Abstract
OBJECTIVES
To examine associations of inflammation with physical function and potential mediation by white matter hyperintensities (WMH) in African-Americans (AA) and European-Americans (EA).
DESIGN
Cross-sectional analysis using linear and logistic models with Generalized Estimating Equations to account for familial clustering, reporting results as regression coefficients (β) and odds ratios (OR) adjusted for education, alcohol, exercise, BMI, hypertension, diabetes, heart disease, cognition, ankle brachial index, race-site and supported interactions.
SETTING
Genetic Epidemiology Network of Arteriopathy-Genetics of Microangiopathic Brain Injury Study cohort.
PARTICIPANTS
AA and EA sibships, ≥2 siblings with hypertension before age 60 (n=1960; 65% female, 51% AA, age 26–91y, 50% obese, 72% hypertensive).
MEASUREMENTS
Inflammation
C-reactive protein (CRP), interleukin-6 (IL6), soluble tumor necrosis factor receptors [sTNFR] 1 and 2; magnetic resonance imaged WMH volumes (cm3). Walking speed (cm/second) over 25 feet and mobility difficulty (any self-reported difficulty walking ½ mile).
RESULTS
In separate models, inflammatory markers were associated with walking speed (sTNFR1: β=−2.74, p<.001; sTNFR2: −1.23, p=.03; CRP β=−1.95, p=.001; IL6 β=−1.24, p=.03) and mobility difficulty (sTNFR1: OR=1.36, p=.001; sTNFR2:OR=1.25, p=.005; CRP OR=1.22, p=.005; IL6 OR=1.18, p=.02); WMH was associated marginally only with sTNFR1 in AA (β=0.07, p=0.06). WMH were associated with walking speed in AA (AA: (β=−3.17, p=0.017; EA: β=−2.23, p=0.17) but not with mobility difficulty (OR=1.10, p=.54). Adjusting for WMH did not change associations.
CONCLUSION
In young-to-old persons with prevalent cardiovascular risk factors, multiple inflammatory biomarkers were associated with slower walking speed independent of microvascular disease in the brain. There was little evidence for mediation by brain WMH. Inflammation may contribute to physical function impairments through pathways other than brain microvascular disease, particularly in AA.
Keywords: inflammation, physical function, white matter hyperintensity, ethnicity
INTRODUCTION
Preserved physical and cognitive functions enable older adults to maintain independent living while impaired physical function, such as slower walking speeds, leads to poorer quality of life, institutionalization, incident disability, higher healthcare costs and mortality in community-dwelling older adults.1–3 The underlying mechanisms contributing to physical function impairments are not known. One hypothesis involves inflammatory-mediated effects on muscle, leading to sarcopenia and muscle weakness.4,5 Higher levels of interleukin-6 (IL-6), high sensitivity C-reactive protein (CRP), and tumor necrosis factor alpha (TNFα) are associated with slower walking speed and may directly affect muscle strength or function.5–7
However, inflammation is also associated with microvascular damage in the brain,8–10 and structural brain abnormalities, including white matter hyperintensities (WMH), are associated with gait instability, slower walking speed and declines in physical function.11–14 It is plausible that inflammation contributes to physical function impairments indirectly through effects on the brain microvascular system that damage motor control mechanisms.
The purpose of this study was to examine relationships of the inflammatory biomarkers CRP, IL-6, and soluble tumor necrosis factor receptors 1 and 2 (sTNFR1 & 2)15 with subjective and objective measures of physical function and potential mediating effects of WMH, indicative of microvascular ischemia, in a biracial cohort with prevalent cardiovascular risk factors.
METHODS
Population
The Genetic Epidemiology Network of Arteriopathy (GENOA) study is a cohort of 3,437 hypertensive adults and their siblings recruited in 1995 from Jackson, Mississippi (all African-Americans [AA]) and Rochester, Minnesota (all European-Americans [EA]). GENOA recruited sibships with the requirement that at least 2 siblings have essential hypertension prior to age 60. Additional siblings were recruited regardless of hypertension status. Inflammatory markers were assayed at the second examination coinciding with the Genetics of Microangiopathic Brain Injury [GMBI] Study, (2001–2006) which also conducted brain magnetic resonance imaging (MRI, n=1660), physical function (n=1960). (Supplementary Figure 1)
Inflammatory Markers
Blood was drawn at each field center after an overnight fast, centrifuged for 10 min at 4°C, aliquoted in 0.5–1 mL volumes of EDTA plasma (or serum for CRP), and stored at −80°C. Frozen samples at the Jackson site were shipped overnight on dry ice to the Mayo Clinic Immunochemical Core Laboratory (Rochester, MN). IL-6, sTNFR1, and sTNFR2 levels were measured using a multiplex assay (SearchLight™, Pierce, Boston, MA); CRP levels were measured using a high sensitivity assay.16 TNF soluble receptor fractions have been validated as sensitive indicators of TNFα system activation and were assayed rather than TNFα levels due to longer half-lives and features of stability over time of the receptors.15,17
Physical Function Assessments
Time to walk 25 feet (7.62 m) at the participants’ usual pace was recorded in an unobstructed corridor with a stop watch and converted to centimeters (cm)/second (s). Subjective mobility difficulty was assessed by asking participants how much difficulty they had walking ½ mile (“None”, “Some,” “A lot,” or “Unable”). Responses were dichotomized as ‘none’ (referent) versus ‘any’.
