Abstract
Background
Hematological reference values are important for the clinical decisions in laboratory diagnosis and monitoring of patients. The correct interpretation of laboratory results depends entirely on the reference intervals that have been established for the locality. But, in sub-Saharan African countries particularly in Ethiopia, locally derived reference intervals were not established and they are forced to use intervals established from western population. Thus this study aimed to establish locally derived hematological reference values that could be used in Northwest Ethiopia.
Methods
A cross sectional study was conducted from April to May 2014 with 120 male and 120 female apparently health adult blood donors at Gondar University Hospital. A structured pretested questionnaire was used for socio demographic and clinical data collection. About 4 ml of blood was collected with EDTA test tube and analyzed using Cell-Dyn 1800 to enumerate the hematological parameters. The data were collected and entered into SPSS version 20 for analysis. Mann–Whitney U test was used to determine reference intervals and Harris and Boyd test was used to determine the reference intervals that need partition. The 95th percentile of measurements was taken as a reference interval.
Results
Median and 95th percentile of WBC for general population were lower than Caucasian population, Addis Ababa, Burkina Faso and Kenya of similar studies. The RBC, Hgb and PCV lower 95% limit values of both sex were lower than studies in Addis Ababa, Kenya, Burkina Faso and text book. While PCV upper limit values higher than the above countries. MCV values of the current study were higher than those countries while MCHC values were lower. Similarly, the absolute values of neutrophils in the current study were lower than Caucasian and Afro Caribbean but higher than African countries and Jamaica but lymphocyte count was higher.
Conclusions
The hematological reference intervals established in this study was different from those reported in other part of Ethiopia or African countries as well as Caucasian population. The RBC, PCV, Hgb and MCHC reference intervals were different in gender. Thus, using of locally determined reference range is advisable.
Electronic supplementary material
The online version of this article (doi:10.1186/s13104-016-2288-8) contains supplementary material, which is available to authorized users.
Keywords: Hematological reference interval, Adult population, Gondar
Background
Hematological reference values are necessary in routine assessments for diagnosis of blood disorders, infectious diseases, immune status, and diseases progression and also in assessment of response to antiretroviral treatment. Locally-derived age-specific hematological reference ranges were also recommended for proper patient managements and interpretation of results [1].
In the use and interpretation of laboratory test results, it is important to understand the limitations of tests. These include the ability of the test to indicate the presence or absence of disease or whether the value in a report is normal or abnormal for a patient. These sounds the knowledge of reference value for a particular laboratory test is very crucial in patient management. The laboratory staff and those requesting tests should know the accepted reference ranges and clinical significance of the results of the quantitative tests performed in the laboratory. This will ensure that significantly abnormal results are detected, checked, reported, and acted on as soon as possible [2].
There is a difference in reference ranges related to age, sex, geographic origin, altitude, and ethnic background in addition to hemoglobin abnormalities (thalassemia, sickle cell disease and hemoglobin C) or pathologic conditions (malaria, HIV and other viral infections) prevented to have one common hematological values for all countries [2–7]. Thus, the use of normal hematological values derived from other African countries could result in incorrect patient management in routine clinical care.
The reference intervals for analysis of hematological parameters during routine clinical activities in Ethiopia or Africa are reference texts generated from Caucasian adult populations living in developed countries [8]. Lower values for hemoglobin, red blood cells, hematocrit, mean corpuscular volumes, platelets and neutrophils and higher monocytes and eosinophils levels were reported in African population compared to similar population in Western counter parts [6, 9–12]. Also there are ample evidences that clinicians and medical researchers should use method-specific reference ranges in their laboratories which account for gender differences and variances in the ethnic composition of the local society [13], but this is not done in developing countries.
An increasing number of clinical trials taking place in Africa are seeking to identify safe and effective prevention and treatment strategies to combat the heavy burden of infectious diseases in this region but there is only attempts to establish standard reference hematological intervals [14], there is lack of well-established reference intervals.
In the absence of locally derived reference intervals, clinicians and researchers have to use reference values of western population’s, thus it is important to establish local hematological reference intervals for appropriate diagnosis, treatment, and follow up of patients. The reference intervals that are currently used in Ethiopia are adopted from textbooks that refer mainly to Caucasian subjects. But one study in Ethiopia conducted in southeast Addis Ababa showed lower WBC and platelet values of healthy HIV negative Ethiopians than the adopted reference values [15]. Thus, the main aim of this study was to establish locally derived hematological reference values that could be used in Northwest Ethiopia.
