholar]
- 11.Even-Sapir E. Imaging of malignant bone involvement by morphologic, scintigraphic, and hybrid modalities. J Nucl Med. 2005;46:1356–1367. [PubMed] [Google Scholar]
- 12.Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47:287–297. [PubMed] [Google Scholar]
- 13.Cook G, Jr, Parker C, Chua S, Johnson B, Aksnes AK, Lewington VJ. 18F-fluoride PET: changes in uptake as a method to assess response in bone metastases from castrate-resistant prostate cancer patients treated with 223Ra-chloride (Alpharadin). EJNMMI Res. 2011;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen CT, Qiu ZL, Han TT, Luo QY. Performance of 18F-fluoride PET or PET/CT for the detection of bone metastases: a meta-analysis. Clin Nucl Med. 2015;40:103–110. [DOI] [PubMed] [Google Scholar]
- 15.Schirrmeister H, Guhlmann A, Elsner K, et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med. 1999;40:1623–1629. [PubMed] [Google Scholar]
- 16.Jambor I, Kuisma A, Ramadan S, et al. Prospective evaluation of planar bone scintigraphy, SPECT, SPECT/CT, 18F-NaF PET/CT and whole body 1.5T MRI, including DWI, for the detection of bone metastases in high risk breast and prostate cancer patients: SKELETA clinical trial. Acta Oncol. 2016;55:59–67. [DOI] [PubMed] [Google Scholar]
- 17.Iagaru A, Mittra E, Yaghoubi SS, et al. Novel strategy for a cocktail 18F-fluoride and 18F-FDG PET/CT scan for evaluation of malignancy: results of the pilot-phase study. J Nucl Med. 2009;50:501–505. [DOI] [PubMed] [Google Scholar]
- 18.Iagaru A, Mittra E, Mosci C, et al. Combined 18F-fluoride and 18F-FDG PET/CT scanning for evaluation of malignancy: results of an international multicenter trial. J Nucl Med. 2013;54:176–183. [DOI] [PubMed] [Google Scholar]
- 19.Minamimoto R, Mosci C, Jamali M, et al. Semiquantitative analysis of the biodistribution of the combined 18F-NaF and 18F-FDG administration for PET/CT imaging. J Nucl Med. 2015;56:688–694. [DOI] [PubMed] [Google Scholar]
- 20.Minamimoto R, Loening A, Jamali M, et al. Prospective comparison of 99mTc-MDP scintigraphy, combined 18F-NaF and 18F-FDG PET/CT, and whole-body MRI in patients with breast and prostate cancer. J Nucl Med. 2015;56:1862–1868. [DOI] [PubMed] [Google Scholar]
- 21.Rajpar S, Fizazi K. Bone targeted therapies in metastatic castration-resistant prostate cancer. Cancer J. 2013;19:66–70. [DOI] [PubMed] [Google Scholar]
- 22.Fischer M, Kampen WU. Radionuclide therapy of bone metastases. Breast Care (Basel). 2012;7:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choudhury AD, Kantoff PW. New agents in metastatic prostate cancer. J Natl Compr Canc Netw. 2012;10:1403–1409. [DOI] [PubMed] [Google Scholar]
- 24.Lam MG, Hoekstra A, de Klerk JM, van Rijk PP, Zonnenberg BA. Radiation safety considerations for the bone seeking radiopharmaceuticals 89SrCl2, 186Re-HEDP and 153Sm-EDTMP. Nuklearmedizin. 2009;48:37–43. [PubMed] [Google Scholar]
- 25.Kurosaka S, Satoh T, Chow E, et al. EORTC QLQ-BM22 and QLQ-C30 quality of life scores in patients with painful bone metastases of prostate cancer treated with strontium-89 radionuclide therapy. Ann Nucl Med. 2012;26:485–491. [DOI] [PubMed] [Google Scholar]
- 26.Tu SM, Millikan RE, Mengistu B, et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet. 2001;357:336–341. [DOI] [PubMed] [Google Scholar]
