Abstract
Imaging of the Warburg effect, which is the principal but not the sole cause for enhanced glucose metabolism in tumors, with PET and 18F-FDG has become the mainstay for the imaging evaluation of several cancers. Despite the seemingly prevalent notion that 18F-FDG PET may not be useful in prostate cancer, relatively limited evidence suggests that this imaging modality can be useful for the evaluation of the extent of metastatic disease and the assessment of the therapy response and prognosis in men with castration-resistant prostate cancer. Incidental high focal 18F-FDG uptake in the prostate gland, although generally rare, may also indicate occult prostate cancer that may need to be further scrutinized. In general, 18F-FDG PET is not useful for initial staging and is of limited utility in the clinical setting of biochemical failure after prior definitive therapy for primary cancer. Although more experience is needed, it appears that the imaging of cellular proliferation with PET and 3′-deoxy-3′-18F-fluorothymidine or 2′-18F-fluoro-5-methyl-1-β-d-arabinofuranosyluracil may also allow for targeted biopsy and localization for focal therapy of aggressive prostate tumors as well as assessment of the therapy response to various standard and novel treatment regimens in patients with metastatic disease.
Keywords: PET/CT, PET, prostate cancer, 18F-FDG, 18F-FLT, 18F-FMAU
Interest in the potential role of PET with several radiotracers targeted to the underlying complex biology of prostate cancer has been increasing. The Warburg effect is a hallmark of cancer and can be reliably interrogated with PET and 18F-FDG. In fact, 18F-FDG PET has now become the mainstay for the imaging evaluation of several cancers. Another important biologic feature in cancer is cellular proliferation. The imaging of cellular proliferation can allow for tumor characterization and early objective assessment of the response to therapy. This article summarizes experience with the utility and limitations of PET for the imaging examination of glucose metabolism and cellular proliferation in prostate cancer.
GLUCOSE METABOLISM
It has often been stated that 18F-FDG PET is not useful in prostate cancer. However, this belief seems to have arisen from some early studies in which 18F-FDG PET was interrogated in the setting of primary tumor diagnosis or staging of the disease, for which the overall results were unsatisfactory (1–3). The utility of 18F-FDG PET appears to depend on the phase of the disease; therefore, it may be quite relevant in one phase of the disease but limited in another phase (4,5).
Incidental High Prostatic 18F-FDG Uptake in Primary Cancer Detection
Reesink et al. assessed the clinical relevance of incidental prostatic lesions with 18F-FDG PET and whether the findings should prompt additional evaluations (6). That investigation involved 108 consecutive men who had bladder cancer and underwent radical cystoprostatectomy. Incidental prostatic uptake was noted in 40% of the cohort; overall, occult prostate cancers were found in 23% of the surgical specimens. The positive and negative predictive values for findings labeled as suspect or indeterminate for prostate cancer were 29% and 79%, respectively. However, the authors’ final conclusions were that overall incidental prostatic uptake on 18F-FDG PET/CT had a low positive predictive value for prostate cancer and that the Gleason score did not correlate with the SUVmax or serum prostate-specific antigen (PSA).
In another study, involving 6,128 male patients who had undergone 18F-FDG PET scans, incidental prostatic 18F-FDG uptake was noted in 1.3% of the patients (7). There was no significant correlation between SUVmax or serum PSA levels and whether the lesions were benign or malignant. Brown et al. reported that focal incidental prostatic uptake with an SUVmax of greater than 6 should be further evaluated with multiparametric MRI (8). A recent systematic review and metaanalysis of 47,925 men in 6 studies reported a pooled prevalence of 1.8% (95% confidence interval [CI], 1.3%–2.3%) for incidental high 18F-FDG uptake in the prostate gland (9). The pooled risks of malignancy in patients who were further evaluated or underwent biopsy (444 patients with incidental prostatic uptake underwent further evaluation and 121 patients underwent biopsy) were 17% (95% CI, 12%–23%) and 62% (95% CI, 54%–71%), respectively. Kang et al. suggested that incidental prostatic uptake on 18F-FDG PET scans should not be ignored and that further investigation, such as PSA determination or additional imaging, should be undertaken; they made this suggestion despite the realization that the level of 18F-FDG accumulation can overlap in normal prostate, benign prostatic hyperplasia, and prostate cancer tissues, which often coexist (10).