Brain Magnetic Resonance Imaging
Brain MRI using 1.5T MR units (GE Medical Systems, Waukesha, WI) was offered to all participants, of whom 1,666 consented and were included. Of these, 6 were missing walking speed and 8 were missing mobility difficulty. Persons with a history of stroke, neurologic disease, or implanted metal devices (n=8), hydrocephalus on imaging (n=3), or poor quality scans (e.g. motion artifact, n=7) were excluded. Interactive image processing steps were performed blinded to participants’ histories. A fully automated algorithm segmented each slice of the multi-slice, edited, fluid-attenuated inversion recovery (FLAIR) sequence (based on image intensity) into voxels, which were assigned either to brain, cerebrospinal fluid, or WMH categories. For WMH volume, the mean absolute error of this method is 6.6%, and the mean test-retest coefficient of variation is 1.4%. WMH volume (cm3) was determined from axial FLAIR images. Each set consisted of contiguous 3-mm interleaved slices with no inter-slice gap.
Potential Confounders
Global cognitive function was assessed using the Mini-Mental State Examination (MMSE)18 via a protocol accordant with the Consortium for the Establishment of a Registry for Alzheimer’s Disease battery. Educational level was recorded as <12 years, 12 years (high school or equivalent degree), some college, or ≥ college degree. “Ever smoked” was defined as those responding they had ever smoked more than 100 cigarettes. Alcohol use was assessed by self-report of “sometimes” versus “never” drinking alcoholic beverages. Hours of moderate or vigorous exercise was self-reported as the number of hours per day in the past week spent doing moderate (e.g. heavy housework) or vigorous activity and multiplied by 7. Height was measured by stadiometer and weight by electronic balance with participants wearing lightweight clothing. Body mass index (BMI) was calculated as weight (kg)/height (m2). The average of the 2nd and 3rd blood pressure measurements, taken in a seated, rested state with appropriately sized cuffs, was used. Self-reports of medical conditions were asked as “Have you ever been told by a physician that you had [condition]?” Hypertension was defined as blood pressure >140/90, self-report, or use of anti-hypertensive medications. Diabetes was defined as fasting glucose ≥126 mg/dl or random >200 mg/dl, self-report, or use of hypoglycemic medications. History of coronary heart disease (CHD) was defined as having a history of myocardial infarction or blocked coronary arteries that required surgery, angioplasty or balloon dilation. Ankle-brachial index (ABI) was measured as the lower of left or right average posterior tibial and dorsalis pedis systolic pressures divided by highest brachial systolic pressure.19
Statistical Analyses
Walking speed was used as a continuous and categorical variable, dichotomized at ≥1 m/sec (good vs poor).20 WMH were log transformed due to skewness. Descriptive statistics were compared using Fisher’s Exact test for categorical and Wilcoxon Rank Sum Tests for continuous variables by walking speed categories. To assess the relationships between inflammatory markers, WMH, and function, we used linear and logistic models as appropriate with generalized estimating equations (GEE), accounting for clustering by sibship. The inflammatory markers sTNFR1, sTNFR2, CRP, and IL-6 were standardized to allow comparisons of relationships across biomarkers with different units of measure. Results are reported as standardized regression coefficients (β) for continuous and odds ratios (OR) for categorical outcomes. Therefore, a β= −3.0 for CRP on the outcome walking speed would be interpreted as for every standard deviation increase in CRP, the expected walking speed would be 3 cm/s slower.
To assess mediation by WMH of the relationships of inflammatory biomarkers to physical function, we constructed separate models using the Kenny-Barron21 approach of examining relationships of (a) the predictor (sTNFR1, sTNFR2, CRP, or IL-6) to the proposed mediator (WMH); (b) the predictor to the outcome (physical function); and (c) the proposed mediator to the outcome. Lastly we compared the association of the predictor to the outcome observed in (b) to the association between the predictor and outcome from a model that also adjusted for the proposed mediator. A mediator should be associated with the predictor (a) and the outcome (c) and should attenuate the relationship of the predictor to the outcome observed in (b). Sensitivity to missing data was examined using inverse proportionately weighted GEE models. All models adjusted for education, alcohol, exercise, BMI, hypertension, diabetes, CHD, MMSE and interactions between race and age, sex, smoking, and ABI. There was little support for differential relationships between inflammatory markers and function by race using race-stratified analyses or interaction terms (race × inflammatory marker interactions all p>0.5) so data for EA and AA were pooled. Additional sensitivity analyses were performed comparing primary models to models that also adjusted for history of stroke and interactions of all variables with race. The results were substantively unchanged.
As a measure of overall dysregulated inflammation, quartiles of each inflammatory marker were constructed and a count of inflammatory markers in the uppermost quartile was tallied (0, 1, 2, ≥ 3 or more). Because there were few participants with all four markers in the top quartile, participants with three or four inflammatory markers in the top quartile were combined. Statistical analyses were performed in Stata 12.0 (StataCorp LP, College Station, TX).
RESULTS
Participants with walking speed <1 m/s were more likely to be older, have less education, lower MMSE, greater WMH volumes, generally worse health, and higher levels of sTNFR1, sTNFR2, CRP, and IL-6 (Table 1). Overall, the mean age of participants was 61.2 years (standard deviation=10.0, range=26–94). AA tended to be older (63.1 (9.5), 26–94 years) than EA (58.9 years (10.2), 29–84 years). The mean WMH volume was 9.0 cm3 (9.2), range=1.2–126, and was higher in AA (10.4 cm3 (11.4), 2.0–126), than in EA (7.7 cm3 (6.4), 1.2–62). In addition, AA had higher levels of CRP (t=−3.37, p=0.0004) and IL-6 (t=−7.17, p<0.0001) but lower sTNFR1 (t=5.73, p<0.0001). EA with slow walking speeds were more likely to be nondrinkers and AA with slow walking speeds were more likely to be women and have a history of CHD.