Methods
Study setting and population
A cross sectional study was conducted from April to June 2014 at Gondar University Hospital blood banking center. The hospital is located in Gondar town which is 740 km from the capital of Ethiopia, Addis Ababa, in the Northwest of Ethiopia. The city has a latitude and longitude of 12°36′N and 37°28′E with an elevation of 2133 meters above sea level. Based on figures from the Central Statistical Agency of Ethiopia in 2007, Gondar has an estimated total population of 231,977. Gondar University Hospital is the only referral hospital with more than 400 beds for North West Ethiopia serving a population of about 5 million. The consecutive nonparametric method was based solely on the ranks of the observations (in order of magnitude) and ignores their measured values. According to the National Committee for Clinical Laboratory Standards (NCCLS), International Federation of Clinical Chemistry (IFCC) and Clinical Laboratory Standards Institute (CLIS) Guideline recommendations, a minimum size of 120 observations from each category is needed [16]. Based on this guide line 120 male and 120 female study participants who pass the exclusion/inclusion criteria were selected conveniently. The reference individuals were selected based on medical examinations, current health status, blood pressure, taking any medication, working with hazardous chemicals, alcohol intake, presence of inherited health disorder in the family, tuberculosis, lymphadenopathy, weight loss, regular exercise, tobacco smoking, allergy manifestation, fever, malaria, HIV, hepatitis B virus surface antigen (HbsAg), hepatitis C virus antibodies (HcAbs), menstruation, pregnancy and use of contraceptives. After written informed consents were taken from study subjects, 4 ml of venous blood samples were collected immediately following the donation in tube containing EDTA and 4 ml bloods with plan tube for serological analysis was taken and proceed.
Laboratory investigation
About 4 ml of venous blood was collected by an experienced laboratory technologist from each subject for hematological parameters analysis. Hematological parameters; total white cell count (WBC), differential white cell count (neutrophils, lymphocytes, eosinophils, monocytes and basophiles), platelet count, red blood cell count (RBC), hemoglobin (Hgb), hematocrit (%), mean cell volume (MCV), mean cell hemoglobin (MCH), mean cell hemoglobin concentration (MCHC), and red cell distribution width (RDW) were determined using the automated blood analyzer Cell-Dyne 1800 (Abbott Laboratories Diagnostics Division, USA).The other 4 ml of blood after clotting the serum was separated and serological tests (HIV, HbsAg, and HcAbs) was assayed. Erythrocyte sedimentation rate (ESR) was done by using westerngreen method for 1 h.
Statistical analysis
Data was cleaned, edited, checked for completeness and entered into Epi-Info version 3.3.5 and then transferred to SPSS version 20 for statistical analysis. The mean, median and standard deviation of hematological reference intervals were determined using descriptive statistics. Mann–Whitney U test was used to determine reference intervals and also Harris and Boyd test was used to determine the reference intervals that need partition (Table 2). The 95th percentile was taken as a reference interval.
Table 2.
Result of Harris and Boyd test, which was performed to see the need for partitioning of Reference interval based on sex
| Parameters | P value | Harris and Boyd | ||
|---|---|---|---|---|
| Z* | Z† | Decision | ||
| WBC (×109/l) | 0.3779 | 3 | 0.4 | No separate RI |
| Neutrophil (%) | 0.7239 | 3 | 0.6 | No separate RI |
| Neutrophils absolute (×109/l) | 0.6331 | 3 | 0.01 | No separate RI |
| Lymphocyte (%) | 0.6750 | 3 | 0.5 | No separate RI |
| Lymphocytes absolute (×109/l) | 0.1705 | 3 | 0.9 | No separate RI |
| Mixed (%) | 0.5375 | 3 | 0.5 | No separate RI |
| Mixed absolute (×109/l) | 0.2784 | 3 | 0.5 | No separate RI |
| Platelet (×109/l) | 0.0223 | 3 | 2.3 | No separate RI |
| RBC (×1012/l) | 0.0016 | 3 | 3.4 | Separate RI |
| Hgb (g/dl) | <0.0001 | 3 | 5.6 | Separate RI |
| PCV (%) | 0.0033 | 3 | 3.4 | Separate RI |
| MCH (pg) | 0.0016 | 3 | 3.1 | Separate RI |
| MCHC (g/dl) | <0.0001 | 3 | 5.0 | Separate RI |
| MCV (fl) | 0.2929 | 3 | 0.3 | No separate RI |
| RDW | 0.9577 | 3 | 0.6 | No separate RI |
| ESR (mm/h) | 0.3283 | 3 | 0.9 | No separate RI |
Z† = critical value, Z* = calculated value, Separate RI are needed only when Z† is greater than Z*, P value is derived from Mann–Whitney U test
Italic values indicate hematological parameters which need partition between male and female (Z† > Z*)
Results
Socio demographic characteristics
A total of 120 male and 120 female adult healthy subjects from Gondar University Hospital Blood Bank Centre were included in this study. The mean age of the study subjects was 24.50 ± 6.65 years with age range from 18 to 50 years and about 69.6% were within age range of 18–25 years. From the study subjects 78.3% were living in Gondar town, 26.7% were married, 58.3% were students and 89.2% were orthodox in religion (Table 1).
Table 1.