Kwon et al. reported that, of 47,109 men who underwent 18F-FDG PET in a 10-y period between 2004 and 2014, 1,335 (2.83%) showed incidental prostatic 18F-FDG uptake and 99 of these men underwent prostate biopsy (11). Prostate cancer occurred in 3.8% of men with serum PSA levels of less than 2.5 ng/mL and in 59.7% of men with serum PSA levels of greater than or equal to 2.5 ng/mL. Multivariable analysis showed that focal lesions (odds ratio, 5.50; P = 0.038), age (odds ratio, 1.06; P = 0.031), and serum PSA levels (odds ratio, 1.28; P = 0.001) were independent predictors of prostate cancer diagnosis. The authors concluded that patients with high 18F-FDG uptake in the prostate should be further evaluated by the measurement of serum PSA and that those with high serum PSA levels should be considered for prostate biopsy. In another Japanese investigation, an incidental prostatic 18F-FDG uptake of 2% in 3,236 cases was reported (12). In the evaluable 49 cases, 16% had prostate cancer, whereas 84% were benign.
Initial Staging
There are few data on the use of 18F-FDG PET/CT for the initial staging of prostate cancer, given the general low avidity of 18F-FDG for primary prostate cancer. Liu reported on a retrospective study of 9 men (mean serum PSA level, 291 ng/mL; SD, 363 ng/mL; range, 6.1–980 ng/mL) who underwent 18F-FDG PET/CT at the time of initial staging of known primary prostate cancer (13). The standard of reference for the PET observations was biopsy, regional diagnostic CT, or whole-body bone scan. Although the sensitivity of 18F-FDG PET/CT for identifying primary cancer was only 33%, metastatic lymph nodes or bone lesions were also detected in 6 of the 9 patients. Liu concluded that, in general, 18F-FDG PET/CT may not be useful for the detection of primary cancer but may be useful for initial staging in certain subgroups of patients with high serum PSA levels.
Beauregard et al. performed 18F-FDG PET/CT for the staging workup of 44 patients with known Gleason sum scores of greater than or equal to 8 (i.e., aggressive tumors) (14). Foci suggesting high 18F-FDG uptake were found in the prostate gland, lymph nodes, and bone in 44%, 13%, and 6% of the patients, respectively. The absence or presence of intraprostatic 18F-FDG uptake was associated with a median cancer-free survival probability of 70.2% or 26.9% (P = 0.0097), respectively.
In the early analysis of the National Oncologic PET Registry data in the United States, involving 2,042 scans, for the initial staging of prostate cancer (the most common cancer type in the initial staging subgroup), 18F-FDG PET/CT had an impact on clinical management in 32% (95% CI, 30.0%–34.1%) of the patients (15).
Biochemical Recurrence
Localization of disease in patients with biochemical recurrence is essential, as it directs appropriate management, which may include salvage therapy with surgery or radiation for local recurrence, systemic therapy for metastatic disease, or both. The American Urologic Association defines biochemical recurrence in postprostatectomy patients as an initial serum PSA level of 0.2 ng/mL or higher, with a second, confirmatory level of greater than 0.2 ng/mL (16). The American Society for Therapeutic Radiology and Oncology consensus definition for biochemical failure after primary external-beam radiotherapy is an increase of 2 ng/mL or more above the nadir PSA level, regardless of hormonal therapy (17). Nonstandard imaging studies should only be considered when the results of standard imaging (99mTc-based bone scintigraphy or contrast-enhanced abdomen and pelvis CT) are negative or equivocal. Multiparametric MRI is also typically used to scrutinize the prostate bed.