Table 1.
Participant Characteristics by Race and Walking Speed (WS)
| Characteristic | European American | African American | |||||
|---|---|---|---|---|---|---|---|
| Good WS (WS ≥ 1 m/sec) |
Poor WS (WS < 1 m/s) |
p-value | Good WS (WS ≥ 1 m/sec) |
Poor WS (WS < 1 m/s) |
p-value | ||
| n=724 (75%) | n=238 (25%) | n=311 (31%) | n=687 (69%) | ||||
| Demographics | Age (years) | 57.0 (9.4) | 66.2 (8.5) | <0.001 | 60.2 (8.7) | 64.1 (8.4) | <0.001 |
|
| |||||||
| Women | 419 (58%) | 146 (61%) | 0.363 | 189 (61%) | 513 (75%) | <0.001 | |
|
| |||||||
| Education | |||||||
|
| |||||||
| <12 Years | 26 (5%) | 25 (12%) | <0.001 | 55 (23%) | 242 (41%) | <0.001 | |
| 12 Years | 278 (49%) | 124 (61%) | <0.001 | 79 (32%) | 199 (34%) | <0.001 | |
| Some College | 114 (20%) | 24 (12%) | <0.001 | 5 (2%) | 13 (2%) | <0.001 | |
| ≥College Degree | 152 (27%) | 30 (15%) | <0.001 | 105 (43%) | 140 (24%) | <0.001 | |
|
| |||||||
| Ever Smoked | 361 (50%) | 105 (44%) | 0.135 | 113 (36%) | 280 (41%) | 0.208 | |
|
| |||||||
| Alcohol | 571 (79%) | 161 (68%) | 0.001 | 118 (38%) | 235 (34%) | 0.254 | |
|
| |||||||
| Exercise (hours/week) | 28.1 (17.6) | 21.0 (15.6) | <0.001 | 10.8 (11.1) | 7.7 (11.3) | <0.001 | |
|
| |||||||
| Comorbidities | Body Mass Index | 30.1 (5.8) | 31.7 (6.0) | <0.001 | 29.9 (5.1) | 31.9 (6.5) | <0.001 |
|
| |||||||
| Hypertension | 500 (69%) | 195 (82%) | <0.001 | 204 (66%) | 513 (75%) | 0.004 | |
|
| |||||||
| Diabetes Mellitus | 63 (9%) | 45 (19%) | <0.001 | 57 (18%) | 198 (29%) | <0.001 | |
|
| |||||||
| Coronary Heart Disease | 54 (7%) | 16 (7%) | 0.775 | 8 (3%) | 49 (7%) | 0.003 | |
|
| |||||||
| Stroke | 8 (1%) | 8 (3%) | 0.035 | 3 (1%) | 38 (6%) | <0.001 | |
|
| |||||||
| Ankle-brachial index | 1.1 (0.1) | 1.1 (0.2) | <0.001 | 1.0 (0.1) | 1.0 (0.1) | 0.015 | |
|
| |||||||
| Brain Structure | White Matter Hyperintensities (cm3) | 7.01 (5.88) | 9.85 (7.41) | <0.001 | 8.91 (9.61) | 10.97 (11.60) | 0.015 |
|
| |||||||
| Cognitive Function | Mini-Mental State Exam | 28.9 (1.5) | 28.5 (1.6) | <0.001 | 27.9 (1.9) | 26.6 (2.6) | <0.001 |
|
| |||||||
| Inflammatory Markers | sTNFR1 | 1283.58 (638.09) | 1576.59 (730.74) | <0.001 | 1066.66 (498.00) | 1211.52 (662.22) | 0.012 |
|
| |||||||
| sTNFR2 | 1892.03 (762.10) | 2156.88 (882.59) | <0.001 | 1770.96 (711.56) | 1907.51 (822.32) | 0.019 | |
|
| |||||||
| C-reactive Protein | 3.79 (4.48) | 5.43 (6.06) | <0.001 | 4.51 (5.59) | 6.12 (6.72) | <0.001 | |
|
| |||||||
| Interleukin-6 | 8.17 (6.64) | 9.36 (6.39) | <0.001 | 8.81 (6.88) | 9.59 (6.45) | 0.001 | |
Wilcoxon Rank Sum Test for continuous variables (mean ± standard deviation) or Fisher’s Exact tests (count (%)) for categorical variables.
MMSE = Mini-Mental State Examination; sTNFR1 = soluble tumor necrosis factor receptor 1; sTNFR2 = soluble tumor necrosis factor receptor 2
Increases in each inflammatory marker were associated with slower walking speed. Walking speed was 2.74 cm/s (95% confidence internal (CI): −3.95, −1.53, p<.001) slower for each standard deviation increase in sTNFR1 and 1.23 cm/s (95% CI: −2.34, −0.12; p=.030) slower for each standard deviation increase in sTNFR2. Each standard deviation increase in CRP was associated with a 1.95 cm/s (95% CI: −3.08, −0.81, p=.001) slower walking speed and IL-6 with a 1.24 m/s slower walking speed (95% CI: −2.39, −0.10; p=.033) (Table 2).
Table 2.