- 27.Bilen MA, Johnson MM, Mathew P, et al. Randomized phase 2 study of bone-targeted therapy containing strontium-89 in advanced castrate-sensitive prostate cancer. Cancer. 2015;121:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Logan KW, Volkert WA, Holmes RA. Radiation dose calculations in persons receiving injection of samarium-153 EDTMP. J Nucl Med. 1987;28:505–509. [PubMed] [Google Scholar]
- 29.Singh A, Holmes RA, Farhangi M, et al. Human pharmacokinetics of samarium-153 EDTMP in metastatic cancer. J Nucl Med. 1989;30:1814–1818. [PubMed] [Google Scholar]
- 30.Turner JH, Claringbold PG, Hetherington EL, et al. A phase I study of samarium-153 ethylenediaminetetramethylene phosphonate therapy for disseminated skeletal metastases. J Clin Oncol. 1989;7:1926–1931. [DOI] [PubMed] [Google Scholar]
- 31.Turner JH, Claringbold PG. A phase II study of treatment of painful multifocal skeletal metastases with single and repeated dose samarium-153 ethylenediaminetetramethylene phosphonate. Eur J Cancer. 1991;27:1084–1086. [DOI] [PubMed] [Google Scholar]
- 32.Tripathi M, Singhal T, Chandrasekhar N, et al. Samarium-153 ethylenediamine tetramethylene phosphonate therapy for bone pain palliation in skeletal metastases. Indian J Cancer. 2006;43:86–92. [DOI] [PubMed] [Google Scholar]
- 33.Coronado M, Redondo A, Coya J, et al. Clinical role of Sm-153 EDTMP in the treatment of painful bone metastatic disease. Clin Nucl Med. 2006;31:605–610. [DOI] [PubMed] [Google Scholar]
- 34.Bayouth JE, Macey DJ, Kasi LP, Fossella FV. Dosimetry and toxicity of samarium-153-EDTMP administered for bone pain due to skeletal metastases. J Nucl Med. 1994;35:63–69. [PubMed] [Google Scholar]
- 35.Sartor O, Reid RH, Bushnell DL, Quick DP, Ell PJ. Safety and efficacy of repeat administration of samarium Sm-153 lexidronam to patients with metastatic bone pain. Cancer. 2007;109:637–643. [DOI] [PubMed] [Google Scholar]
- 36.Heron DE, Brufsky A, Beriwal S, Kurman M. Myelotoxicity of samarium Sm 153 lexidronam in patients receiving prior treatment with chemotherapy or radiotherapy. Ann Oncol. 2008;19:1639–1643. [DOI] [PubMed] [Google Scholar]
- 37.Baczyk M, Czepczynski R, Milecki P, Pisarek M, Oleksa R, Sowinski J. 89Sr versus 153Sm-EDTMP: comparison of treatment efficacy of painful bone metastases in prostate and breast carcinoma. Nucl Med Commun. 2007;28:245–250. [DOI] [PubMed] [Google Scholar]
- 38.Montesano T, Giacomobono S, Acqualagna G, et al. Our experience on pain palliation of bone metastasis with Sr-89 or Sm-153 in cancer patients resistant to a conventional analgesic therapy: a retrospective study. Clin Ter. 2009;160:193–199. [PubMed] [Google Scholar]
- 39.Pinto A, Cruz P. Radium-223 chloride: a new treatment option for metastatic castration-resistant prostate carcinoma. Drugs R D. 2012;12:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drake CG, Sharma P, Gerritsen W. Metastatic castration-resistant prostate cancer: new therapies, novel combination strategies and implications for immunotherapy. Oncogene. 2014;33:5053–5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bishr M, Saad F. Overview of the latest treatments for castration-resistant prostate cancer. Nat Rev Urol. 2013;10:522–528. [DOI] [PubMed] [Google Scholar]
- 42.Hafeez S, Parker C. Radium-223 for the treatment of prostate cancer. Expert Opin Investig Drugs. 2013;22:379–387. [DOI] [PubMed] [Google Scholar]
- 43.Malik Z, Payne H, Ansari J, et al. Evolution of the treatment paradigm for patients with metastatic castration-resistant prostate cancer. Adv Ther. 2013;30:1041–1066. [DOI] [PubMed] [Google Scholar]