In a study of 18F-FDG PET, a sensitivity of 75% and a specificity of 100% for the detection of pelvic lymph node metastases were reported; validation was based on histopathologic examination of the surgically harvested nodes (18). Jadvar et al. reported the findings of a prospective investigation on the potential utility of 18F-FDG PET/CT and 18F-NaF PET/CT for the detection of occult metastases in 37 men with PSA relapse (range, 0.5–40.2 ng/mL) and strictly negative results on standard imaging studies (19). The 18F-FDG PET/CT detection rate was only 8.1% in the setting of biochemical recurrence. In another recent investigation, involving 28 patients with PSA relapse after definitive primary therapy (82.1% radical prostatectomy and 17.9% external-beam radiation therapy), the sensitivity and specificity of 18F-FDG PET/CT were 61.6% and 75%, respectively (20). Schöder et al. reported a positive detection rate of 31% in this clinical setting (21). In another comparative study of 18F-FDG and 11C-choline, the sensitivities of 11C-choline and 18F-FDG were 60.6% and 31%, respectively (22). The sensitivities increased for both radiotracers, to 80% and 40%, respectively, when the serum PSA levels were greater than 1.9 ng/mL. On the basis of current experience, it appears that, in general, 18F-FDG PET has limited utility in this clinical setting.
Response Assessment in Metastatic Disease
Prostate cancer is a remarkably heterogeneous disease; therefore, a personalized approach to tailored treatment is most desired. Such an approach demands surrogate imaging markers that can portray the disease activity accurately before, during, and after treatment as well as dependence on specific response criteria that are used in data analysis, such as RECIST 1.0, RECIST 1.1, or PERCIST 1.0 (23,24). Tumor 18F-FDG uptake generally decreases with successful treatment (androgen deprivation or chemotherapy), although imaging findings may be discordant with those of other manifestations of disease, including changes in the levels of serum PSA or circulating tumor cells (25).
Simoncic et al. compared dynamic 18F-NaF and 18F-FDG PET/CT for assessment of the response to zibotentan in men with bone metastases from prostate cancer (26). Late (2-wk break after 4 wk of therapy, i.e., wk 6) 18F-NaF and 18F-FDG uptake responses were correlated, but earlier uptake responses (4 wk of therapy) were unrelated, suggesting that 18F-NaF uptake and 18F-FDG uptake in the setting of response assessment may be spatially disjointed and that these radiotracers may provide complementary information. Other studies have shown that 18F-FDG uptake in metastatic lesions declines with successful androgen deprivation therapy or chemotherapy (Fig. 1) (27,28). Although these preliminary studies are encouraging, there is clearly a need for additional experience in this clinical scenario.
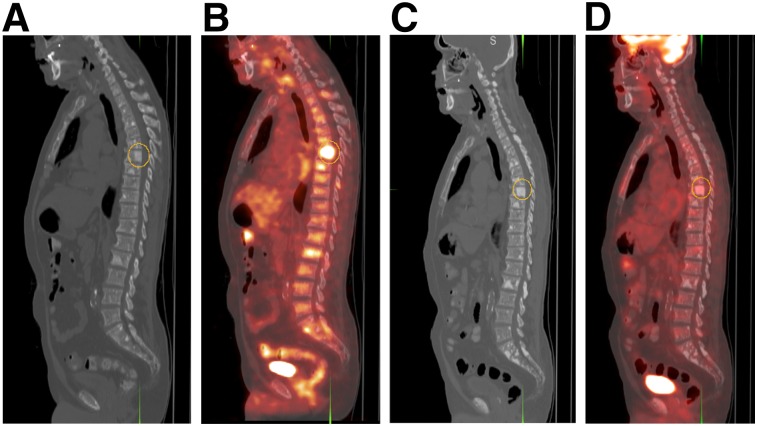
FIGURE 1.
69-y-old man with castration-resistant metastatic prostate cancer before (A and B) and after (C and D) treatment with docetaxel. Note general decline in metabolic activity in bone lesions (index thoracic spine lesion SUVmax, denoted by circular region of interest, declined from 5.0 to 2.1), compatible with favorable response to chemotherapy and concordant with decline in serum PSA level from 98 ng/mL before therapy to 21 ng/mL after therapy. (A and C) Sagittal CT at bone window level. (B and D) Fused 18F-FDG PET/CT.
Assessment of Prognosis
Recently, there has been increasing emphasis on the prognostic utility of various imaging studies in cancer, in terms of accuracy for the prediction of an outcome of interest, which can help with clinical management decisions and with assessment of the comparative effectiveness of various conventional and emerging treatment strategies. In the clinical setting of prostate cancer, these outcome measures may include, but are not limited to, time to biochemical recurrence (time to PSA progression), time to first metastasis, time to symptomatic progression, time to initiation of cytotoxic chemotherapy, time to radiographic progression, time to castration resistance state, progression-free survival, metastasis-free survival, disease-specific survival, and overall survival (29).