Relationships between Inflammatory Markers and Physical Function or White Matter Hyperintensity (WMH) Volumes and between WMH Volumes and Physical Function
| Walking Speed | Mobility Difficulty | WMH (EA) | WMH (AA) | |
|---|---|---|---|---|
| β, p-value (95% CI) | OR, p-value (95% CI) | β, p-value (95% CI) | β, p-value (95% CI) | |
| sTNFR1 | −2.743 p<0.001 | 1.360 p=0.001 | 0.004 p=0.836 | 0.070 p=0.055 |
| (−3.95,−1.53) | (1.13,1.63) | (−0.03,0.04) | (−0.00,0.14) | |
|
| ||||
| sTNFR2 | −1.230 p=0.030 | 1.247 p=0.005 | 0.016 p=0.392 | 0.042 p=0.229 |
| (−2.34,−0.12) | (1.07,1.45) | (−0.02,0.05) | (−0.03,0.11) | |
|
| ||||
| CRP | −1.946 p=0.001 | 1.219 p=0.005 | 0.001 p=0.959 | 0.013 p=0.563 |
| (−3.08,−0.81) | (1.06,1.40) | (−0.04,0.04) | (−0.03,0.06) | |
|
| ||||
| IL-6 | −1.245 p=0.033 | 1.179 p=0.021 | −0.012 p=0.565 | 0.029 p=0.264 |
| (−2.39,−0.10) | (1.02,1.36) | (−0.05,0.03) | (−0.02,0.08) | |
|
| ||||
| WMH in EA | −2.233 p=0.166 | 1.522 p=0.103 | ||
| (−5.39,0.92) | (0.92,2.52) | |||
|
| ||||
| WMH in AA | −3.173 p=0.017 | 1.101 p=0.541 | ||
| (−5.78,−0.57) | (0.81,1.50) | |||
Inflammatory biomarkers are standardized. WMH are log-transformed. Adjusted for education, alcohol, exercise, BMI, HTN, DM, CHD, and MMSE, race-site (except where race-stratified) and interactions (in pooled analyses) between race and age, sex, smoking, and ABI. Pooled additionally adjusted for race.
sTNFR1 = soluble tumor necrosis factor receptor 1; sTNFR2 = soluble tumor necrosis factor receptor 2; CRP = C-reactive protein; IL-6 = interleukin=6; WMH = white matter hyperintensities; EA = European American; AA = African American
Similar associations were observed between inflammatory markers and self-reported mobility difficulty in adjusted models. Each standard deviation increase in sTNFR1 (OR=1.36, 95% CI 1.13, 1.63; p=.001), sTNFR2 (OR=1.25, 95% CI 1.07, 1.45; p=.005), CRP (OR=1.22, 95% CI 1.06, 1.40; p=.005), and IL-6 (OR=1.18, 95% CI 1.02, 1.36; p=.021) was associated with greater odds of reporting mobility difficulty (Table 2).
Slower walking speed was associated with higher WMH volumes in AA (β= −3.17, 95% CI −5.78, −0.57; p=0.017) but not EA (β= −2.23, 95% CI −5.39, 0.92; p=.166) although estimates were of similar magnitude (Table 2). Self-reported mobility difficulty was not associated with WMH in either AA or EA. WMH volume was not statistically associated with inflammatory markers, suggesting that effects of inflammation on function in this cohort were not mediated by WMH. Therefore, WMH was treated as an adjustor variable in the following results.
Table 3 shows the associations of each inflammatory marker with functional outcomes, adjusted for the same variables as in Table 2 and for WMH. Adjusting for WMH did not change the association of the inflammatory biomarkers with either physical function outcome. Only the relationship of sTNFR2 with walking speed lost statistical significance when adjusting for WMH, with little change in the beta coefficient (β=−1.23, 95% CI: −2.34, −0.12, p=.030 versus β=−1.12, −2.27, 0.04, p=.058). We similarly found no evidence of mediation by WMH in sensitivity analyses examining only older (>65 years) adults. Similar mediation results were found in sensitivity analyses including only participants with complete data.
Table 3.
The Relationship of Inflammation with Physical Function with and without Adjusting for White Matter Hyperintensities (WMH)
| Walking Speed (cm/s) | Mobility Difficulty | |||
|---|---|---|---|---|
| β, p-value | OR, p-value | |||
| Inflammatory Marker | (95% CI) | (95% CI) | ||
| m1: Without WMH n=1960 |
m2: With WMH n=1660 |
m1: Without WMH n=1961 |
m2: With WMH n=1958 |
|
| sTNFR1 | −2.743 p<0.001 | −2.524 p<0.001 | 1.360 p=0.001 | 1.320 p=0.007 |
| (−3.95,−1.53) | (−3.83,−1.22) | (1.13,1.63) | (1.08,1.61) | |
|
| ||||
| sTNFR2 | −1.230 p=0.030 | −1.118 p=0.058 | 1.247 p=0.005 | 1.223 p=0.014 |
| (−2.34,−0.12) | (−2.27,0.04) | (1.07,1.45) | (1.04,1.44) | |
|
| ||||
| CRP | −1.946 p=0.001 | −2.460 p<0.001 | 1.219 p=0.005 | 1.200 p=0.018 |
| (−3.08,−0.81) | (−3.72,−1.20) | (1.06,1.40) | (1.03,1.40) | |
|
| ||||
| IL-6 | −1.245 p=0.033 | −1.399 p=0.034 | 1.179 p=0.021 | 1.227 p=0.011 |
| (−2.39,−0.10) | (−2.69,−0.11) | (1.02,1.36) | (1.05,1.44) | |
m1: Model 1, Adjusted for education, alcohol, exercise, BMI, HTN, DM, CHD, and MMSE, race-site and interactions between race and age, sex, smoking, and ABI.