- 44.Mukherji D, Omlin A, Pezaro C, Shamseddine A, de Bono J. Metastatic castration-resistant prostate cancer (CRPC): preclinical and clinical evidence for the sequential use of novel therapeutics. Cancer Metastasis Rev. 2014;33:555–566. [DOI] [PubMed] [Google Scholar]
- 45.Harrison MR, Wong TZ, Armstrong AJ, George DJ. Radium-223 chloride: a potential new treatment for castration-resistant prostate cancer patients with metastatic bone disease. Cancer Manag Res. 2013;5:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pacilio M, Ventroni G, De Vincentis G, et al. Dosimetry of bone metastases in targeted radionuclide therapy with alpha-emitting 223Ra-dichloride. Eur J Nucl Med Mol Imaging. 2016;43:21–33. [DOI] [PubMed] [Google Scholar]
- 47.Chittenden SJ, Hindorf C, Parker CC, et al. A phase 1, open-label study of the biodistribution, pharmacokinetics, and dosimetry of 223Ra-dichloride in patients with hormone-refractory prostate cancer and skeletal metastases. J Nucl Med. 2015;56:1304–1309. [DOI] [PubMed] [Google Scholar]
- 48.Dauer LT, Williamson MJ, Humm J, et al. Radiation safety considerations for the use of 223RaCl2 DE in men with castration-resistant prostate cancer. Health Phys. 2014;106:494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nilsson S, Strang P, Aksnes AK, et al. A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur J Cancer. 2012;48:678–686. [DOI] [PubMed] [Google Scholar]
- 50.Parker CC, Pascoe S, Chodacki A, et al. A randomized, double-blind, dose-finding, multicenter, phase 2 study of radium chloride (Ra 223) in patients with bone metastases and castration-resistant prostate cancer. Eur Urol. 2013;63:189–197. [DOI] [PubMed] [Google Scholar]
- 51.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. [DOI] [PubMed] [Google Scholar]
- 52.Kluetz PG, Pierce W, Maher VE, et al. Radium Ra 223 dichloride injection: U.S. Food and Drug Administration drug approval summary. Clin Cancer Res. 2014;20:9–14. [DOI] [PubMed] [Google Scholar]
- 53.Wissing MD, van Leeuwen FW, van der Pluijm G, Gelderblom H. Radium-223 chloride: extending life in prostate cancer patients by treating bone metastases. Clin Cancer Res. 2013;19:5822–5827. [DOI] [PubMed] [Google Scholar]
- 54.Romero Otero J, Garcia Gomez B, Campos Juanatey F, Touijer KA. Prostate cancer biomarkers: an update. Urol Oncol. 2014;32:252–260. [DOI] [PubMed] [Google Scholar]
- 55.Jadvar H, Quinn DI, Conti PS. One-year postapproval clinical experience with radium-223 dichloride in patients with metastatic castrate-resistant prostate cancer. Cancer Biother Radiopharm. 2015;30:195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Etchebehere EC, Milton DR, Araujo JC, Swanston NM, Macapinlac HA, Rohren EM. Factors affecting 223Ra therapy: clinical experience after 532 cycles from a single institution. Eur J Nucl Med Mol Imaging. 2016;43:8–20. [DOI] [PubMed] [Google Scholar]
- 57.Etchebehere EC, Araujo JC, Fox PS, Swanston NM, Macapinlac HA, Rohren EM. Prognostic factors in patients treated with 223Ra: the role of skeletal tumor burden on baseline 18F-fluoride PET/CT in predicting overall survival. J Nucl Med. 2015;56:1177–1184. [DOI] [PubMed] [Google Scholar]
- 58.Rohren EM, Etchebehere EC, Araujo JC, et al. Determination of skeletal tumor burden on 18F-fluoride PET/CT. J Nucl Med. 2015;56:1507–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fahey F, Zukotynski K, Jadvar H, et al. Proceedings of the Second NCI–SNMMI Workshop on Targeted Radionuclide Therapy. J Nucl Med. 2015;56:1119–1129. [DOI] [PubMed] [Google Scholar]