In an investigation of 42 men with primary prostate cancer, Oyama et al. showed that patients with higher primary tumor uptake had a significantly poorer prognosis than did patients with tumors that showed lower 18F-FDG uptake (30). Meirelles et al. compared the prognostic values of bone scans and 18F-FDG PET in a prospective imaging trial of 43 men with metastatic castration-resistant prostate cancer (31). Overall survival correlated inversely with the SUVmax of the osseous lesions, with median survival times of 14.4 mo for SUVmax of greater than 6.10 and 32.8 mo for SUVmax of less than or equal to 6.10 (P = 0.002). Although a calculated bone scan index was also prognostic (14.7 mo and 28.2 mo for bone scan indices of >1.27 and <1.27, respectively; P = 0.004), in the multivariate analysis, only SUVmax was an independent factor for predicting survival.
Jadvar et al. reported on a prospective study of 87 men who had metastatic castration-resistant prostate cancer, underwent 18F-FDG PET/CT, and were then monitored for overall survival (32). In the multivariate analysis after adjustment for prognostic clinical confounders (age, serum PSA level, serum alkaline phosphatase level, use of pain medication, prior chemotherapy, and Gleason score at initial diagnosis), the sum of the SUVmax for up to 25 metabolically active lesions (lymph node, bone, and soft-tissue metastases) was statistically significant, with a hazard ratio of 1.01 (95% CI, 1.001–1.020; P = 0.053), for predicting overall survival. Specifically, the moving hazard of death in relation to the sum of the SUVmax, interpreted as the chance of death per person per month, showed a marked upward shift of the curve (i.e., increased chance of death) for a sum of the SUVmax of greater than 20.
In another retrospective investigation, the association of CT patterns and glycolytic activity of prostate cancer bone metastases with overall survival was investigated in 38 patients (33). The number of lesions on CT or 18F-FDG PET, but not the intensity of 18F-FDG uptake, was associated with overall survival.
Aside from differences in methodology between the study of Jadvar et al. and the study of Vargas et al., the central hypothesis remains the same: that both the number of lesions and the intensity or aggressiveness of the “worst” lesion will be independent prognostic variables (34).
CELLULAR PROLIFERATION
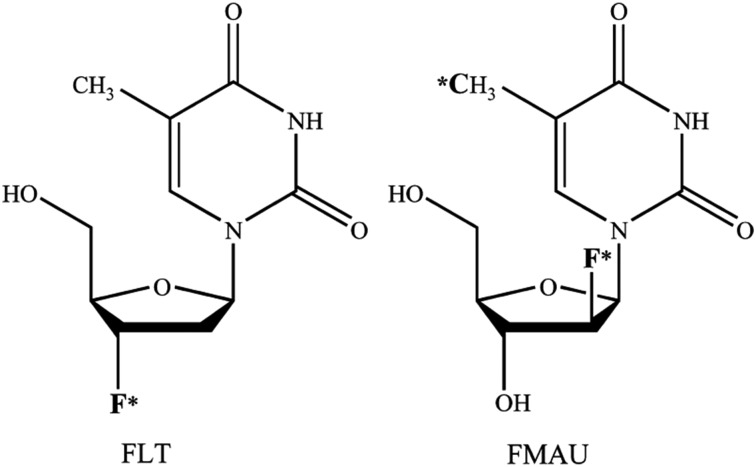
The imaging of cellular proliferation provides valuable information about the rate of tumor growth, which can be important in tumor characterization (e.g., indolent vs. aggressive), and early assessment of the response to therapy (35). PET in conjunction with radiotracers that track the thymidine salvage pathway of DNA synthesis has been studied for the noninvasive imaging–based assessment of cellular proliferation in cancer (36–38). Experience with 2 radiotracers that have been used in preclinical and pilot clinical studies of prostate cancer, 3′-deoxy-3′-18F-fluorothymidine (18F-FLT) and 2′-18F-fluoro-5-methyl-1-β-d-arabinofuranosyluracil (18F-FMAU), is briefly highlighted here (Fig. 2).