The socio demographic characteristics of adult of Gondar participated in the study
| Variables | Socio demographic characteristics | Frequency | Percentage (%) |
|---|---|---|---|
| Sex | Male | 120 | 50 |
| Female | 120 | 50 | |
| Age group (years) | 18–25 | 167 | 69.6 |
| 26–35 | 51 | 21.2 | |
| >35 | 22 | 9.2 | |
| Address | Gondar | 188 | 78.9 |
| Around gondar | 52 | 21.1 | |
| Marital status | Married | 64 | 26.7 |
| Single | 174 | 72.5 | |
| Divorced | 2 | 0.8 | |
| Religion | Orthodox | 214 | 89.2 |
| Muslim | 22 | 9.2 | |
| Protestant | 1 | 0.4 | |
| Others | 3 | 1.2 | |
| Occupation | Civil servant | 21 | 8.8 |
| Private | 36 | 15.0 | |
| House wife | 6 | 2.5 | |
| Farmer | 37 | 15.4 | |
| Students | 120 | 58.3 |
Hematological reference interval
In order to determine hematological parameters that need combined or partition reference interval based on sex, Harris and Boyd test was used. Basing this test partitioned reference interval was determined for RBC, PCV, Hgb, MCH and MCHC (Table 2). Median and 95th percentile of WBC, absolute neutrophils, absolute lymphocytes, absolute mixed cell, platelet, MCV, RDW and ESR were 5.1 (3.2–8.8 × 109/l), 2.7 (1.6–5.1 × 109/l), 1.9 (1–3.5 × 109/l), 0.5 (0.2–1 × 109/l), 264 (128–432 × 109/l), 92 (85–100 fl); 14 (12–17%) and 5 (0–15 mm/h) respectively for the general population (Table 3).
Table 3.
Median, IQR, range and 95th percentile of reference interval, P values of hematological parameters for adult in Gondar, northwestern Ethiopia
| Parameters | N | Median (IQR) | Range (min–max) | RI (95th percentile) | Lower limit 90% CI | Upper limit 90% CI |
|---|---|---|---|---|---|---|
| WBC (×109/l) | 240 | 5.1 (4.2–6.3) | 2.7–9.5 | 3.2–8.8 | 3.2 (2.9–3.5) | 8.8 (8.3–9.1) |
| Neutrophil (%) | 240 | 53 (48–58) | 30–77 | 36–69 | 36 (33.8–39.1) | 69 (66.9–76.5) |
| Neutrophils absolute (×109/l) | 240 | 2.7 (2.1–3.5) | 1.3–7.3 | 1.6–5.1 | 1.6 (1.3–1.7) | 5.1 (4.6–6.2) |
| Lymphocyte (%) | 240 | 38 (33–42) | 17–56 | 22–55 | 22 (18.2–24.9) | 55 (52.4–55.9) |
| Lymphocytes absolute (×109/l) | 240 | 1.9 (1.5–2.3) | 0.70–5.0 | 1–3.5 | 1 (0.8–1.1) | 3.5 (3.2–4.2) |
| Mixed (%) | 240 | 9 (8–11) | 4–18 | 6–13 | 6 (5.4–6.4) | 13 (13.0–18.0) |
| Mixed absolute (×109/l) | 240 | 0.50 (0.4–0.6) | 0.2–1.2 | 0.2–1 | 0.2 (0.2–0.3) | 1.0 (0.9–1.2) |
| MCV (fl) | 240 | 92 (90–94) | 78–105 | 85–100 | 85 (80.3–85.1) | 100 (99–102) |
| Platelet (×109/l) | 240 | 264 (210–312) | 110–531 | 128–432 | 128 (115–140) | 432 (403–496) |
| ESR (mm/h) | 240 | 5.0 (0–5) | 0–20 | 0–15 | 0 (0–0) | 15 (15–20) |
| RDW | 240 | 14 (13–14) | 12–18 | 12–17 | 12 (12–12.7) | 17 (15.5–18.3) |
| RBC (×1012/l) | ||||||
| Male | 120 | 5.01 (4.56–5.59) | 3.12–6.95 | 3.53–6.93 | 3.53 (3.12–3.9) | 6.93 (6.45–6.95) |
| Female | 120 | 4.8 (4.2–5.25) | 3.0–6.50 | 3.45–6.25 | 3.45 (3.0–3.57) | 6.25 (6.08–6.5) |
| Hgb (g/dl) | ||||||
| Male | 120 | 14.2 (13.1–15.5) | 11.0–19.1 | 11.5–18.0 | 11.5 (11–11.9) | 18.0 (17.9–19.1) |
| Female | 120 | 12.9 (12.0–14.3) | 10.9–16.9 | 11.0–16.7 | 11.0 (10.9–11.3) | 16.7 (16.2–16.9) |
| PCV (%) | ||||||
| Male | 120 | 46.9 (43.2–50.0) | 34.5–60.2 | 36.2–58.6 | 36.2 (34.5–37.6) | 58.6 (57.0–60.2) |
| Female | 120 | 45.2 (40.4–48) | 30.8–59.0 | 32.1–56.6 | 32.1 (30.8–36) | 56.6 (52.1–59) |
| MCH (pg) | ||||||
| Male | 120 | 29.0 (28–30) | 26–34.0 | 26.6–33.3 | 26.6 (25.7–27.1) | 33.3 (32–34) |
| Female | 120 | 28.6 (27.7–29.2) | 25.6–33.1 | 25.8–32.8 | 25.8 (25.6–26.4) | 32.8 (31–33.1) |
| MCHC (g/dl) | ||||||
| Male | 120 | 31.3 (30.6–32.4) | 28.0–36.5 | 29.5–34.4 | 29.5 (28–30) | 34.4 (33.6–36.5) |
| Female | 120 | 30.8 (30.0–31.2) | 28.0–34.7 | 28.5–34.4 | 28.5 (28–29) | 34.4 (32.5–34.7) |
Box and Whisker plots indicate the effect of sex on hematological parameters of the study participants (Fig. 1). The median and 95th percentile reference intervals for RBC, Hgb, PCV, MCH and MCHC were 5.01 (3.53–6.93 × 1012/l), 46.9 (36.2–58.6%), 14.2 (11.5–18 g/dl) and 31.3 (29.5–34.4 g/dl) respectively for males and 4.8 (3.45–6.25 × 1012/l), 45.2 (32.1–56.6%), 12.9 (11–16 g/dl) and 30.8 (28.5–34.4 g/dl) respectively for females (Table 3).
