Abstract
Background
Retention of children in HIV care is essential for prevention of disease progression and mortality.
Methods
Retrospective cohort of children (0 to <15 years) initiating antiretroviral treatment (ART) at health facilities in Kenya, Mozambique, Rwanda and Tanzania, January 2005–June 2011. Retention was defined as the proportion of children known to be alive and attending care at their initiation facility; lost to follow-up (LTF) was defined as no clinic visit for > 6 months. Cumulative incidence of ascertained survival and retention after ART initiation was estimated through 24 months using Kaplan-Meier methods. Factors associated with LTF and death were assessed using Cox proportional hazard modeling.
Results
17,712 children initiated ART at 192 facilities: median age was 4.6 years (IQR: 1.9–8.3), median CD4 was 15% (IQR: 10–20) for children < 5 years and 265 cells/uL (IQR: 111–461) for children ≥ 5 years. At 12 and 24 months, 80% and 72% of children were retained with 16% and 22% LTF and 5% and 7% known deaths respectively. Retention ranged from 71–95% and 62–93% at 12 and 24 months across countries, and was lowest for children < 1 year (51% at 24 months). LTF and death were highest in children < 1 year of age and children with advanced disease.
Conclusion
Retention was lowest in young children and differed across country programs. Young children and those with advanced disease are at highest risk for LTF and death. Further evaluation of patient- and program-level factors is needed to improve health outcomes.
Keywords: HIV, retention, pediatric, antiretrovirals
INTRODUCTION
Scale up of pediatric HIV care and treatment across sub-Saharan Africa (SSA), where over 90% of children living with HIV reside1, has been substantial with more than 387,000 children reported as initiating antiretroviral therapy (ART) by December 20102. Treatment responses of children on ART in resource-limited settings have been robust3–9 with high rates of viral suppression and immune reconstitution as well as improved survival10–15. However, the mortality rate of children on ART in resource-limited settings is considerably higher than the rate in developed countries, 8.0 versus 0.9 deaths per 100 child-years (p < 0.001) respectively16. Reasons for this disparity include both biomedical and programmatic factors such as advanced disease at time of presentation, fragility of infants and younger children, other infectious co-morbidities and malnutrition, delays in ART initiation, and suboptimal retention in care4,13.
Retention of HIV-infected children in care is essential for prevention of HIV-related morbidity and mortality through timely ART initiation, monitoring and management of disease progression and treatment failure, and provision of medications and supportive care. Pediatric programs in SSA report retention ranging from 77–89% at 12 and 24 months11,14,15,17–19 which may jeopardize long-term health outcomes20. Both patient and programmatic factors influencing retention need to be identified to improve outcomes and inform future interventions.
We examined retention, lost to follow-up (LTF) and death among 17,712 children, less than 15 years of age, initiating ART at 192 health facilities in Kenya, Mozambique, Rwanda, and Tanzania from January 2005 through June 2011. The objectives of this analysis were to determine the proportion of children who were retained, LTF and died at 12 and 24 months, to assess variation in retention outcomes by country, and to identify patient- and facility-level factors associated with these outcomes.
METHODS
Study Population
We conducted a retrospective cohort analysis of all children, < 15 years of age, initiating ART at 192 HIV care facilities in Kenya, Mozambique, Rwanda and Tanzania from January 2005–June 2011. All facilities received support from ICAP, a President’s Emergency Plan for AIDS Relief (PEPFAR) implementing partner that has been supporting HIV care and treatment in SSA since 2005. ICAP is a nongovernmental organization at the Mailman School of Public Health at Columbia University that supports scale-up of HIV care and treatment through facility mentorship of facility staff, renovation of laboratory and health facilities, creation and support of monitoring and evaluation tools and practices, and other technical assistance21. All health facilities included in this analysis participate in the Identifying Optimal Models of HIV Care and Treatment Study (5U2GPS001537-03)22 which uses routinely collected patient- and facility-level data to measure patient and program outcomes. All facilities had electronic patient-level databases, which are password protected, and encrypted de-identified databases are transferred to ICAP offices every quarter where they are aggregated for analysis. Clinical and laboratory data was recorded by facility staff onto paper records and clerks then data was transferred from paper records into an electronic patient database; data quality assessments were done every 6 months to assess for completeness and accuracy of data entry.
Eligibility criteria for ART initiation followed each country’s national guidelines which reflect WHO recommendations23,24. Per WHO 2006 guidelines, children < 5 years were eligible for ART if they had WHO Stage III/IV, CD4 percentage < 25% (<12 months), CD4 percentage <20 % (12–35 months) or CD4 percentage < 15% (36–59 months). For children > 5 years old, ART eligibility included 1) WHO Stage IV, 2) WHO stage III and CD4 < 350 cells/uL, or 3) CD4< 200 cells/uL irrespective of WHO Stage23. From 2006 to 2011, all four countries began adopting revised WHO guidelines, including recommendations for ART for all HIV-infected children < 1 year25–28, later updated to < 2 years regardless of CD4 measurement24,29,30. Standard of care for HIV care and ART regimens followed national guidelines, which recommended ART for eligible children beginning in 200623. Recommended facility follow-up was a minimum of every 3 months with semi-annual CD4 testing. Access to CD4 testing was not always available but improved over time with increased access to CD4 analyzers and decentralization of laboratories.
Definitions and Outcomes
Follow-up time on ART was estimated as the time between date of ART initiation and either documented transfer, death, LTF, or completion of the observation period. Children were considered retained if they were known to be alive and continuing ART at their initiation facility. Mortality was passively ascertained from documentation of death in patient records. LTF was defined as having no recorded visit for 6 months with no visits after the last missed visit, as per by Chi et al31, and non-retention was defined as either death or LTF. Children who were LTF were censored 15 days after their last recorded visit. Children documented to have transferred to another facility were censored at their recorded date of transfer.
Individual-level factors were chosen based on available data across all country datasets and factors known or suspected through previous research to influence retention and survival. The CD4 percent or cell count and WHO stage taken closest to the date of ART initiation, within a window of 3 months prior to or up to one month after ART initiation, was used to determine disease severity at ART initiation. Clinical stage and immunologic category were defined according to 2006 WHO guidelines: severe immunodeficiency is defined as CD4 < 25% or < 1500 cells/uL in children ≤ 11 months, CD4 < 20% or < 750 cells/uL in children age 12 to 35 months, CD4 < 15% or < 350 cells/uL in children age 36 to 59 months and CD4 < 15% or < 200 cells/uL in children > 5 years23. To investigate the potential influence of malnourishment in our population, we constructed weight-for-age z- scores and categorized children as z-score ≤ or > −2 standard deviations32. No reliable data was available for TB status or treatment at the time of ART initiation. A new category of “severe illness” was created to include any child with severe immunodeficiency, defined above, or WHO Stage IV at time of ART initiation because of the high proportion of missing CD4 measurements and WHO stage.
Facilities were defined as primary or secondary/tertiary. A primary facility is any health center; secondary and tertiary facilities are district, regional, or national hospitals. Facility location was categorized into urban (capital and other large cities with city administration and political bodies), semi-urban (big and small towns, peri-urban areas, growth areas, and mining communities), and rural (small towns, and farming areas).
Statistical Analysis
Cumulative incidence of non-retention, LTF, and reported death through 24 months after ART initiation were estimated using Kaplan-Meier techniques. In the primary analysis, Cox Proportional Hazards models accounting for within-clinic correlation between patients with robust sandwich error terms were used to assess the association between measured patient and facility-level characteristics and LTF and documented death after ART initiation. Individual-level factors include age, point of entry, weight-for-age z-score at ART initiation, CD4 measure at ART initiation, WHO stage at ART initiation, and year of ART initiation; facility-level factors include country, facility type and setting.