FIGURE 2.
Chemical structures of 18F-FLT and 18F-FMAU. F* denotes position of 18F for 18F-FLT and 18F-FMAU; *C denotes position of 11C for 11C-FMAU. (Reprinted with permission of (37).)
18F-FLT
18F-FLT is the most studied cellular proliferation PET tracer. It is phosphorylated by thymidine kinase 1, is retained in proliferating cells without DNA incorporation, and can be described with a 3-compartment model (39,40). Normal biodistribution is characterized by relatively high uptake in the liver and bone marrow, with the urinary bladder receiving the highest dose through renal excretion (41). Other than data from a few preclinical animal studies, few data on the potential utility of 18F-FLT in human prostate cancer are available, perhaps because of the high physiologic localization of 18F-FLT in normal bone marrow—the most common site for prostate tumor metastases. Nevertheless, a preclinical micro-PET study demonstrated a significant decline in 18F-FLT uptake after docetaxel treatment in 22Rv1 hormone-refractory prostate tumors implanted in athymic mice (42). The conclusion was that 18F-FLT might be a useful tracer for the early assessment of anticancer therapy with docetaxel in patients with castration-resistant prostate cancer.
18F-FMAU
18F-FMAU is phosphorylated by thymidine kinase and incorporated into DNA (43). Tehrani et al. showed that this thymidine analog was preferentially phosphorylated by mitochondrial thymidine kinase 2 rather than cytosolic thymidine kinase 1 (44). The fact that 18F-FMAU shows little accumulation in bone renders it an ideal PET radiotracer in prostate cancer (Fig. 3) (45). Jadvar et al. showed that there may be an association between androgen signaling and thymidine metabolism and that 18F-FMAU PET may be useful in prostate tumor characterization (46). A possible explanation may be the androgen control of mitochondrial function, which may include thymidine kinase 2 enzymatic activity (47). A pilot observational study of 18F-FMAU PET in 3 men with prostate cancer confirmed the tumor retention of 18F-FMAU in local prostate recurrence and in metastatic lesions with barely visible activity in the urinary bladder and normal bone (48). Moreover, on average, 95% of the blood activity cleared within 10 min after 18F-FMAU administration, and about 70% of the activity in the urine was intact 18F-FMAU at 60 min after injection. Jadvar et al. also recently initiated a pilot study to assess the potential utility of 18F-FMAU in image-targeted biopsy with software-based fusion of PET, transrectal ultrasound, and multiparametric MRI of the prostate gland (Fig. 4) (49). This hybrid imaging methodology may allow for improved localization and characterization of tumors for targeted biopsy and focal therapy (50).
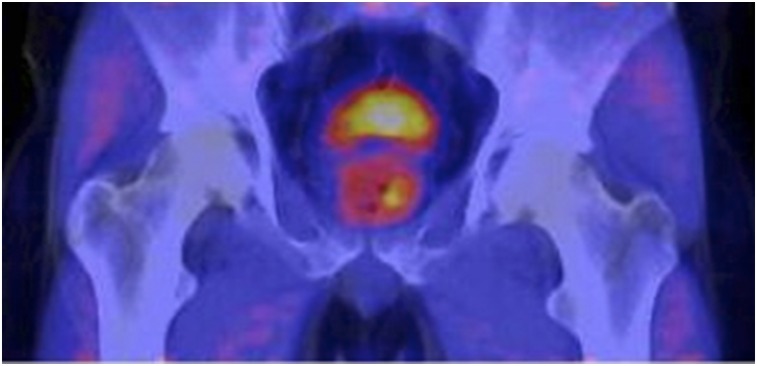
FIGURE 3.
Normal biodistribution of 18F-FMAU. Note relatively high tracer uptake in liver and renal cortex; moderate uptake in salivary glands, thyroid, heart, spleen, and urinary bladder; and relatively low uptake in bone marrow.
FIGURE 4.
61-y-old man who had elevated serum PSA level (10.5 ng/mL) and had undergone standard transrectal ultrasound biopsy, with negative results. Axial PET/CT with 18F-FMAU demonstrated focally increased tracer uptake in left base of prostate gland. PET/multiparametric MRI–directed biopsy revealed atypical small acinar proliferation suggestive of early malignancy.