Fig. 1.
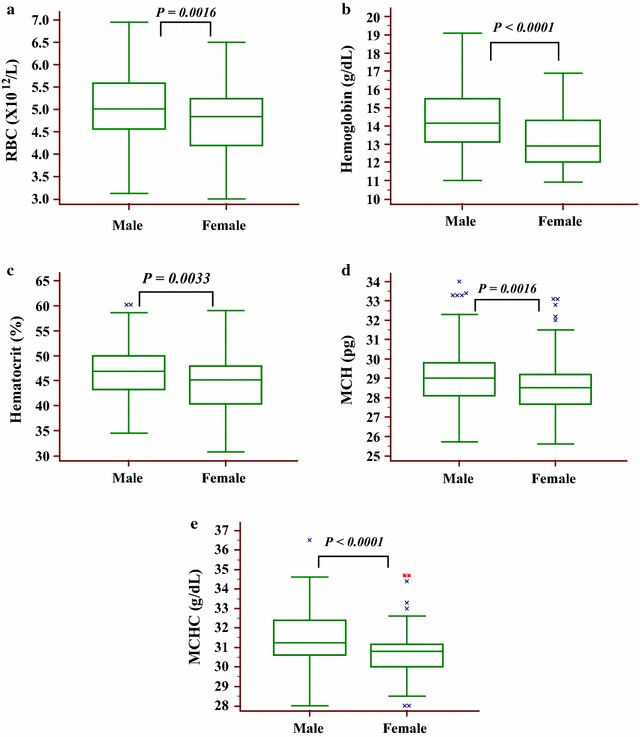
Box and Whisker plots indicating the effect of sex on hematological parameters of the study participants. a Red blood cells, b Hemoglobin, c Hematocrit, d Mean corpuscular hemoglobin, e Mean corpuscular hemoglobin concentration
Discussion
This study showed a significant gender differences for the RBC parameters (RBC, Hgb, PCV, MCH and MCHC). The finding agrees with the well-established fact that males have higher values than female. While there was no significant differences between genders with regard to WBC parameters, platelet counts, ESR, MCV and RDW. This was also in agreement with previously carried out studies in Addis Ababa and other African countries [1, 6, 15, 17–19] (Tables 4, 5 and 6). The significant difference between male and female may be due to biological and physiological factors such as the influence of the hormone androgen on erythropoiesis and also due to menstrual blood loss in females
Table 4.