Ethical Considerations
This study is part of the Identifying Optimal Models of HIV Care and Treatment, which was approved by the Columbia University Medical Center IRB, the US Centers for Disease Control and Prevention, PEPFAR’s Office of the Global AIDS Coordinator (OGAC), and each participating country’s national ethics committee.
RESULTS
Characteristics of HIV Care Programs
From January 2005 through June 2011, 37,154 children were enrolled in HIV services at 192 facilities in Kenya, Rwanda, Tanzania, and Mozambique (Table 1). A total of 17,712 children initiated ART with the largest cohort in Mozambique (7,226) and the smallest in Rwanda (2,170). The number of children initiated on ART increased annually from 585 in 2005 to 3,653 in 2010, the last full year of observation, with the number of facilities increasing from 75 to 192. Half of the facilities (54%) were in primary-level health centers as compared to secondary (district/regional hospitals) or tertiary-level facilities (teaching or national referral hospitals). Half of facilities (49%) were in rural locations (Table 1) and the majority of facilities were government-supported.
Table 1.
Characteristic of children age < 15 years initiating ART (N = 17,712) and HIV treatment facilities (N=193) in Kenya, Mozambique, Rwanda and Tanzania, January 2005 through June 2011
| Overall | Kenya | Mozambique | Rwanda | Tanzania | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | ||
| Total population | 456,192 | (100%) | 105,313 | (23.1%) | 242,045 | (53.1%) | 44,450 | (9.7%) | 64,384 | (14.1%) | |
| Adult | 419,038 | (91.9%) | 94,064 | (89.3%) | 225,036 | (93.0%) | 40,476 | (91.1%) | 59,462 | (92.4%) | |
| Children | 37,154 | (8.1%) | 11,249 | (10.7%) | 17,009 | (7.0%) | 3,974 | (8.9%) | 4,922 | (7.6%) | |
|
Children initiating ART |
17,712 | (47.7%) | 5,710 | (50.8%) | 7,226 | (42.5%) | 2,170 | (54.6%) | 2,606 | (52.9%) | |
| Individual-level characteristics | |||||||||||
| Female | 9,026 | (51.0%) | 2,870 | (50.3%) | 3,809 | (52.7%) | 1,047 | (48.2%) | 1,330 | (51.0%) | |
| Age | |||||||||||
| 0 to < 1 yr | 2,151 | (12.1%) | 441 | (7.7%) | 1,301 | (18.0%) | 147 | (6.8%) | 262 | (10.1%) | |
| 1 to < 2 yrs | 2,526 | (14.3%) | 532 | (9.3%) | 1,499 | (20.7%) | 191 | (8.8%) | 304 | (11.7%) | |
| 2 – < 5 yrs | 4,641 | (26.2%) | 1,509 | (26.4%) | 1,942 | (26.9%) | 577 | (26.6%) | 613 | (23.5%) | |
| 5 to < 10 yrs | 5,335 | (30.1%) | 2,041 | (35.7%) | 1,734 | (24.0%) | 707 | (32.6%) | 853 | (32.7%) | |
| 10 to < 15 yrs | 3,059 | (17.3%) | 1,187 | (20.8%) | 750 | (10.4%) | 548 | (25.3%) | 574 | (22.0%) | |
| Point of entry into HIV care | |||||||||||
| PMTCT | 862 | (4.9%) | 247 | (4.3%) | 177 | (2.4%) | 198 | (9.1%) | 240 | (9.2%) | |
| TB clinic | 444 | (2.5%) | 381 | (6.7%) | 25 | (0.3%) | 12 | (0.6%) | 26 | (1.0%) | |
| VCT/PICT* | 13,791 | (77.9%) | 4,512 | (79.0%) | 5,798 | (80.2%) | 1,960 | 90.3%) | 1,521 | (58.4%) | |
| Missing | 2,615 | (14.8%) | 570 | (10.0%) | 1,226 | (17.0%) | - | - | 819 | (31.4%) | |
| Weight-for-age z-score (CDC Standard) | |||||||||||
| ≤ −2 | 5,866 | (33.1%) | 2,070 | (36.2%) | 1,605 | (22.2%) | 795 | (36.6%) | 1,396 | (53.6%) | |
| >2 | 5,624 | (31.8%) | 2,208 | (38.7%) | 2,075 | (28.7%) | 432 | (19.9%) | 909 | (34.9%) | |
| Missing | 6,222 | (35.1%) | 1,432 | (25.1%) | 3,546 | (49.1%) | 943 | (43.5%) | 301 | (11.6%) | |
| WHO stage prior to ART initiation | |||||||||||
| I | 1,970 | (11.1%) | 782 | (13.7%) | 723 | (10.0%) | 316 | 14.6%) | 149 | (5.7%) | |
| II | 3,829 | (21.6%) | 1,808 | (31.7%) | 1,191 | (16.5%) | 440 | (20.3%) | 390 | (15.0%) | |
| III | 6,672 | (37.7%) | 2,219 | (38.9%) | 2,331 | (32.3%) | 1,036 | (47.7%) | 1,086 | (41.7%) | |
| IV | 1,845 | (10.4%) | 257 | (4.5%) | 734 | (10.2%) | 172 | (7.9%) | 682 | (26.2%) | |
| Missing | 3,396 | (19.2%) | 644 | (11.3%) | 2,247 | (31.1%) | 206 | (9.5%) | 299 | (11.5%) | |
| CD4 % at ART initiation among patients < 5 years of age (N = 9,318) | |||||||||||
| median (IQR) | 3,029 | 15% (10–20%) |
576 | 16% (10–21%) |
2,453 | 15% (10–19%) |
. | . | . | . | |
| Missing | 6,289 | (67%) | 1,906 | (77%) | 2,289 | (48%) | (100%) | (100%) | |||
| CD4 count (cells/mL) at ART initiation among patients 5–15 years of age (N = 8,394) | |||||||||||
| median (IQR) | 5,234 | 265 (111–461) |
1,972 | 222 (77–397) |
1,491 | 289 (127–499) |
1061 | 337 (206–574) |
710 | 196 (81–378) |
|
| < 100 | 1,229 | (14.6%) | 571 | (17.7%) | 327 | (13.2%) | 131 | (10.4%) | 200 | (14.0%) | |
| 100–200 | 816 | (9.7%) | 337 | (10.4%) | 198 | (8.0%) | 120 | (9.6%) | 161 | (11.3%) | |