CONCLUSION
Incidental high focal 18F-FDG uptake in the prostate gland is rare but may identify previously unknown prostate tumors. 18F-FDG PET is generally not useful for staging known disease and has limited value in patients with biochemical recurrence. Castration-resistant metastatic disease is often metabolically active, and limited evidence currently suggests that 18F-FDG PET may be useful for assessment of treatment response and prognosis. Imaging of cellular proliferation with 18F-FMAU may allow for the localization of aggressive primary tumors, which may then be amenable to focal therapy of localized prostate cancer.
DISCLOSURE
Hossein Jadvar was supported in part by National Institutes of Health grants R01-CA111613, R21-CA142426, R21-EB017568, and P30-CA014089. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Salminen E, Hogg A, Binns D, Frydenberg M, Hicks R. Investigations with FDG PET scanning in prostate cancer show limited value for clinical practice. Acta Oncol. 2002;41:425–429. [DOI] [PubMed] [Google Scholar]
- 2.Liu IJ, Zafar MB, Lai YH, et al. Fluorodeoxyglucose positron emission tomography studies in diagnosis and staging of clinically organ-confined prostate cancer. Urology. 2001;57:108–111. [DOI] [PubMed] [Google Scholar]
- 3.Hofer C, Laubenbacher C, Block T, et al. Fluorine-18-fluorodeoxyglucose positron emission tomography is useless for detection of local recurrence after radical prostatectomy. Eur Urol. 1999;36:31–35. [DOI] [PubMed] [Google Scholar]
- 4.Jadvar H. Molecular imaging of prostate cancer with [F-18] fluorodeoxyglucose PET. Nat Rev Urol. 2009;6:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jadvar H. Imaging evaluation of prostate cancer with 18F-fluorodeoxyglucose PET/CT: utility and limitations. Eur J Nucl Med Mol Imaging. 2013;40(suppl 1):S5–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reesink DJ, Fransen van de Putte EE, Vegt E, et al. Clinical relevance of incidental prostatic lesions on FDG-positron emission tomography/computerized tomography: should patients receive further evaluation? J Urol. November 17, 2015 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7.Sahin E, Elboga U, Kalender E, Basibuyuk M, Demir HD, Celen YZ. Clinical significance of incidental FDG uptake in the prostate gland detected by PET/CT. Int J Clin Exp Med. 2015;8:10577–10585. [PMC free article] [PubMed] [Google Scholar]
- 8.Brown AM, Lindenberg ML, Sankineni S, et al. Does focal incidental 18F-FDG uptake in the prostate gland have significance? Abdom Imaging. 2015;40:3222–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertagna F, Sadeghi R, Giovanella L, et al. Incidental uptake of 18F-fluorodeoxyglucose in the prostate gland: systematic review and meta-analysis on prevalence and risk of malignancy. Nuklearmedizin. 2014;53:249–258. [DOI] [PubMed] [Google Scholar]
- 10.Kang PM, Seo WI, Lee SS, et al. Incidental abnormal FDG uptake in the prostate on 18-fluoro-2-deoxyglucose positron emission tomography-computed tomography scans. Asian Pac J Cancer Prev. 2014;15:8699–8703. [DOI] [PubMed] [Google Scholar]
- 11.Kwon T, Jeong IG, You D, et al. Prevalence and clinical significance of incidental 18F-fluorodeoxyglucose uptake in prostate. Korean J Urol. 2015;56:288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seino H, Ono S, Miura H, et al. Incidental prostate 18F-FDG uptake without calcification indicates possibility of prostate cancer. Oncol Rep. 2014;31:1517–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y. Diagnostic role of fluorodeoxyglucose positron emission tomography-computed tomography in prostate cancer. Oncol Lett. 2014;7:2013–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beauregard JM, Blouin AC, Fradet V, et al. FDG-PET/CT for pre-operative staging and prognostic stratification of patients with high-grade prostate cancer at biopsy. Cancer Imaging. 2015;15:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillner BE, Siegel BA, Shields AF, et al. Relationship between cancer type and impact of PET and PET/CT on intended management: findings of the National Oncologic PET Registry. J Nucl Med. 2008;49:1928–1935. [DOI] [PubMed] [Google Scholar]