m2: Model 2, Adjusted for variables in “m1” plus WMH
Abbreviations: sTNFR1 = soluble tumor necrosis factor receptor 1; sTNFR2 = soluble tumor necrosis factor receptor 2; CRP = C-reactive protein; IL-6 = interleukin-6; WMH = white matter hyperintensities
Having more inflammatory markers in the highest quartile was associated with slower walking speed (Table 4). Both race-stratified and pooled analyses are shown. Having one inflammatory marker in the top quartile was associated with a 3.2 cm/s decrease in walking speed (β=−3.24, 95% CI −5.95, −0.54; p=0.019), two markers with a 4.4 cm/s decrease (β=−4.37, 95% CI −7.68, −1.06; p=0.010), and three or four markers with a 7.0 cm/s decrease (β=−7.02, 95% CI −11.25, −2.78; p=0.001). Having three or four markers in the upper quartile was also associated with greater odds of mobility difficulty (OR=2.7, 95% CI 1.47, 5.05; p=0.001).
Table 4.
Relationship of Physical Function with Increasing Numbers of Inflammatory Markers in Highest Quartiles Stratified by Race and Pooled Results
| Walking Speed (cm/s) | Mobility Difficulty | |||||
|---|---|---|---|---|---|---|
| # Inflammatory Markers in Top Quartile | # Inflammatory Markers in Top Quartile | |||||
| 1 | 2 | 3 or 4 | 1 | 2 | 3 or 4 | |
| GEE | GEE | GEE | GEE | GEE | GEE | |
| β, p-value | β, p-value | β, p-value | OR, p-value | OR, p-value | OR, p-value | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| EA | −1.86 p=0.328 | −4.95 p=0.036 | −7.04 p=0.016 | 0.97 p=0.931 | 1.83 p=0.114 | 2.87 p=0.029 |
| (−5.59,1.87) | (−9.56,−0.33) | (−12.78,−1.30) | (0.43,2.15) | (0.86,3.89) | (1.12,7.39) | |
|
| ||||||
| AA | −4.65 p=0.018 | −3.87 p=0.101 | −6.97 p=0.021 | 0.88 p=0.597 | 1.29 p=0.360 | 2.71 p=0.018 |
| (−8.48,−0.81) | (−8.50,0.76) | (−12.91,−1.03) | (0.54,1.42) | (0.75,2.22) | (1.18,6.20) | |
|
| ||||||
| Pooled | −3.24 p=0.019 | −4.37 p=0.010 | −7.02 p=0.001 | 0.90 p=0.623 | 1.46 p=0.099 | 2.72 p=0.001 |
| (−5.95,−0.54) | (−7.68,−1.06) | (−11.25,−2.78) | (0.59,1.36) | (0.93,2.27) | (1.47,5.05) | |
Adjusted for education, alcohol, exercise, BMI, HTN, DM, CHD, and MMSE, race-site (except where race-stratified) and interactions (in pooled analyses) between race and age, sex, smoking, and ABI. Pooled analyses additionally adjusted for race.
GEE = Generalized Estimating Equation; EA = European American; AA = African American
We conducted additional sensitivity analyses to examine effects of missing data (Supplementary Table 2). EA who were missing walking speed had higher BMI, lower MMSE, more prevalent stroke, and higher IL-6. Otherwise, there were inconsistent patterns across characteristics for EA missing physical function assessments, with some sharing similar characteristics to those with good (≥1m/s) walking speed (smoking status, hypertension), those with poor (<1m/s) walking speed (alcohol intake), and others who had characteristics between those with good and poor walking speed (age, education, exercise, diabetes, sTNFR1, sTNFR2, CRP). AA who did not have walking speed assessments were less educated, had generally poorer health, lower MMSE scores, and higher WMH volumes (Supplementary Table 3). In addition, all inflammatory biomarker levels were highest among AA who did not complete the walking assessment. Sensitivity analyses examining potential effects of missingness are shown in Supplementary Table 4, with essentially no substantive change in results.
DISCUSSION
This study demonstrates that higher levels of inflammation, both individual biomarkers and having more inflammatory biomarkers in upper quartiles, are associated with poor subjective and objective measures of physical function. The results were independent of several potential confounders. In addition, the data did not support mediation of these findings by brain microvascular disease in African American or European Americans, suggesting that separate pathways involving inflammation and brain structure may affect physical function. The clinical implications could be quite substantial. For example, the difference in walking speed for persons with the highest versus the lowest sTNFR1 levels would translate to a difference of 9 cm/s. A 10 cm/s faster walking speed is associated with a 12% lower mortality in older adults22, and 5 cm/s is considered a meaningful change.23
Prior studies have reported that inflammation can impair insulin action, modify hormone secretion and hormone receptor transduction, impair endothelial function and energy regulation, or contribute to microvascular changes in the vascular system,24–28 which may contribute to muscle weakness, reduced endurance, ineffective cell metabolism and energy production, and impaired central nervous system control of motor movements. Although other studies have shown associations between structural brain abnormalities and physical function measures,29–31 we only observed associations between WMH and walking speed in the AA participants. This could be due to the younger age of the cohort and AA having higher inflammation and more WMH. In addition, EA had faster gait speeds than AA and may have been above the threshold of detection in this younger cohort. Previous studies have also demonstrated lower physical function and greater disability in AA compared to EA.32,33 Potentially, higher levels of inflammation among AA could contribute to disparities in physical function.