| 200–350 | 1,305 | (15.5%) | 479 | (14.8%) | 357 | (14.4%) | 325 | (25.9%) | 144 | (10.1%) | |
| ≥ 350 | 1,884 | (22.4%) | 585 | (18.1%) | 609 | (24.5%) | 485 | (38.6%) | 205 | (14.4%) | |
| Missing | 3,160 | (37.6%) | 1,256 | (38.9%) | 993 | (40.0%) | 194 | (15.5%) | 717 | (50.2%) | |
| Severe illness at ART initiation** | |||||||||||
| No | 9,864 | (55.7%) | 3757 | (65.8%) | 2985 | (41.3%) | 1667 | (76.8%) | 1455 | (55.8%) | |
| Yes | 6,103 | (34.5%) | 1534 | (26.9%) | 3216 | (44.5%) | 403 | (18.6%) | 950 | (36.5%) | |
| Missing | 1,745 | (9.9%) | 419 | (7.3%) | 1025 | (14.2%) | 100 | (4.6%) | 201 | (7.7%) | |
| Year of ART initiation | |||||||||||
| 2005 | 585 | (3.3%) | 160 | (2.8%) | 177 | (2.4%) | 139 | (6.4%) | 109 | (4.2%) | |
| 2006 | 1,846 | (10.4%) | 620 | (10.9%) | 666 | (9.2%) | 325 | (15.0%) | 235 | (9.0%) | |
| 2007 | 2,970 | (16.8%) | 945 | (16.5%) | 1202 | (16.6%) | 468 | (21.6%) | 355 | (13.6%) | |
| 2008 | 3,180 | (18.0%) | 1074 | (18.8%) | 1213 | (16.8%) | 411 | (18.9%) | 482 | (18.5%) | |
| 2009 | 3,760 | (21.2%) | 1298 | (22.7%) | 1536 | (21.3%) | 331 | (15.3%) | 595 | (22.8%) | |
| 2010 | 3,653 | (20.6%) | 1112 | (19.5%) | 1653 | (22.9%) | 365 | (16.8%) | 523 | (20.1%) | |
| 2011 (Jan–June) | 1,718 | (9.7%) | 501 | (8.8%) | 779 | (10.8%) | 131 | (6.0%) | 307 | (11.8%) | |
| Facility-level characteristics | |||||||||||
| Number of facilities | 192 | (100%) | 69 | (35.9%) | 31 | (16.1%) | 42 | (21.9%) | 50 | (26.0%) | |
| Facility-type | |||||||||||
| Primary | 103 | (53.6%) | 35 | (50.7%) | 18 | (58.1%) | 31 | (73.8%) | 19 | (38.0%) | |
| Secondary/tertiary | 89 | (46.4%) | 34 | (49.3%) | 13 | (41.9%) | 11 | (26.2%) | 31 | (62.0%) | |
| Facility location | |||||||||||
| Urban | 35 | (18.2%) | 1 | (1.4%) | 23 | (74.2%) | 11 | (26.2%) | 0 | (0.0%) | |
| Semi-Urban | 63 | (32.8%) | 21 | (30.4%) | 2 | (6.5%) | 2 | (4.8%) | 38 | (76.0%) | |
| Rural | 94 | (49.0%) | 47 | (68.1%) | 6 | (19.4%) | 29 | (69.0%) | 12 | (24.0%) | |
Voluntary counseling and testing (VCT), Provider initiated counseling and testing (PICT)
Severe illness includes any child with WHO stage IV or severe immunodeficiency, defined as CD4 < 25% or < 1500cells/uL for children < 12 months, CD4 < 20% or < 750 cells/uL in children 12–35 months, CD4 < 15% or <350 cells/uL in children 36–59 months, and CD4 < 15% or < 200 cells/uL in children > 5 years (WHO 2006 guidelines)
Patient Characteristics
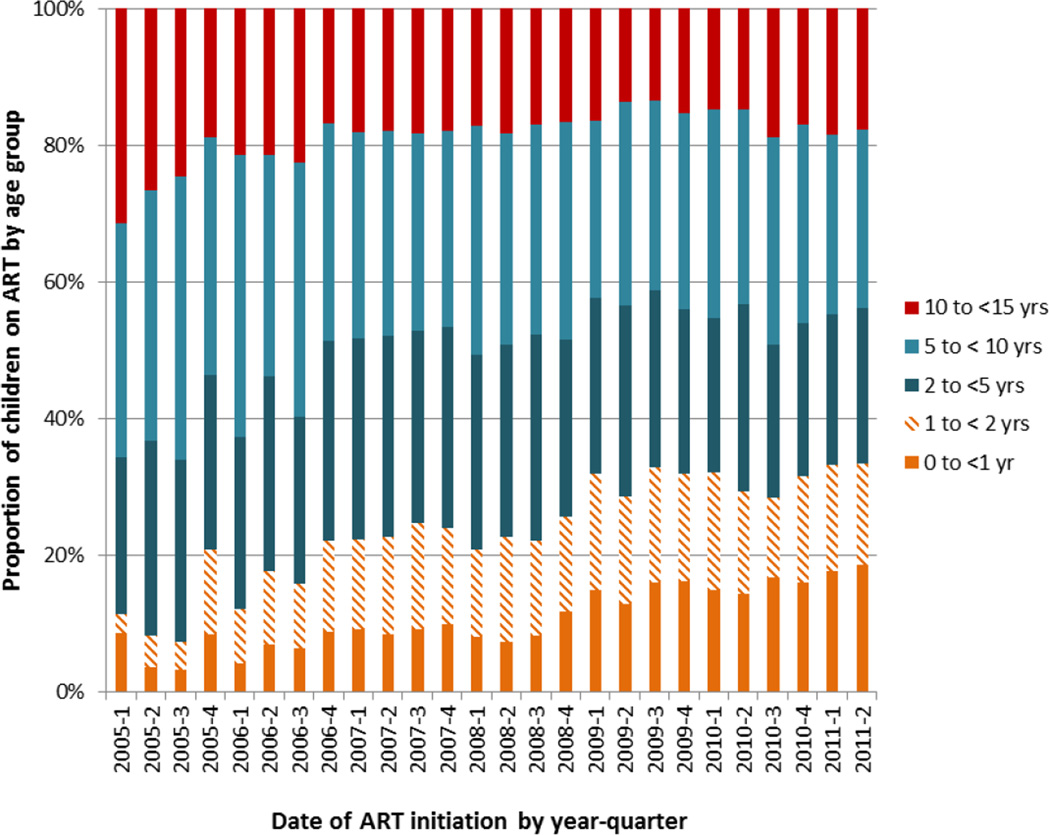
Median follow-up time for children initiated on ART was 598 days (IQR: 245–1,106). The median age at ART initiation was 4.6 years (IQR: 1.9–8.3) with a similar distribution of females (51%) and males. Forty-eight percent of children had WHO Stage III/IV disease, and 32% of children had severe immunodeficiency (67% of children < 5 years and 38% of children ≥ 5 years). Thirty-three percent of children had a weight-for-age z-score of ≤ −2. Among the 8,263 children with recorded CD4 measurements (33% for children < 5 years and 62% of children ≥ 5 years) at ART initiation, the median CD4 was 15% (IQR: 10–20) for children < 5 years and 265 cells/uL (IQR:111–461) for children ≥ 5 years (Table 1). Overall, 34.5% of children were classified with severe illness at time of ART initiation (Table 1) and the proportion of children < 1 year classified with severe illness decreased from 56% in 2006 to 18% in 2011 (Supplemental Table 1). Over time, the proportion of children < 2 years of age initiated on ART increased from 12% of all children in 2005 to 33% in 2011 (Figure 1). The majority of all children reported entry into HIV care through voluntary counseling and testing or provider initiated counseling and testing (Table 1). First-line ART regimens for 95% of children included two nucleoside reverse transcriptase inhibitors plus one non-nucleoside reverse transcriptase inhibitor.
Figure 1.
Trends in age distribution among children age < 15 years (N=17,712) at ART initiation at 192 facilities in Kenya, Mozambique, Rwanda, and Tanzania, January 2005 through June 2011
Country programs varied in the proportion of young (< 2 years) and severely ill children. Rwanda reported the smallest proportion of young children, 15.6% as compared to 38.7% in Mozambique. Rwanda also reported the smallest proportion of children with severe illness at 18.6% compared to 44.5% in Mozambique, 36.5% in Tanzania, and 26.9% in Kenya. Tanzania had the largest proportion of children with documented WHO Stage IV, 26.2%, as compared to Kenya (4.5%); however over 30% of children in Mozambique did not have WHO stage recorded.