Comparison of median, 95% reference range and Mean ± SD of hematological parameters in this study and others
| Parameter | Gondar (current study) | Addis Ababa [17] | Kenya [6] | Burkina Faso (22) | Wintrobe [2] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Median (95% range) | Mean ± SD | Median (95% range) | Mean ± SD | Median (95% range) | Mean ± SD | Median (95% range) | Mean ± SD | Median (95% range) | Mean ± SD | |
| WBC (×109) | Female | 5.1 (3.2–8.8) | 5.3 ± 1.4 | 5.9 (3–12.2) | 6.2 ± 2.2 | 4.9 (3.0–9.1) | NA | 5.2 (3.4–7.4) | NA | NA (4.3–10) | 7.2 ± NA |
| Male | 5.9 (3–9.8) | 6.0 ± 1.8 | 4.3 (2.7–7.5) | NA | 5.1 (3.2–9.2) | NA | NA (4.3–100) | 7.2 ± NA | |||
| Platelet (×109) | Female | 264 (128–432) | 267 ± 76 | 193 (98–352) | 202 ± 67 | 251 (124–444) | NA | 252 (159–356) | NA | NA (150–450) | NA |
| Male | 203 (97–324) | 207 ± 62 | 218 (115–366) | NA | 217 (127–365) | NA | NA (150–450) | NA | |||
| Neutrophil (absolute) (×109) | Female | 2.7 (1.6–5.1) | 2.8 ± 0.9 | NA | NA | 1.96 (0.99–5.6) | NA | NA | NA | NA | NA |
| Male | NA | NA | 1.78 (0.88–4.3) | NA | NA | NA | NA | NA | |||
| Neutrophil (%) | Female | 53 (36–69) | 53 ± 8 | NA | NA | 42 (20–70) | NA | 46 (30–62) | NA | NA | NA |
| Male | NA | NA | 42 (20–70) | NA | 43 (27–64) | NA | NA | NA | |||
| Lymphocyte (absolute) (×109) | Female | 1.9 (1–3.5) | 2 ± 0.7 | 1.7 (1.09–3.5) | NA | 2.16 (1.29–4.0) | NA | 2.2 (1.4–3.2) | NA | NA | NA |
| Male | (0.96–3.5) | NA | 1.86 (1.12–3.2) | NA | 2.1 (1.3–4.0) | NA | NA | NA | |||
| Lymphocyte (%) | Female | 38 (22–55) | 37 ± 8 | (17–59) | 36 ± NA | 45 (20–60) | NA | 43 (27–57) | NA | NA | NA |
| Male | (17–59) | 36 ± NA | 45 (20–60) | NA | 44 (26–60) | NA | NA | NA | |||
| Mix cells (absolute) (×109) | Female | 0.5 (0.2–1) | 0.5 ± 0.2 | NA | NA | NA | NA | NA | NA | NA | NA |
| Male | NA | NA | NA | NA | NA | NA | NA | NA | |||
| Mixed cells (%) | Female | 9 (6–13) | 10 ± 2 | NA | NA | NA | NA | NA | NA | NA | NA |
| Male | NA | NA | NA | NA | NA | NA | NA | NA | |||
WBC white blood cells, SD standard deviation, NA not applicable
Table 5.
Comparison of median, 95% reference range and Mean ± SD of hematological parameters in this study and others
| Parameter | Gondar (current study) | Addis Ababa [17] | Kenya [6] | Burkina Faso (22) | Wintrobe [2] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median (95% range) | Mean ± SD | Median (95% range) | Mean ± SD | Median (95% range) | Mean ± SD | Median (95% range) | Mean ± SD | Median (95% range) | Mean ± SD | |
| RBC (×1012) | ||||||||||
| Female | 4.8 (3.45–6.25) | 4.7 ± 0.725 | 4.5 (3.7–5.2) | 4.5 ± 0.4 | 4.8 (3.7–5.6) | NA | 4.2 (3.5–4.9) | NA | NA (4.2–5.5) | 4.8 ± NA |
| Male | 5.01 (3.53-6.93) | 0.09 ± 0.79 | 5 (4.3–5.9) | 5.1 ± 0.4 | 5.3 (4.4–6.3) | NA | 4.7 (3.8–5.6) | NA | NA (4.5–6.3) | 5.4 ± NA |
| PCV (%) | ||||||||||
| Female | 45.2 (32.1–56.6) | 44.2 ± 5.84 | 42.1 (35.3–48.8) | 42 ± 3.2 | 40 (30–50) | NA | 35 (30–39) | NA | NA (37–47) | 42 ± NA |
| Male | 46.9 (36.2–58.6) | 6.8 ± 5.8 | 48.2 (41.6–55.11) | 48.3 ± 3.7 | 47 (40–50) | NA | 39 (34–46) | NA | NA (41–51) | 46 ± NA |
| Hgb (g/dl) | ||||||||||
| Female | 12.9 (11–16.7) | 13.2 ± 1.6 | 14.9 (12.2–16.6) | 14.3 ± 3.4 | 8.44 (5.9–10) | NA | 12 (9.8–13.5) | NA | NA (12–16) | 14 ± NA |
| Male | 14.2 (11.5–18) | 14.4 ± 1.8 | 16.1 (13.9–18.3) | 16.1 ± 1.1 | 9.9 (8.3–11.3) | NA | 13.5 (11.3–15.6) | NA | NA (14–18) | 16 ± NA |
| MCV (fl) | ||||||||||
| Female | 92 ± 3.7 | NA | NA | 84.9 (66–95.7) | NA | 84 (75–92) | NA | NA | NA | |
| Male | 92 (85 –100) | NA | NA | 88 (71.4–98.2) | NA | 85 (73–94) | NA | NA | NA | |
| MCH (pg) | ||||||||||
| Female | 28.6 (25.8–32.8) | 28.5 ± 1.46 | NA | NA | NA | NA | 28 (24–32) | NA | NA | NA |
| Male | 29 (26.6–33.3) | 29.1 ± 1.54 | NA | NA | NA | NA | 29 (25–34) | NA | NA | NA |
| MCHC (g/dl) | ||||||||||
| Female | 30.8 (28.5–34.4) | 30.6 ± 1.2 | NA | NA | 33.9 (32.2–35.2) | NA | 34.2 (31.4–36.1) | NA | NA | NA |
| Male | 31.3 (29.5–34.4) | 31.5 ± 1.3 | NA | NA | 34.2 (32.4–35.3) | NA | 34.6 (31.7–36.3) | NA | NA | NA |
Table 6.