- 16.Cookson MS, Aus G, Burnett AL, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–545. [DOI] [PubMed] [Google Scholar]
- 17.Roach M, III, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendation of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. [DOI] [PubMed] [Google Scholar]
- 18.Chang CH, Wu HU, Tsai JJ, et al. Detecting metastatic pelvic lymph nodes by 18F-2-deoxyglucose positron emission tomography in patients with prostate specific antigen relapse after treatment for localized prostate cancer. Urol Int. 2003;70:311–315. [DOI] [PubMed] [Google Scholar]
- 19.Jadvar H, Desai B, Ji L, et al. Prospective evaluation of 18FNaF and 18F-FDG PET/CT in detection of occult metastatic disease in biochemical recurrence of prostate cancer. Clin Nucl Med. 2012;37:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Öztürk H, Karapolat I. 18F-fluorodeoxyglucose PET/CT for detection of disease in patients with prostate-specific antigen relapse following radical treatment of a local-stage prostate cancer. Oncol Lett. 2016;11:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schöder H, Herrmann K, Gönen M, et al. 2-[18F]fluoro-2-deoxyglucose positron emission tomography for the detection of disease in patients with prostate-specific antigen relapse after radical prostatectomy. Clin Cancer Res. 2005;11:4761–4769. [DOI] [PubMed] [Google Scholar]
- 22.Richter JA, Rodríguez M, Rioja J, et al. Dual tracer 11C-choline and FDG-PET in the diagnosis of biochemical prostate cancer relapse after radical treatment. Mol Imaging Biol. 2010;12:210–217. [DOI] [PubMed] [Google Scholar]
- 23.Jadvar H. Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J Nucl Med. 2011;52:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jadvar H, Desai B, Ji L, et al. RECIST 1.0, PERCIST 1.0 and PSA treatment response criteria in metastatic castrate-resistant prostate cancer [abstract]. In: Radiological Society of North America 99th Scientific Assembly and Annual Meeting; December 1–6, 2013; Chicago, IL.
- 25.Jadvar H, Desai B, Ji L, et al. Comparison of RECIST 1.0, PERCIST 1.0 and PCWG2 treatment response criteria in metastatic castrate-sensitive prostate cancer [abstract]. In: Society of Nuclear Medicine and Molecular Imaging Annual Meeting; June 6–10, 2015; Baltimore, MD.
- 26.Simoncic U, Perlman S, Liu G, Staab MJ, Straus JE, Jeraj R. Comparison of NaF and FDG PET/CT for assessment of treatment response in castrate-resistant prostate cancers with osseous metastases. Clin Genitourin Cancer. 2015;13:e7–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oyama N, Akino H, Suzuki Y, et al. FDG PET for evaluating the change of glucose metabolism in prostate cancer after androgen ablation. Nucl Med Commun. 2001;22:963–969. [DOI] [PubMed] [Google Scholar]
- 28.Jadvar H, Desai B, Quinn D, et al. Treatment response assessment of metastatic prostate cancer with FDG PET/CT [abstract]. J Nucl Med. 2011;52(suppl 1):1908. [Google Scholar]
- 29.Jadvar H. Prognostic utility of PET in prostate cancer. PET Clin. 2015;10:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oyama N, Akino H, Suzuki Y, et al. Prognostic value of 2-deoxy-2-[F-18]fluoro-d-glucose positron emission tomography imaging for patients with prostate cancer. Mol Imaging Biol. 2002;4:99–104. [DOI] [PubMed] [Google Scholar]