Retention, LTF and Mortality at 12 and 24 months
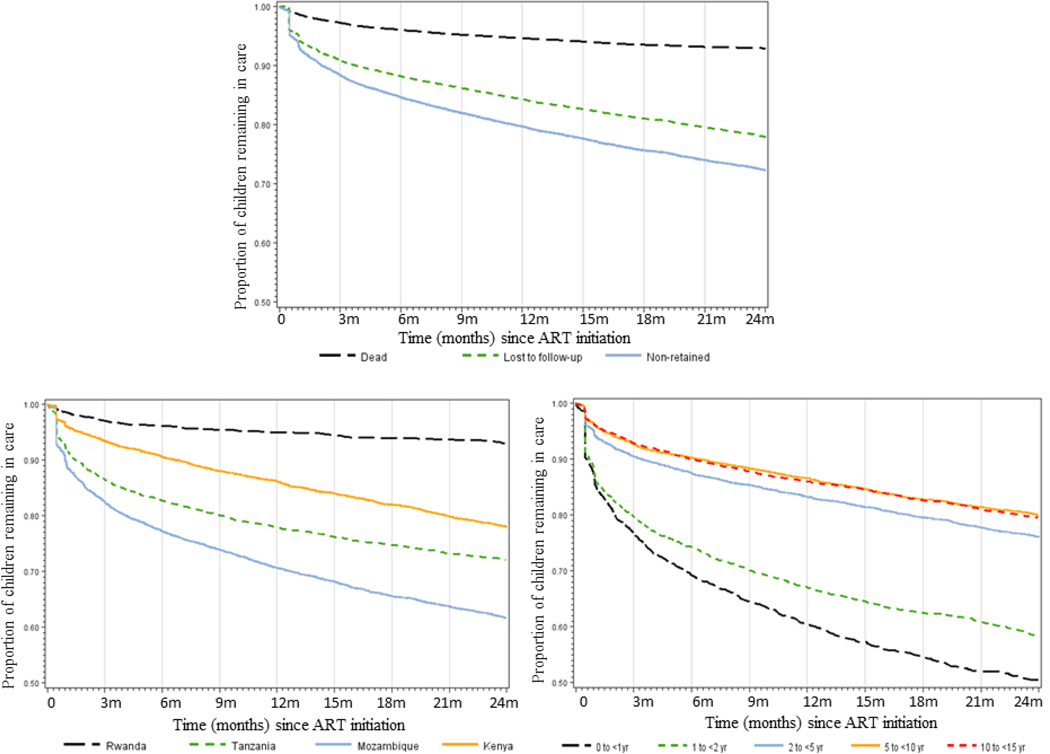
Among those initiated on ART, 80% and 72% of children were retained at 12 and 24 months (Table 2, Figure 2a). At 12 and 24 months, 16% and 22% of children were LTF, while 5% and 7% had documented deaths (Table 2). LTF and mortality rates were highest in the first 6 months after ART initiation (26.3 and 9.1 per 100 person-years, respectively), declining through 12 months (18.4 and 6.3 per 100 person-years) and 24 months (14.2 and 4.5 per 100 person-years). Retention varied substantially across countries, ranging from 71–95% at 12 months and 62–93% at 24 months (Table 2, Figure 2b). Compared to children in Rwanda, children in Mozambique had 16 fold higher rate of LTF (adjusted HR 16.8, 95% CI 8.9–32.0) and children in Tanzania had over two times higher death rate (adjusted HR 2.6, 95% CI 1.8–3.6). All country programs experienced the largest drop in retention during the first six months after ART initiation. Across all age groups and time periods, the proportion of children who were LTF was higher than the proportion of known deaths, except for Rwanda. The overall proportion of documented transfers was similar across programs: 18% Kenya, 12% Mozambique, 19% Rwanda, and 15% Tanzania.
Table 2.
Retention, loss to follow-up, and death at 12 and 24 months after ART initiation: overall, by country, and by age group
| Retention after ART initiation | |||||||||||||
| overall | <1 year | 1 to <2 years | 2 to <5 years | 5 to <10 years | 10 to <15 years | ||||||||
| N | 12 month s |
24 month s |
12 month s |
24 month s |
12 month s |
24 month s |
12 month s |
24 month s |
12 month s |
24 month s |
12 month s |
24 month s |
|
| Overall |
17,71 2 |
80% | 72% | 61% | 51% | 67% | 58% | 83% | 76% | 87% | 80% | 86% | 80% |
| Kenya |
5,710 |
86% | 78% | 70% | 58% | 81% | 69% | 87% | 80% | 89% | 82% | 88% | 80% |
| Mozambiq ue |
7,226 |
71% | 62% | 54% | 43% | 60% | 51% | 78% | 69% | 81% | 73% | 78% | 68% |
| Rwanda |
2,170 |
95% | 93% | 86% | 82% | 91% | 88% | 96% | 94% | 97% | 95% | 96% | 94% |
| Tanzania |
2,606 |
78% | 72% | 65% | 58% | 63% | 56% | 80% | 73% | 83% | 78% | 83% | 78% |
| Loss to follow-up after ART initiation | |||||||||||||
| overall | <1 year | 1 to <2 years | 2 to <5 years | 5 to <10 years | 10 to <15 years | ||||||||
| N | 12 month s |
24 month s |
12 month s |
24 month s |
12 month s |
24 month s |
12 month s |
24 month s |
12 month s |
24 month s |
12 month s |
24 month s |
|
| Overall |
17,71 2 |
16% | 22% | 30% | 39% | 26% | 34% | 14% | 20% | 11% | 17% | 10% | 15% |
| Kenya |
5,710 |
11% | 19% | 26% | 37% | 17% | 28% | 11% | 18% | 9% | 16% | 9% | 16% |
| Mozambiq ue |
7,226 |
24% | 32% | 36% | 44% | 33% | 41% | 19% | 27% | 16% | 24% | 19% | 27% |
| Rwanda |
2,170 |
1% | 2% | 4% | 6% | 2% | 2% | 1% | 1% | 1% | 2% | 1% | 1% |
| Tanzania |
2,606 |
14% | 18% | 24% | 30% | 23% | 27% | 13% | 19% | 11% | 15% | 10% | 14% |
| Measured death after ART initiation | |||||||||||||
| overall | <1 year | 1 to <2 years | 2 to <5 years | 5 to <10 years | 10 to <15 years | ||||||||
| N | 12 month s |
24 month s |
12 month s |
24 month s |
12 month s |
24 month s |
12 month s |
24 month s |
12 month s |
24 month s |
12 month s |
24 month s |
|
| Overall |
17,71 2 |
5% | 7% | 13% | 18% | 9% | 12% | 4% | 5% | 3% | 4% | 4% | 6% |
| Kenya |
5,710 |
3% | 3% | 6% | 7% | 3% | 4% | 2% | 2% | 2% | 3% | 4% | 4% |
| Mozambiq ue |
7,226 |
7% | 9% | 16% | 23% | 10% | 13% | 4% | 6% | 3% | 4% | 4% | 7% |
| Rwanda |
2,170 |
3% | 5% | 9% | 11% | 6% | 9% | 3% | 4% | 2% | 3% | 3% | 4% |
| Tanzania |
2,606 |
9% | 11% | 14% | 17% | 19% | 23% | 7% | 10% | 7% | 9% | 8% | 10% |
Table percentages obtained using product-limit estimates. LTF defined as not having a recorded clinic visit within the last 6 months of clinic follow-up. Children who were LTF were censored 15 days after their last recorded visit. Children documented to have transferred to another facility were censored at their recorded date of transfer.
Figure 2.
Cumulative incidence curves of retention, lost to follow-up and death among children age < 15 years initiating ART in Kenya, Mozambique, Rwanda, and Tanzania (2a), retention by country (2b) and retention by age group (2c)
Retention was lowest among children < 1 year, 61% and 51% at 12 and 24 months, and highest in children age 5 to < 10 years, 87% and 80% respectively (Table 2, Figure 2c). There was no consistent improvement in retention, LTF or death in children < 1 year over time by calendar year although LTF appeared to be marginally lower in later years (Supplemental Table 2).