Comparisons of median and 95% reference range with Eastern and Southern Africa (18), Tanzania (19), Togo (20) and Uganda (21) of African countries
| Parameters | Gondar | Togo | Tanzania | Uganda | Eastern and southern Africa |
|---|---|---|---|---|---|
| Median (95% range) | Median (95% range) | Median (95% range) | Median (95% range) | Median (95% range) | |
| WBC (×109/l) | 5.1 (3.2–8.8) | 4.1 (1.9–10.1) | 4.6 (3.0–7.9) | 4.9 (2.8–8.2) | 3.1–9.1 |
| Neutrophils (Abs) | 2.7 (1.6–5.1) | 1.6 (0.5–5.4) | 2.2 (1.1–4.7) | NA | 1.0–5.3 |
| Neutrophils (%) | 53 (36–69) | NA | 48.1 (32.0–69.1) | 38.9 (22.2–59.3) | 25–66 |
| Lymphocyte (Abs) | 1.9 (1–3.5) | 2.1 (1.1–4.3) | 1.8 (1.1–3.0) | NA | 1.2–3.7 |
| Lymphocyte (%) | 38 (22–55) | NA | 40.5 (20.8–56.7) | 44.4 (26.7–61.2) | 23–59 |
| Mixed (Abs) | 0.5 (0.2–1) | NA | 0.5 (0.3–1.1) | NA | NA |
| Mixed (%) | 9 (6–13) | NA | 10.3 (5.6–19.8) | NA | NA |
| Platelet (×109/l) | 264 (128–432) | 239 (120–443) | 244 (150–398) | 218.5 (109–384) | 126–438 |
| MCV (fl) | 92 (85–100) | 84.5 (80–99) | 89.4 (77.6–98.1) | 85 (71–97) | 68–98 |
| RBC (×1012/l) | |||||
| Male | 4.8 (3.45–6.25) 5.0 | 5.0 (3.3–6.4) | 5.21 (4.41–6.27) | 5.0 (3.8–6.1) | 4.0–6.4 |
| Female | 1 (3.53–6.93) | 4.5 (3.1–6.0) | 4.69 (3.84–5.59) | 4.4 (3.3–5.3) | 3.8–5.6 |
| Hgb (g/dl) | |||||
| Male | 12.9 (11–16.7) | 15.8 (10.0–18.4) | 15.4 (13.7–17.7) | 14.5 (11.6–17.1) | 12.2–17.7 |
| Female | 14.2 (11.5–18) | 13.0 (10.3–17.1) | 13.5 (11.1–15.7) | 12.8 (9.8–16.2) | 9.5–15.8 |
| PCV (%) | |||||
| Male | 45.2 (32.1–56.6) | 42.8 (28.0–54.0) | 46.6 (40.2–53.7) | 42.6 (33.8–49.5) | 35.0–50.8 |
| Female | 46.9 (36.2–58.6) | 38.1 (28.0–47.0) | 41.5 (36.2–46.8) | 37.8 (28.3–46.8) | 29.4–45.4 |
| MCH (pg) | |||||
| Male | 28.6 (25.8–32.8) | 29.7 (26–36) | 30.0 (23.1–33.2) | 29.2 (23.0–33.8) | NA |
| Female | 29 (26.6–33.3) | 29.3 (25–37) | 29.3 (24.2–33.1) | 29.5 (24.8–32.7) | NA |
| MCHC (%) | |||||
| Male | 30.8 (28.5–34.4) | 35.1 (29–39) | 33.3 (30.6–35.1) | 34.2 (32.4–35.3) | NA |
| Female | 31.3 (29.5–34.4) | 35.1 (30–41) | 32.7 (30.4–34.8) | 34.2 (33.0–35.5) | NA |
| RDW (%) | |||||
| Male | 14 (12–17) | NA | NA | 12.8 (10.9–16.8) | NA |
| Female | 14 (12–17) | NA | NA | 12.7 (11.0–17.3) | NA |
Abs absolute
The 95th percentile reference interval of the current study lower limit of RBC in both sexes, were lower than studies conducted in Addis Ababa Ethiopia, Kenya, Burkina Faso and text books while the upper limit was higher than the above mentioned studies (Tables 4, 5 and 6). Comparison of hematological results with this study indicated that similarity could not be found based on geographical proximity, this may be due to difference in altitude, method and instrument used for analysis [6, 8, 15, 18].