- 31.Meirelles GS, Schoder H, Ravizzini GC, et al. Prognostic value of baseline [18F]fluorodeoxyglucose positron emission tomography and 99mTc-MDP bone scan in progressing metastatic prostate cancer. Clin Cancer Res. 2010;16:6093–6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jadvar H, Desai B, Ji L, et al. Baseline 18F-FDG PET/CT parameters as imaging biomarkers of overall survival in castrate-resistant metastatic prostate cancer. J Nucl Med. 2013;54:1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vargas HA, Wassberg C, Fox JJ, et al. Bone metastases in castration-resistant prostate cancer: associations between morphologic CT patterns, glycolytic activity, and androgen receptor expression on PET and overall survival. Radiology. 2014;271:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jadvar H, Groshen SG, Quinn DI. Association of overall survival with glycolytic activity of castrate-resistant prostate cancer metastases. Radiology. 2015;274:624–625. [DOI] [PubMed] [Google Scholar]
- 35.Mankoff DA, Shields AF, Krohn KA. PET imaging of cellular proliferation. Radiol Clin North Am. 2005;43:153–167. [DOI] [PubMed] [Google Scholar]
- 36.Nimmagadda S, Shields AF. The role of DNA synthesis imaging in cancer in the era of targeted therapeutics. Cancer Metastasis Rev. 2008;27:575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bading JR, Shields AF. Imaging of cell proliferation: status and prospects. J Nucl Med. 2008;49(suppl 2):64S–80S. [DOI] [PubMed] [Google Scholar]
- 38.Tehrani OS, Shields AF. PET imaging of proliferation with pyrimidines. J Nucl Med. 2013;54:903–912. [DOI] [PubMed] [Google Scholar]
- 39.Shields AF, Briston DA, Chandupatla S, et al. A simplified analysis of [18F]3′-deoxy-3′-fluorothymidine metabolism and retention. Eur J Nucl Med Mol Imaging. 2005;32:1269–1275. [DOI] [PubMed] [Google Scholar]
- 40.Shields AF, Grierson JR, Dohmen BM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4:1334–1336. [DOI] [PubMed] [Google Scholar]
- 41.Vesselle H, Grierson J, Peterson LM, Muzi M, Mankoff DA, Krohn KA. 18F-fluorothymidine radiation dosimetry in human PET imaging studies. J Nucl Med. 2003;44:1482–1488. [PubMed] [Google Scholar]
- 42.Oyama N, Hasegawa Y, Kiyono Y, et al. Early response assessment in prostate carcinoma by 18F-fluorothymidine following anticancer therapy with docetaxel using preclinical tumor models. Eur J Nucl Med Mol Imaging. 2011;38:81–89. [DOI] [PubMed] [Google Scholar]
- 43.Sun H, Mangner TJ, Collins JM, Muzik O, Douglas K, Shields AF. Imaging DNA synthesis in vivo with 18F-FMAU and PET. J Nucl Med. 2005;46:292–296. [PubMed] [Google Scholar]
- 44.Tehrani OS, Douglas KA, Lawhorn-Crews JM, Shields AF. Tracking cellular stress with labeled FMAU reflects changes in mitochondrial TK2. Eur J Nucl Med Mol Imaging. 2008;35:1480–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shields AF. Positron emission tomography measurement of tumor metabolism and growth: its expanding role in oncology. Mol Imaging Biol. 2006;8:141–150. [DOI] [PubMed] [Google Scholar]
- 46.Jadvar H, Yap LP, Park R, et al. [18F]-2′-fluoro-5-methyl-1-beta-d-arabinofuranosyluracil (18F-FMAU) in prostate cancer: initial preclinical observations. Mol Imaging. 2012;11:426–432. [PMC free article] [PubMed] [Google Scholar]
- 47.Doeg KA, Polomski LL, Doeg LH. Androgen control of mitochondrial and nuclear DNA synthesis in male sex accessory tissue of castrate rats. Endocrinology. 1972;90:1633–1638. [DOI] [PubMed] [Google Scholar]
- 48.Sun H, Sloan A, Mangner TJ, et al. Imaging DNA synthesis with [18F]FMAU and positron emission tomography in patients with cancer. Eur J Nucl Med Mol Imaging. 2005;32:15–22. [DOI] [PubMed] [Google Scholar]
- 49.Jadvar H, Chen K, Ukimura O. Targeted prostate gland biopsy with combined TRUS, mpMRI and 18F-FMAU PET-CT. Clin Nucl Med. 2015;40:e426–e428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jadvar H. Imaging cellular proliferation in prostate cancer with positron emission tomography. Asia Ocean J Nucl Med Biol. 2015;3:72–76. [PMC free article] [PubMed] [Google Scholar]