Self-selected walking speed may provide an estimate of efficiency and reserve in multiple systems, including cardiopulmonary, musculoskeletal, peripheral and central nervous systems, hormones and inflammation among others, that interact to control mobility.34–38 However, controlling for several comorbidities that have been associated with poorer physical function37,39,40 had little effect on the relationship between inflammatory markers and physical function, consistent with the view that frailty and functional decline are affected by, but also distinct from, comorbidity.41 The foremost biomarkers of inflammation emphasized in previous studies of inflammation and physical function are CRP and IL-6.5–7,38–46 The current study adds to our knowledge of relationships between inflammation and physical function by including biomarkers of TNFα activity. Few studies examined markers of TNFα activity and most have used TNFα rather than soluble receptors of TNF.6,42,46 However, the latter appear more stable over time and better reflect TNF activity.17,47 Some studies of TNFα failed to show associations with physical performance measures6,42,48 while those using sTNFR1 and sTNFR2 assays demonstrated poor function with higher levels of sTNFR1 and sTNFR249 as do ours. Hence the current findings corroborate studies supporting a role of multiple inflammatory biomarkers’ involvement in impaired physical function, including those with cardiovascular risk factors.
Some limitations of this study warrant discussion. The cross-sectional design prohibits inferences about causality. However, the lack of mediation remains of interest and should be less affected by the cross-sectional design. The young age of the cohort could limit the ability to detect relationships due to early, small changes in gait characteristics that speed does not capture; additionally, the younger age may have limited the WMH exposure, particularly among EA. However, WMH were associated with walking speed and inflammation in AA, and the robust findings that walking speed was associated with all inflammatory markers even in this young cohort suggests further studies in mid-life are warranted to better understand mechanistic pathways. Differences by race could result from regional differences or other explanations as all AA participants were from Jackson, MS and all EA were from Rochester, MN. However, the differences in inflammatory biomarker levels and functional outcomes between EA and AA are consistent with other studies.32,50 This study adds important information given the limited data on inflammation and physical function in racially diverse populations.
In summary, in a biracial cohort of young to old participants with prevalent cardiovascular risk factors, inflammation was negatively associated with subjective and objective measures of physical function. The data did not support meditation by microvascular lesions in the brain and provide additional support for the hypothesis that inflammation has direct biological effects on physical function in EA and AA. Interventions to reduce structural brain changes and inflammation in mid-life or earlier may help preserve physical function, particularly in AA with prevalent cardiovascular risk factors.
Supplementary Material
Figure 1.
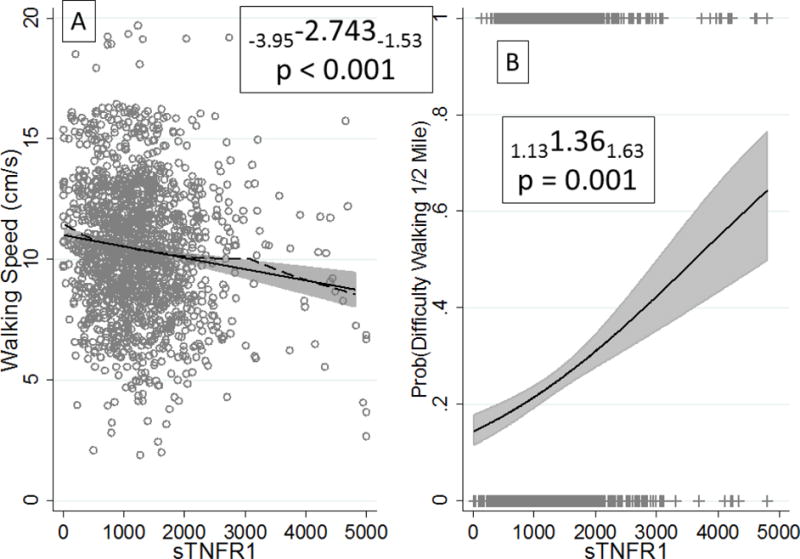
Associations of Soluble Tumor Necrosis Factor 1 with Walking Speed (Panel A) and Probability of Difficulty Walking ½ Mile (Panel B). Panel A reports beta coefficients from multiple linear regression, and panel B reports odds ratios from logistic regression, both with confidence intervals in subscripts for a 1 standard deviation (SD) increase in sTNFR1 (SD=711). Both models were adjusted for education, alcohol, exercise, BMI, HTN, DM, CHD, and MMSE, race-site, and interactions between race and age, sex, smoking, and ABI.
Acknowledgments
Funding Source: National Institutes of Health Grants U01-HL054463, R01-HL87660, HL-81331, M01 RR00585.
Sponsor’s Role: The sponsor played no role in the design, methods, subject recruitment, data collections, analysis, or preparation of paper.