The lower limit of hemoglobin reference interval in this study was lower than a study conducted in Addis Ababa and text books while higher than studies conducted in Kenya and Burkina Faso for both genders. On the other hand the upper limit of Hgb was higher than Addis Ababa, text books, Kenya, and Burkina Faso. This difference may be due to altitude and ethnic variations. Thus, it may not be possible, even, to have one standard reference between two localities in the same county as experienced in Kenya. Similarly the lower limit reference intervals of PCV in both sexes was lower than study conducted in Addis Ababa, text books and higher than studies conducted in Kenya and Burkina Faso. While the upper limit was higher than studies carried out in Addis Ababa, Kenya, Burkina Faso and text books [6, 8, 15, 18]. Likewise of RBC and Hgb the difference in the reference interval values might arise from differences in altitude and ethnical variations.
The current study reference interval values of MCV are generally higher than values indicated in different African countries. The lower limit values of MCH in both sexes in this study was higher than a study conducted in Burkina Faso, but the upper limit was comparable, but the established values of MCHC in both sexes was generally lower than values indicated in different African countries [6, 18]. The WBCs reference intervals established for the general population in the current study was lower than the values described for Caucasian population in different text books, Addis Ababa, Burkina Faso and Kenya. However, median and 95 percentile ranges of neutrophils and lymphocytes of this study compared with similar study conducted on Caucasian, African, Afro-Caribbean and Jamaican population, neutrophils were lower than Caucasian and Afro-Caribbean, but higher than African and Jamaica. Lymphocyte count in the current study was higher than Caucasian and African black population [6, 8, 15, 18]. The difference between the current result and other findings might be due to geographical differences, environment, diet, and ethnic background [16].
The median and 95th percentile range values of ESR was at normal range with no difference for male and female as all the recruited subjects were healthy, not exposed to inflammatory conditions associated with systemic inflammatory diseases [20].
Conclusions
Haematological reference intervals established from adult healthy population of Northwest Ethiopia (Gondar and surrounding areas) were different from Caucasian and African counties. The haematological intervals were also different from previous results obtained in other part of Ethiopia. Intervals for red blood cell count, hematocrit values, hemoglobin concentration, MCH and MCHC were different for male and female but there was no difference in WBC parameters, PLT, MCV, RDW and ESR.Further studies on haematological intervals for all age groups are recommended for locally derived standard haematological intervals (Additional file 1).
Authors’ contributions
AY: Recruited the patients, collected and analyzed the data and wrote the draft of the manuscript. BE: Conceived the study, obtained ethics papers, supervised the collection of data and revised the draft. BT: Involve in proposal development, data collection and entry for analysis. MA: Interpreted the collected data and wrote the draft along with BE. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to extend their appreciation to Gondar University Hospital, Blood banking and ART laboratory professionals for their help in the collection, processing and analyzing of laboratory tests and University of Gondar for giving this opportunity to conduct this study. Also authors want to express their great thanks to the study participants for their patience and cooperation.
Competing interests
The authors declare that they have no competing interests.
Availability of data and material
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was conducted after ethical approval was obtained from University of Gondar, School of Biomedical and Laboratory Sciences Ethical Committee. Informed written consent was taken from study participants before enrollment in the study. Then the objective of this research was explained to the study participants. Those willing to participate were included. Participation in the study was voluntary blood donors and refusal was possible. To ensure confidentiality of data, study subjects were identified using codes and unauthorized persons were not able to access the collected data. The study participants’ serological results were reported by blood banking manager to the physician for proper management and counseling.
Abbreviations
- AIDS
acquired immune deficiency syndrome
- BMI
body mass index
- CLSI
Clinical Laboratory Standard Institution
- EDTA
ethylene diamine tetra-acetic acid
- HbsAgs
hepatitis B virus surface antigen
- HcAbs
hepatitis C virus antibodies
- Hgb
hemoglobin
- HIV
human immunodeficiency virus
- IFCC
International Federation of Clinical Chemistry
- LPT
lymphocyte
- MCH
mean corpuscular hemoglobin
- MCHC
mean corpuscular hemoglobin concentration
- MCV
mean corpuscular volume
- NCCLS
National Committee for Clinical Laboratory Standards
- NTL
neutrophils
- PCV
packed cell volume
- PLT
platelet
- RBC
red blood cells
- RDW
red cell distribution width
- RIs
reference intervals
- WBC
white blood cells
Additional file
Additional file 1. Questionnaire for data collection of a study on Hematological reference intervals determination in adults at Gondar university hospital, Northwest Ethiopia.
Contributor Information
Aregawi Yalew, Email: yalewaregawi@gmail.com.
Betelihem Terefe, Email: betch.nym@gmail.com.
Meseret Alem, Email: mesey4839@gmail.com.
Bamlaku Enawgaw, Email: bamlak21@gmail.com.