Factors associated with LTF and death
Multivariable analysis was done to assess factors associated with LTF and death including country, age at ART initiation, severe illness at ART initiation, weight-for-age z-score at ART initiation, year of ART initiation, point of entry into HIV care, and facility type and location. The adjusted hazard ratio for LTF and death were highest in children < 1 year (HRLTF =2.0, 95% CI 1.7–2.4; HRDeath= 3.4, 95% CI: 2.6–4.6) (Table 3). Compared to children with less advanced disease, children with severe illness had higher rate of death (HRDeath =1.6, 95% 1.4–1.9) but similar rate of LTF (HRLTF = 0.99, 95% CI 0.86–1.13) (Table 3). Among older children, CD4 count was a strong predictor of LTF and death; children ≥ 5 years with CD4 < 100 cells/uL had nearly three times higher death rates compared with children initiating ART at CD4 > 350 cells/uL. Children with missing CD4 counts had 2.3 times the rate of death compared to children with a CD4 > 350 cells/uL; and children missing WHO stage or severe illness information were also more likely than other children to be LTF or to be documented deaths. There was no evidence of differences in LTF, or death by point of entry or facility type (Table 3). There was no consistent evidence for reduced LTF, or death by year of ART initiation. No facility-level factors were found to have a significant impact on the risk of LTF or death.
Table 3.
Factors associated with loss to follow-up and death among children age < 15 years (N=17,712) in Kenya, Mozambique, Rwanda, and Tanzania
| Loss to follow-up | Measured Death | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude | Adjusted1 | Crude | Adjusted1 | ||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| Country | |||||||||
| Kenya | 12.57 | (6.88–22.98) | 9.53 | (4.96–18.33) | 0.81 | (0.61–1.07) | 0.84 | (0.61–1.16) | |
| Rwanda | |||||||||
| Tanzania | 10.72 | (5.86–19.63) | 7.83 | (3.77–16.26) | 2.67 | (2.1–3.39) | 2.57 | (1.82–3.62) | |
| Mozambique | 19.82 | (10.33–38.01) | 16.83 | (8.86–31.96) | 2.13 | (1.52–2.97) | 1.71 | (1.30–2.26) | |
| Age at ART initiation | |||||||||
| 0 to < 1yr | 2.59 | (2.24–2.99) | 2.02 | (1.73–2.36) | 4.08 | (2.96–5.63) | 3.41 | (2.55–4.56) | |
| 1 to < 2 yrs | 2.13 | (1.83–2.48) | 1.71 | (1.47–1.98) | 2.68 | (2.14–3.35) | 2.16 | (1.74–2.68) | |
| 2 to < 5 yrs | 1.23 | (1.10–1.37) | 1.13 | (1.00–1.28) | 1.11 | (0.91–1.36) | 1.11 | (0.91–1.35) | |
| 5 to < 10 yrs | |||||||||
| 10 to < 15 yrs | 0.95 | (0.84–1.08) | 1.04 | (0.93–1.16) | 1.41 | (1.12–1.75) | 1.23 | (0.97–1.54) | |
| Point of entry | |||||||||
| PMTCT | 0.88 | (0.63–1.23) | 0.82 | (0.67–0.99) | 1.56 | (1.15–2.11) | 1.03 | (0.78–1.36) | |
| TB clinic | 0.92 | (0.63–1.36) | 1.07 | (0.80–1.44) | 0.86 | (0.51–1.44) | 1.62 | (1.02–2.55) | |
| Other HCT | |||||||||
| missing | 1.11 | (0.94–1.32) | 0.90 | (0.78–1.03) | 1.44 | (1.12–1.86) | 1.10 | (0.86–1.41) | |
| Weight-for-age z-score (CDC Standard) | |||||||||
| ≤ −2 | 1.18 | (1.04–1.33) | 1.34 | (1.21–1.47) | 2.62 | (2.19–3.13) | 2.36 | (1.98–2.80) | |
| >2 | reference | ||||||||
| Missing | 1.56 | (1.15–2.13) | 1.42 | (1.19–1.70) | 2.80 | (2.21–3.55) | 2.42 | (1.89–3.09) | |
| WHO stage prior to ART initiation | |||||||||
| I | 1.12 | (0.89–1.41) | 1.03 | (0.87–1.21) | 0.61 | (0.46–0.82) | 0.65 | (0.47–0.88) | |
| II | 0.92 | (0.76–1.12) | 0.90 | (0.78–1.02) | 0.60 | (0.48–0.75) | 0.75 | (0.59–0.94) | |
| III | |||||||||
| IV | 1.34 | (1.12–1.6) | 1.16 | (0.98–1.36) | 2.20 | (1.78–2.7) | 1.60 | (1.34–1.92) | |
| Missing | 2.24 | (1.34–3.75) | 1.75 | (1.21–2.53) | 1.82 | (1.24–2.66) | 1.49 | (1.07–2.06) | |
| CD4 count (cells/uL) at ART initiation for children > 5 years of age2 | |||||||||
| < 100 | 0.79 | (0.58–1.06) | 1.16 | (0.95–1.41) | 1.87 | (1.41–2.48) | 2.78 | (1.92–4.03) | |
| 100–199 | 0.72 | (0.55–0.94) | 0.96 | (0.78–1.18) | 1.14 | (0.84–1.55) | 1.28 | (0.81–2.05) | |
| 200–349 | 0.65 | (0.53–0.80) | 0.84 | (0.70–1.02) | 0.85 | (0.62–1.17) | 1.36 | (0.94–1.98) | |
| >350 | |||||||||
| Missing | 1.05 | (0.75–1.48) | 1.23 | (0.90–1.68) | 1.38 | (1.00–1.92) | 2.27 | (1.62–3.19) | |
| Severe illness at ART initiation3 | |||||||||
| Severe | 1.30 | (1.07–1.57) | 0.99 | (0.86–1.13) | 1.96 | (1.61–2.40) | 1.60 | (1.36–1.89) | |
| Not severe | |||||||||
| Missing | 2.33 | (1.64–3.32) | 1.71 | (1.37–2.14) | 2.27 | (1.57–3.28) | 1.70 | (1.27–2.27) | |
| Year of ART initiation | |||||||||
| 2005 | 0.73 | (0.52–1.04) | 0.86 | (0.62–1.19) | 1.21 | (0.8–1.82) | 1.30 | (0.82–2.07) | |
| 2006 | 0.78 | (0.65–0.94) | 0.83 | (0.69–0.99) | 0.95 | (0.68–1.33) | 1.07 | (0.80–1.43) | |
| 2007 | |||||||||
| 2008 | 1.17 | (1.03–1.34) | 1.24 | (1.10–1.39) | 0.95 | (0.76–1.18) | 0.99 | (0.80–1.22) | |
| 2009 | 1.18 | (0.86–1.62) | 1.09 | (0.80–1.49) | 1.07 | (0.84–1.36) | 0.98 | (0.77–1.25) | |
| 2010 | 1.28 | (0.85–1.95) | 1.19 | (0.77–1.84) | 0.98 | (0.75–1.28) | 0.94 | (0.73–1.21) | |
| 2011 | 0.97 | (0.61–1.52) | 0.87 | (0.54–1.42) | 1.19 | (0.87–1.64) | 1.11 | (0.80–1.54) | |
| Facility type | |||||||||
| Primary | 1.22 | (0.64–2.33) | 1.08 | (0.60–1.96) | 1.17 | (0.78–1.74) | 1.00 | (0.74–1.36) | |
| Secondary/Tertiary | reference | ||||||||
| Facility location | |||||||||
| Rural | 0.43 | (0.24–0.79) | 0.84 | (0.56–1.26) | 0.75 | (0.5–1.12) | 1.29 | (0.96–1.73) | |
| Semi-Urban | 0.85 | (0.5–1.44) | 1.61 | (0.96–2.69) | 0.81 | (0.56–1.18) | 1.09 | (0.76–1.55) | |
| Urban | reference | ||||||||
Unless otherwise notes, predictors in adjusted models include country, age at ART initiation, point of entry, severe sickness at ART initiation (see below), year of ART initiation, facility type, and facility location.