Conflict of Interest Checklist
| Elements of Financial/Personal Conflicts | B Windham | S Wilkening | S Lirette | I Kullo | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | ||||
| Grants/Funds | X | X | X | X | ||||
| Honoraria | X | X | X | X | ||||
| Speaker Forum | X | X | X | X | ||||
| Consultant | X | X | X | X | ||||
| Stocks | X | X | X | X | ||||
| Royalties | X | X | X | X | ||||
| Expert Testimony | X | X | X | X | ||||
| Board Member | X | X | X | X | ||||
| Patents | X | X | X | X | ||||
| Personal Relationship | X | X | X | X | ||||
| Elements of Financial/Personal Conflicts | S Turner | M Griswold | T Mosley | |||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | |||||
| Grants/Funds | X | X | X | |||||
| Honoraria | X | X | X | |||||
| Speaker Forum | X | X | X | |||||
| Consultant | X | X | X | |||||
| Stocks | X | X | X | |||||
| Royalties | X | X | X | |||||
| Expert Testimony | X | X | X | |||||
| Board Member | X | X | X | |||||
| Patents | X | X | X | |||||
| Personal Relationship | X | X | X | |||||
Footnotes
Author Contributions: Concept and design: Windham, Griswold. Acquisition of subjects and data: Mosley. Analysis and interpretation of data: Windham, Wilkening, Lirette, Kullo, Turner, Griswold, Mosley. Drafting of manuscript: Windham, Wilkening. Critical revision of manuscript: Lirette, Kullo, Turner, Griswold, Mosley.
References
- 1.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-Extremity Function in Persons over the Age of 70 Years as a Predictor of Subsequent Disability. The New England journal of medicine. 1995;332:556–62. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 3.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cannon JG. Cytokines in aging and muscle homeostasis. The journals of gerontology Series A, Biological sciences and medical sciences. 1995;50 doi: 10.1093/gerona/50a.special_issue.120. Spec No:120-3. [DOI] [PubMed] [Google Scholar]
- 5.Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. Journal of the American Geriatrics Society. 2002;50:1947–54. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 6.Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. The journals of gerontology Series A, Biological sciences and medical sciences. 2004;59:242–8. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 7.Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. The journals of gerontology Series A, Biological sciences and medical sciences. 2000;55:M709–15. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 8.Fornage M, Chiang YA, O’Meara ES, et al. Biomarkers of Inflammation and MRI-Defined Small Vessel Disease of the Brain: The Cardiovascular Health Study. Stroke; a journal of cerebral circulation. 2008;39:1952–9. doi: 10.1161/STROKEAHA.107.508135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raz N, Yang Y, Dahle CL, Land S. Volume of white matter hyperintensities in healthy adults: contribution of age, vascular risk factors, and inflammation-related genetic variants. Biochimica et biophysica acta. 2012;1822:361–9. doi: 10.1016/j.bbadis.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali MS, Starke RM, Jabbour PM, et al. TNF-alpha induces phenotypic modulation in cerebral vascular smooth muscle cells: implications for cerebral aneurysm pathology. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013 doi: 10.1038/jcbfm.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guttmann CR, Benson R, Warfield SK, et al. White matter abnormalities in mobility-impaired older persons. Neurology. 2000;54:1277–83. doi: 10.1212/wnl.54.6.1277. [DOI] [PubMed] [Google Scholar]
- 12.Rosano C, Kuller LH, Chung H, et al. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. Journal of the American Geriatrics Society. 2005;53:649–54. doi: 10.1111/j.1532-5415.2005.53214.x. [DOI] [PubMed] [Google Scholar]
- 13.Baezner H, Blahak C, Poggesi A, et al. Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology. 2008;70:935–42. doi: 10.1212/01.wnl.0000305959.46197.e6. [DOI] [PubMed] [Google Scholar]
- 14.de Laat KF, van Norden AG, Gons RA, et al. Gait in elderly with cerebral small vessel disease. Stroke; a journal of cerebral circulation. 2010;41:1652–8. doi: 10.1161/STROKEAHA.110.583229. [DOI] [PubMed] [Google Scholar]
- 15.Aderka D, Sorkine P, Abu-Abid S, et al. Shedding kinetics of soluble tumor necrosis factor (TNF) receptors after systemic TNF leaking during isolated limb perfusion. Relevance to the pathophysiology of septic shock The Journal of clinical investigation. 1998;101:650–9. doi: 10.1172/JCI694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim CX, Bailey KR, Klee GG, et al. Sex and ethnic differences in 47 candidate proteomic markers of cardiovascular disease: the Mayo Clinic proteomic markers of arteriosclerosis study. PloS one. 2010;5:e9065. doi: 10.1371/journal.pone.0009065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aderka D, Engelmann H, Shemer-Avni Y, et al. Variation in serum levels of the soluble TNF receptors among healthy individuals. Lymphokine and cytokine research. 1992;11:157–9. [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch At, Haskal ZJ, Hertzer NR, Bakal CW, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 20.Cesari M, Kritchevsky SB, Newman AB, et al. Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging And Body Composition Study. Journal of the American Geriatrics Society. 2009;57:251–9. doi: 10.1111/j.1532-5415.2008.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of personality and social psychology. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 22.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. Journal of the American Geriatrics Society. 2006;54:743–9. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 24.Roubenoff R, Hughes VA. Sarcopenia: current concepts. The journals of gerontology Series A, Biological sciences and medical sciences. 2000;55:M716–24. doi: 10.1093/gerona/55.12.m716. [DOI] [PubMed] [Google Scholar]