References
- 1.Zeh C, Amornkul PN, Inzaule S, Ondoa P, Oyaro B, Mwaengo DM, et al. Population-based biochemistry, immunologic and hematological reference values for adolescents and young adults in a rural population in Western Kenya. Plos ONE. 2011;6(6):e21040. doi: 10.1371/journal.pone.0021040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bain BJ. Ethnic and sex differences in the total and differential white cell count and platelet count. J Clin Pathol. 1996;49(8):664–666. doi: 10.1136/jcp.49.8.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alson OR, Ratsitorahina M, Pfister P, Laganier R, Dromigny J. Estimation of normal hemogram values in Madagascar. Archives de l” Institut Pasteur de Madagascar. 2000;66(1–2):68–71. [PubMed] [Google Scholar]
- 4.Menard D, Mandeng MJ, Tothy MB, Kelembho EK, Gresenguet G, Talarmin A. Immunohematological reference ranges for adults from the Central African Republic. Clin Diagn Lab Immunol. 2003;10(3):443–445. doi: 10.1128/CDLI.10.3.443-445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lugada ES, Mermin J, Kaharuza F, Ulvestad E, Were W, Langeland N, et al. Population-based hematologic and immunologic reference values for a healthy Ugandan population. Clin Diagn Lab Immunol. 2004;11(1):29–34. doi: 10.1128/CDLI.11.1.29-34.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kibaya RS, Bautista CT, Sawe FK, Shaffer DN, Sateren WB, Scott PT, et al. Reference ranges for the clinical laboratory derived from a rural population in Kericho, Kenya. Plos ONE. 2008;3(10):e3327. doi: 10.1371/journal.pone.0003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adetifa I, Hill P, Jeffries D, Jackson-Sillah D, Ibanga H, Bah G, et al. Haematological values from a Gambian cohort—possible reference range for a West African population. Int J Lab Hematol. 2009;31(6):615–622. doi: 10.1111/j.1751-553X.2008.01087.x. [DOI] [PubMed] [Google Scholar]
- 8.Greer JP, Wintrobe MM. Wintrobe’s clinical hematology. 11. Philadelphia: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 9.Gill G, England A, Marshal C. Low platelet counts in Zambians. Trans R Soc Trop Med Hyg. 1979;73(1):111–112. doi: 10.1016/0035-9203(79)90142-1. [DOI] [PubMed] [Google Scholar]
- 10.Tugume SB, Piwowar EM, Lutalo T, Mugyenyi PN, Grant RM, Mangeni FW, et al. Hematological reference ranges among healthy Ugandans. Clin Diagn Lab Immunol. 1995;2(2):233–235. doi: 10.1128/cdli.2.2.233-235.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urquhart N, Capildeo K, Sargeant L, Wharfe G, Hisada M, Hanchard B. White blood cell counts in healthy Jamaican adults. West Indian Med J. 2008;57(2):147–151. [PubMed] [Google Scholar]
- 12.Lawrie D, Coetzee LM, Becker P, Mahlangu J, Stevens W, Glencross DK. Local reference ranges for full blood count and CD4 lymphocyte count testing. South Afr Med J. 2009;99(4):243–248. [PubMed] [Google Scholar]
- 13.Wakeman L, Al-Ismail S, Benton A, Beddall A, Gibbs A, Hartnell S, et al. Robust, routine haematology reference ranges for healthy adults. Int J Lab Hematol. 2007;29(4):279–283. doi: 10.1111/j.1365-2257.2006.00883.x. [DOI] [PubMed] [Google Scholar]
- 14.Karita E, Ketter N, Price MA, Kayitenkore K, Kaleebu P, Nanvubya A, et al. CLSI-derived hematology and biochemistry reference intervals for healthy adults in eastern and southern Africa. Plos ONE. 2009;4(2):e4401. doi: 10.1371/journal.pone.0004401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsegaye A, Messele T, Tilahun T, Hailu E, Sahlu T, Doorly R, et al. Immunohematological reference ranges for adult Ethiopians. Clin Diagn Lab Immunol. 1999;6(3):410–414. doi: 10.1128/cdli.6.3.410-414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical and laboratory standard institution (CLSI). How to define and determine reference intervals in the clinical laboratory; Approved Guideline-Second Eedition. C28-A2: vol.15 No.4.
- 17.Kueviakoe IM, Segbena AY, Jouault H, Vovor A, Imbert M. Hematological reference values for healthy adults in Togo. ISRN Hematol. 2010;2011:736062. doi: 10.5402/2011/736062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Böhler T, Kynast-Wolf G, Coulibaly B, Siè A, Kapaun A. Gender-specific distribution of hematological parameters in adults living in Nouna, Burkina Faso. Open Hematol J. 2008;2:1–4. doi: 10.2174/1874276900802010001. [DOI] [Google Scholar]
- 19.Allan R, Alexander M. A sex difference in the leucocyte count. J Clin Pathol. 1968;21(6):691–694. doi: 10.1136/jcp.21.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brigden ML. Clinical utility of the erythrocyte sedimentation rate. Am Fam Phys. 1999;60(5):1443–1450. [PubMed] [Google Scholar]