Analyses for CD4 count at ART initiation are restricted to children 5 years and older, and adjusted analyses include country, age at ART initiation, point of entry, year of ART initiation, facility type, and facility location.
Severe illness includes any child with WHO Stage IV illness or severe immunodeficiency, defined as CD4 < 25% or < 1500 cells/uL in children ≤ 11 months, CD4 < 20% or < 750 cells/uL in children age 12 to 35 months, CD4 < 15% or < 350 cells/uL in children age 36 to 59 months and CD4 < 15% or < 200 cells/uL in children > 5 years (WHO Guidelines 2006)
Results were also stratified by country (Supplemental Table 3) and similar factors are associated with increased risk of LTF and death across all countries. Sensitivity analyses using random effects multilevel Cox Proportional Hazards models, in addition to the fixed effects model presented, yielded no substantial differences in the magnitude of the hazard ratios presented in Table 3.
DISCUSSION
To our knowledge, this is the largest reported cohort of children on ART across multiple African countries, which reflects the scale-up of HIV care and treatment across Sub Saharan Africa. At 12 and 24 months, 80% and 72% of children were retained in care, 16% and 22% were LTF and 5% and 7% were known to have died. The true proportion of children who have died is likely much higher than reported values given some children who are categorized as LTF are undocumented deaths18,33. While this study’s outcomes are similar to other cohorts7,11,14,15,17–19,22,34,35, there was substantial variation within specific populations of children, particularly the youngest (< 1 year) and those with advanced disease, and across country programs.
One of the most striking findings of this study is the outcomes seen among the youngest children initiating ART (< 1 year of age). While young age, as well as advanced disease, has been associated with increased risk of LTF and death4,7,13–15,17,36–39, the fact that only half of children who initiated ART during infancy were retained in care at 24 months and 18% have died is sobering (Table 2). While these estimates can be considered an improvement from the reports in the pre-ART era (50% mortality at 24 months), the mortality rates remain unacceptably high in the context of ART40.
From 2005 through 2011 we observed an increase in the proportion of young children (both infants < 1 year and young children 1 to 2 years) initiating ART (Figure 1) and a concomitant decrease in the severity of illness among infants (Supplemental Table 1). These findings reflect improved access to early diagnostic testing in the four countries41, as well as implementation of the WHO guidelines recommending treatment of all children < 1 year of age, later revised to < 2 years of age. However, despite a decrease in the proportion of infants categorized as severely ill at time of ART initiation from 2005 to 2011, as noted, there was no concurrent improvement in retention or mortality over time (Supplemental Table 2). We would anticipate improved outcomes with the enrollment of healthier children and the accrual of benefits from early treatment5,6,10. While it is encouraging to see greater numbers of young children initiating earlier treatment, further research is needed to identify other factors impacting health outcomes among this highly vulnerable group of children. These findings also underscore the ongoing need for special attention for infants to be promptly diagnosed, initiated on treatment and retained in care.
Another striking finding of this study is the fact that country programs contributing to this analysis achieved vastly different outcomes, with retention at 24 months ranging from 62–93%. Moreover, within countries we observed substantial heterogeneity in both retention and mortality. All facilities received technical support through ICAP and implemented the same general model of care, which emphasizes early infant diagnosis, family-focused care, involvement of peer-educators, and active follow-up. However, possible reasons explaining such differences in country outcomes likely include a combination of national influences, such as national leadership, access to health services and requirements for medical record documentation, as well as epidemiological and program factors such as HIV seroprevalence (ranging from 2.9% in Rwanda to 11.5% in Mozambique42), patient caseload, provider-patient ratios, and decentralization of services. Since the proportion of documented transfers is fairly similar across programs, variability in quality of documentation of transfers may explain some but not all of the variation seen in retention.
The high retention observed in Rwanda is consistent with other studies investigating retention in HIV programs in this country43–46. The ICAP-supported program in Rwanda, in comparison to the other country programs included in this study, had a smaller proportion of young (age < 2 years) and severely ill children at time of ART initiation. Also, the Rwanda program is smaller with a lower patient caseload, 2,170 children in Rwanda as compared to 7,226 children in ICAP-Mozambique (Table 1), that has been associated with lower LTF in resource limited settings47,48. Smaller programs may have higher staff to patient ratios, shorter wait times, and more staff and time for active patient follow-up, which has been shown to significantly decrease LTF49–51. Finally, better documentation may explain some of the differences seen among countries. In Rwanda, the proportion of children who are LTF is lower than the proportion of known deaths, for which the reverse is true in other country programs. The category of LTF serves as a combination category of children who have been truly lost to follow-up and others who have undocumented deaths or transfers. Rwanda’s results are encouraging as they illustrate what is achievable in public programs and suggest that improvement is obtainable. Additional evaluation of program and facility-level factors, not available in this dataset, to explain variations seen across countries is clearly warranted.
There are a number of strengths to this analysis. The first is that it is the largest, single program, multi-country analysis reporting outcomes of children on ART over the period of PEPFAR scale-up beginning in 2005. The results are likely generalizable to other PEPFAR-supported pediatric HIV programs and reflect the diversity in outcomes from rapid scale-up of ART for children across SSA. The use of patient-level data allows analysis within age categories and links patient-level data to facility data. Finally, we were able to assess retention, LTF and death across a relatively long follow-up period of 24 months.
An important limitation of this analysis is the amount of missing data for patient characteristics at time of ART initiation, which may be due to poor documentation as well as lack of clinical staging and limited access to CD4 testing. The large amount of missing data prevented the use of imputation techniques. Instead, we chose to include “missing” as a separate category in our regression analyses to investigate whether children with missing information differed from children with complete information in outcomes of interest, and indeed children with missing data appear to be at increased risk for LTF or death. This is not surprising as clinicians report that some children entering HIV care programs attend only one clinic visit and do not return to complete full enrollment evaluation including CD4 assessment. Second, this study only includes ICAP-supported pediatric HIV facilities that have capacity for electronic patient-level database, which may represent higher-resourced facilities as compared to others. A total of 192 facilities were included representing 31% of ICAP-supported care and treatment facilities in Kenya (24%), Rwanda (88%), Mozambique (47%), and Tanzania (39%) and regional differences within countries were not analyzed. Finally, this analysis does not include HIV-exposed infants, HIV-infected children who have not enrolled in care, or those who have enrolled in care but have not initiated ART—all groups which have lower estimated retention rates12,22,34,52–54.
It is established that the benefits of ART on children in resource-limited settings are comparable with those in developed countries3–9; yet, these benefits are not currently realized in many pediatric HIV programs, such as those presented in this analysis. This study builds upon the evidence that overall retention, LTF and death are suboptimal, and young children and those with advanced disease are at highest risk for LTF and death. Despite encouraging results suggesting enrollment of a larger proportion of younger children and a smaller proportion of those with severe illness, we do not yet see substantial improvement in reported retention, LTF or death of young children. The vast differences across country programs illustrate that improved retention is achievable. However, additional attention to prompt diagnosis, early ART initiation, active follow-up of children who miss appointments and improved documentation of known transfers and deaths are urgent priorities for pediatric HIV programs.