- 25.Walston J. Frailty–the search for underlying causes. Science of aging knowledge environment: SAGE KE. 2004;2004:pe4. doi: 10.1126/sageke.2004.4.pe4. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed T, Haboubi N. Assessment and management of nutrition in older people and its importance to health. Clinical interventions in aging. 2010;5:207–16. doi: 10.2147/cia.s9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brooks-Asplund EM, Tupper CE, Daun JM, Kenney WL, Cannon JG. Hormonal modulation of interleukin-6, tumor necrosis factor and associated receptor secretion in postmenopausal women. Cytokine. 2002;19:193–200. doi: 10.1006/cyto.2002.1963. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Zhou M, Brand J, Huang L. Inflammation Activates the Interferon Signaling Pathways in Taste Bud Cells. The Journal of Neuroscience. 2007;27:10703–13. doi: 10.1523/JNEUROSCI.3102-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gouw AA, Flier WM, Straaten ECW, et al. Simple versus complex assessment of white matter hyperintensities in relation to physical performance and cognition: the LADIS study. Journal of neurology. 2006;253:1189–96. doi: 10.1007/s00415-006-0193-5. [DOI] [PubMed] [Google Scholar]
- 30.Rosano C, Brach J, Studenski S, Longstreth WT, Jr, et al. Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology. 2007;29:193–200. doi: 10.1159/000111582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosano C, Brach J, Longstreth WT, Jr, Newman AB. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology. 2006;26:52–60. doi: 10.1159/000089240. [DOI] [PubMed] [Google Scholar]
- 32.Mendes de Leon CF, Barnes LL, Bienias JL, et al. Racial disparities in disability: recent evidence from self-reported and performance-based disability measures in a population-based study of older adults. The journals of gerontology Series B, Psychological sciences and social sciences. 2005;60:S263–71. doi: 10.1093/geronb/60.5.s263. [DOI] [PubMed] [Google Scholar]
- 33.Blanco I, Verghese J, Lipton RB, et al. Racial differences in gait velocity in an urban elderly cohort. Journal of the American Geriatrics Society. 2012;60:922–6. doi: 10.1111/j.1532-5415.2012.03927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrucci L, Ble A, Bandinelli S, Windham BG, Simonsick EM. Inflammation: The Fire of Frailty? In: Carey JR, Robine J-M, Michel JP, editors. Longevity and Frailty. New York: Springer-Verlag; 2006. pp. 91–8. [Google Scholar]
- 35.Jones LM, Waters DL, Legge M. Walking speed at self-selected exercise pace is lower but energy cost higher in older versus younger women. Journal of physical activity & health. 2009;6:327–32. doi: 10.1123/jpah.6.3.327. [DOI] [PubMed] [Google Scholar]
- 36.Mian OS, Thom JM, Ardigo LP, Narici MV, Minetti AE. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta physiologica. 2006;186:127–39. doi: 10.1111/j.1748-1716.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- 37.Volpato S, Blaum C, Resnick H, et al. Comorbidities and impairments explaining the association between diabetes and lower extremity disability: The Women’s Health and Aging Study. Diabetes care. 2002;25:678–83. doi: 10.2337/diacare.25.4.678. [DOI] [PubMed] [Google Scholar]
- 38.Cappola AR, Xue QL, Ferrucci L, et al. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. The Journal of clinical endocrinology and metabolism. 2003;88:2019–25. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- 39.Fried LF, Lee JS, Shlipak M, et al. Chronic kidney disease and functional limitation in older people: health, aging and body composition study. Journal of the American Geriatrics Society. 2006;54:750–6. doi: 10.1111/j.1532-5415.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 40.McDermott MM, Liu K, Ferrucci L, et al. Circulating blood markers and functional impairment in peripheral arterial disease. Journal of the American Geriatrics Society. 2008;56:1504–10. doi: 10.1111/j.1532-5415.2008.01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. The journals of gerontology Series A, Biological sciences and medical sciences. 2004;59:255–63. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 42.Brinkley TE, Leng X, Miller ME, et al. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. The journals of gerontology Series A, Biological sciences and medical sciences. 2009;64:455–61. doi: 10.1093/gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuo HK, Bean JF, Yen CJ, et al. Linking C-reactive protein to late-life disability in the National Health and Nutrition Examination Survey (NHANES) 1999–2002. The journals of gerontology Series A, Biological sciences and medical sciences. 2006;61:380–7. doi: 10.1093/gerona/61.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDermott MM, Greenland P, Green D, et al. D-dimer, inflammatory markers, and lower extremity functioning in patients with and without peripheral arterial disease. Circulation. 2003;107:3191–8. doi: 10.1161/01.CIR.0000074227.53616.CC. [DOI] [PubMed] [Google Scholar]
- 45.Verghese J, Holtzer R, Lipton RB, et al. High-sensitivity C-reactive protein and mobility disability in older adults. Age Ageing. 2012;41:541–5. doi: 10.1093/ageing/afs038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verghese J, Holtzer R, Oh-Park M, et al. Inflammatory markers and gait speed decline in older adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2011;66:1083–9. doi: 10.1093/gerona/glr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aderka D, Engelmann H, Maor Y, Brakebusch C, et al. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. The Journal of experimental medicine. 1992;175:323–9. doi: 10.1084/jem.175.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verghese J, Holtzer R, Oh-Park M, et al. Inflammatory Markers and Gait Speed Decline in Older Adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66A:1083–9. doi: 10.1093/gerona/glr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Penninx BW, Abbas H, Ambrosius W, et al. Inflammatory markers and physical function among older adults with knee osteoarthritis. The Journal of rheumatology. 2004;31:2027–31. [PubMed] [Google Scholar]
- 50.Visser M, Pahor M, Taaffe DR, et al. Relationship of Interleukin-6 and Tumor Necrosis Factor-α With Muscle Mass and Muscle Strength in Elderly Men and Women: The Health ABC Study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2002;57:M326–M32. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