Supplementary Material
Acknowledgments
We thank all children and staff at the HIV care and treatment facilities included in this analysis. We would also like to thank the Optimal Model Country team member in Kenya (Mark Hawkens, Davies Kimanga, Emily Koech, William Reidy, John Elijah Thiongo, Lucy Wanjiku), Mozambique (, Maria Fernanda Sardella Alvim, Américo Rafi Assan, Amy L. Boore, Kebba M. Jobarteh, Jose Mizela, Antonio Mussa, Carla Xavier), Rwanda (Maria Lahuerta, David Lowrance, Emmanuel Manzi, Njeri Micheu, Fernando Morales, Jules Mugabo, Veronicah Mugisha, William Nagaba, John Pierre Nyemazi, Pratima Raghunathan, Sabin Nsanzimana, Ruben Sahabo), and Tanzania (Annette Almeida, Gretchen Antelman, Gilly Arthur, Ahmed Khatib, Bonita Kilama, Redempta Mbatia, Mohammed Mfaume, Harriet Nuwabaga-Biribonwoha, Geoffrey Somi Ayele Zewde Woldehana).
Sources of support for this work: This work was supported by The President’s Emergency Plan for AIDS Relief; the US Centers for Disease Control and Prevention (Grant number: 5U2GPS001537-03).
Footnotes
Meetings: Preliminary analysis of data in this manuscript was presented at the 19th Conference on Retroviruses and Opportunistic Infections. Retention of HIV+ Children on ART in ICAP-supported HIV Care and Treatment Programs, Abstract # 959. Seattle, Washington. March 5–8, 2012.
Disclaimers and Disclosures: none
Conflicts of Interest: The authors have no conflicts of interests to declare.
References
- 1.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2010. Geneva: 2010. Available at: http://www.unaids.org/globalreport/global_report.htm. [Google Scholar]
- 2.WHO, UNAIDS, UNICEF. Global HIV/AIDS Response, Epidemic update and health sector progress towards universal access, progress report 2011. Geneva: 2011. Available at: http://www.who.int/hiv/pub/progress_report2011/en/index.html. [Google Scholar]
- 3.Ciaranello AL, Chang Y, Margulis AV, et al. Effectiveness of pediatric antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. Clin Infect Dis. 2009 Dec 15;49(12):1915–1927. doi: 10.1086/648079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008 Aug;8(8):477–489. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 5.Edmonds A, Yotebieng M, Lusiama J, et al. The effect of highly active antiretroviral therapy on the survival of HIV-infected children in a resource-deprived setting: a cohort study. PLoS Med. 2011 Jun;8(6):e1001044. doi: 10.1371/journal.pmed.1001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desmonde S, Coffie P, Aka E, et al. Severe morbidity and mortality in untreated HIV-infected children in a paediatric care programme in Abidjan, Cote d'Ivoire, 2004–2009. BMC Infect Dis. 2011;11:182. doi: 10.1186/1471-2334-11-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rouet F, Fassinou P, Inwoley A, et al. Long-term survival and immuno-virological response of African HIV-1-infected children to highly active antiretroviral therapy regimens. AIDS. 2006 Nov 28;20(18):2315–2319. doi: 10.1097/QAD.0b013e328010943b. [DOI] [PubMed] [Google Scholar]
- 8.Fassinou P, Elenga N, Rouet F, et al. Highly active antiretroviral therapies among HIV-1-infected children in Abidjan, Cote d'Ivoire. AIDS. 2004 Sep 24;18(14):1905–1913. doi: 10.1097/00002030-200409240-00006. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien DP, Sauvageot D, Olson D, et al. Treatment outcomes stratified by baseline immunological status among young children receiving nonnucleoside reverse-transcriptase inhibitor-based antiretroviral therapy in resource-limited settings. Clin Infect Dis. 2007 May 1;44(9):1245–1248. doi: 10.1086/513433. [DOI] [PubMed] [Google Scholar]
- 10.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008 Nov 20;359(21):2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.KIDS-ART-LINC. Low risk of death, but substantial program attrition, in pediatric HIV treatment cohorts in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2008 Dec 15;49(5):523–531. doi: 10.1097/QAI.0b013e31818aadce. [DOI] [PubMed] [Google Scholar]
- 12.Anaky MF, Duvignac J, Wemin L, et al. Scaling up antiretroviral therapy for HIV-infected children in Cote d'Ivoire: determinants of survival and loss to programme. Bull World Health Organ. 2010 Jul 1;88(7):490–499. doi: 10.2471/BLT.09.068015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007 Oct 24;298(16):1888–1899. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 14.Davies MA, Keiser O, Technau K, et al. Outcomes of the South African National Antiretroviral Treatment Programme for children: the IeDEA Southern Africa collaboration. S Afr Med J. 2009 Oct;99(10):730–737. [PMC free article] [PubMed] [Google Scholar]
- 15.Sauvageot D, Schaefer M, Olson D, Pujades-Rodriguez M, O'Brien DP. Antiretroviral therapy outcomes in resource-limited settings for HIV-infected children <5 years of age. Pediatrics. 2010 May;125(5):e1039–e1047. doi: 10.1542/peds.2009-1062. [DOI] [PubMed] [Google Scholar]
- 16.Peacock-Villada E, Richardson BA, John-Stewart GC. Post-HAART outcomes in pediatric populations: comparison of resource-limited and developed countries. Pediatrics. 2011 Feb;127(2):e423–e441. doi: 10.1542/peds.2009-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekouevi DK, Azondekon A, Dicko F, et al. 12-month mortality and loss-to-program in antiretroviral-treated children: The IeDEA pediatric West African Database to evaluate AIDS (pWADA), 2000–2008. BMC Public Health. 2011;11:519. doi: 10.1186/1471-2458-11-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenner L, Brinkhof MW, Keiser O, et al. Early mortality and loss to follow-up in HIV-infected children starting antiretroviral therapy in Southern Africa. J Acquir Immune Defic Syndr. 2010 Aug;54(5):524–532. doi: 10.1097/QAI.0b013e3181e0c4cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellis J, Molyneux EM. Experience of anti-retroviral treatment for HIV-infected children in Malawi: the 1st 12 months. Ann Trop Paediatr. 2007 Dec;27(4):261–267. doi: 10.1179/146532807X245643. [DOI] [PubMed] [Google Scholar]
- 20.Mugavero MJ, Amico KR, Westfall AO, et al. Early retention in HIV care and viral load suppression: implications for a test and treat approach to HIV prevention. J Acquir Immune Defic Syndr. 2012 Jan 1;59(1):86–93. doi: 10.1097/QAI.0b013e318236f7d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ICAP. Columbia University; [Accessed June 1, 2012]. Available at: http://www.columbia-icap.org/ [Google Scholar]
- 22.Lahuerta M, Lima J, Elul B, et al. Patients enrolled in HIV care in Mozambique: baseline characteristics and follow-up outcomes. J Acquir Immune Defic Syndr. 2011 Nov 1;58(3):e75–e86. doi: 10.1097/QAI.0b013e31822ac0a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO. Antiretroviral therapy for HIV infection in infants and children in resource-limited settings: towards universal access. Geneva: 2006. Available at: http://www.who.int/hiv/pub/guidelines/WHOpaediatric.pdf. [Google Scholar]
- 24.WHO. Geneva: 2010. Antiretroviral therapy for HIV infection in infants and children: towards universal access, revision 2010. Available at: http://whqlibdoc.who.int/publications/2010/9789241599801_eng.pdf. [PubMed] [Google Scholar]
- 25.WHO. Report of the WHO Technical Reference Group, Paediatric HIV/ART Care Guideline Group Meeting. Geneva: 2008. Apr 10–11, [Google Scholar]
- 26.Vaz P, Macassa E, Santos P, et al. National Guideline on Treatment for HIV-infected Children, Mozambique. Maputo: 2008. [Google Scholar]
- 27.The United Republic of Tanzania MoHaSW-NACP. National Guideline for the Management of HIV and AIDS, Third Edition. Dar es Salam: 2008. [Google Scholar]
- 28.National AIDS/STI Control Program K. Guidelines for antiretroviral therapy in Kenya, 4th edition. Nairobi: Ministry of Medical Services, Republic of Kenya; 2011. [Google Scholar]
- 29.Vaz P, Macassa E, Santos P, et al. National Guideline on Treatment for HIV-infected children, Mozambique. Maputo: 2011. [Google Scholar]
- 30.The United Republic of Tanzania MoHaSW-NACP. National Guideline for the Management of HIV and AIDS, Fourth Edition. Dar es Salam: 2012. [Google Scholar]
- 31.Chi BH, Yiannoutsos CT, Westfall AO, et al. Universal definition of loss to follow-up in HIV treatment programs: a statistical analysis of 111 facilities in Africa, Asia, and Latin America. PLoS Med. 2011 Oct;8(10):e1001111. doi: 10.1371/journal.pmed.1001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000 Jun 8;(314):1–27. [PubMed] [Google Scholar]
- 33.McGuire M, Munyenyembe T, Szumilin E, et al. Vital status of pre-ART and ART patients defaulting from care in rural Malawi. Trop Med Int Health. 2010 Jun;15(Suppl 1):55–62. doi: 10.1111/j.1365-3156.2010.02504.x. [DOI] [PubMed] [Google Scholar]
- 34.Braitstein P, Katshcke A, Shen C, et al. Retention of HIV-infected and HIV-exposed children in a comprehensive HIV clinical care programme in Western Kenya. Trop Med Int Health. 2010 Jul;15(7):833–841. doi: 10.1111/j.1365-3156.2010.02539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asfawesen GY, Solomie J, Bisirat T, Berhanu GM, Mebratu B, Rahlenbeck S. Outcome in a paediatric cohort receiving ART in Addis Abeba, Ethiopia. Acta Paediatr. 2011 Aug;100(8):1164–1167. doi: 10.1111/j.1651-2227.2011.02243.x. [DOI] [PubMed] [Google Scholar]
- 36.Wamalwa DC, Obimbo EM, Farquhar C, et al. Predictors of mortality in HIV-1 infected children on antiretroviral therapy in Kenya: a prospective cohort. BMC Pediatr. 2010;10:33. doi: 10.1186/1471-2431-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markers for predicting mortality in untreated HIV-infected children in resource-limited settings: a meta-analysis. AIDS. 2008 Jan 2;22(1):97–105. doi: 10.1097/01.aids.0000302262.51286.a5. [DOI] [PubMed] [Google Scholar]
- 38.Fetzer BC, Hosseinipour MC, Kamthuzi P, et al. Predictors for mortality and loss to follow-up among children receiving anti-retroviral therapy in Lilongwe, Malawi. Trop Med Int Health. 2009 Aug;14(8):862–869. doi: 10.1111/j.1365-3156.2009.02315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bong CN, Yu JK, Chiang HC, et al. Risk factors for early mortality in children on adult fixed-dose combination antiretroviral treatment in a central hospital in Malawi. AIDS. 2007 Aug 20;21(13):1805–1810. doi: 10.1097/QAD.0b013e3282c3a9e4. [DOI] [PubMed] [Google Scholar]
- 40.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004 Oct 2–8;364(9441):1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 41.Chatterjee A, Tripathi S, Gass R, et al. Implementing services for Early Infant Diagnosis (EID) of HIV: a comparative descriptive analysis of national programs in four countries. BMC Public Health. 2011;11:553. doi: 10.1186/1471-2458-11-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.UNAIDS website. [Accessed April 1, 2012]; Available at: http://www.unaids.org/en/regionscountries/countries. [Google Scholar]
- 43.Lowrance DW, Ndamage F, Kayirangwa E, et al. Adult clinical and immunologic outcomes of the national antiretroviral treatment program in Rwanda during 2004–2005. J Acquir Immune Defic Syndr. 2009 Sep 1;52(1):49–55. doi: 10.1097/QAI.0b013e3181b03316. [DOI] [PubMed] [Google Scholar]
- 44.Franke MF, Stulac SN, Rugira IH, et al. High human immunodeficiency virus-free survival of infants born to human immunodeficiency virus-positive mothers in an integrated program to decrease child mortality in rural Rwanda. Pediatr Infect Dis J. 2011 Jul;30(7):614–616. doi: 10.1097/INF.0b013e31820a599e. [DOI] [PubMed] [Google Scholar]
- 45.van Griensven J, De Naeyer L, Uwera J, Asiimwe A, Gazille C, Reid T. Success with antiretroviral treatment for children in Kigali, Rwanda: experience with health center/nurse-based care. BMC Pediatr. 2008;8:39. doi: 10.1186/1471-2431-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rich ML, Miller AC, Niyigena P, et al. Excellent clinical outcomes and high retention in care among adults in a community-based HIV treatment program in rural Rwanda. J Acquir Immune Defic Syndr. 2012 Mar 1;59(3):e35–e42. doi: 10.1097/QAI.0b013e31824476c4. [DOI] [PubMed] [Google Scholar]
- 47.Fatti G, Grimwood A, Mothibi E, Shea J. The effect of patient load on antiretroviral treatment programmatic outcomes at primary health care facilities in South Africa: a multicohort study. J Acquir Immune Defic Syndr. 2011 Sep 1;58(1):e17–e19. doi: 10.1097/QAI.0b013e318229baab. [DOI] [PubMed] [Google Scholar]
- 48.Lambdin BH, Micek MA, Koepsell TD, et al. Patient volume, human resource levels, and attrition from HIV Treatment programs in central Mozambique. J Acquir Immune Defic Syndr. 2011 Jul 1;57(3):e33–e39. doi: 10.1097/QAI.0b013e3182167e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomson KA, Cheti EO, Reid T. Implementation and outcomes of an active defaulter tracing system for HIV, prevention of mother to child transmission of HIV (PMTCT), and TB patients in Kibera, Nairobi, Kenya. Trans R Soc Trop Med Hyg. 2011 Jun;105(6):320–326. doi: 10.1016/j.trstmh.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 50.Tweya H, Gareta D, Chagwera F, et al. Early active follow-up of patients on antiretroviral therapy (ART) who are lost to follow-up: the 'Back-to-Care' project in Lilongwe, Malawi. Trop Med Int Health. 2010 Jun;15(Suppl 1):82–89. doi: 10.1111/j.1365-3156.2010.02509.x. [DOI] [PubMed] [Google Scholar]
- 51.Nash D, Wu Y, Elul B, Hoos D, El Sadr W. Program-level and contextual-level determinants of low-median CD4+ cell count in cohorts of persons initiating ART in eight sub-Saharan African countries. AIDS. 2011 Jul 31;25(12):1523–1533. doi: 10.1097/QAD.0b013e32834811b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nyandiko WM, Otieno-Nyunya B, Musick B, et al. Outcomes of HIV-exposed children in western Kenya: efficacy of prevention of mother to child transmission in a resource-constrained setting. J Acquir Immune Defic Syndr. 2010 May 1;54(1):42–50. doi: 10.1097/QAI.0b013e3181d8ad51. [DOI] [PubMed] [Google Scholar]
- 53.Ioannidis JP, Taha TE, Kumwenda N, et al. Predictors and impact of losses to follow-up in an HIV-1 perinatal transmission cohort in Malawi. Int J Epidemiol. 1999 Aug;28(4):769–775. doi: 10.1093/ije/28.4.769. [DOI] [PubMed] [Google Scholar]
- 54.van Kooten Niekerk NK, Knies MM, Howard J, et al. The first 5 years of the family clinic for HIV at Tygerberg Hospital: family demographics, survival of children and early impact of antiretroviral therapy. J Trop Pediatr. 2006 Feb;52(1):3–11. doi: 10.1093/tropej/fmi047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.