Summary
Background
Annual low-dose CT screening for lung cancer has been recommended for high-risk individuals, but the necessity of yearly low-dose CT in all eligible individuals is uncertain. This study examined rates of lung cancer in National Lung Screening Trial (NLST) participants who had a negative prevalence (initial) low-dose CT screen to explore whether less frequent screening could be justified in some lower-risk subpopulations.
Methods
We did a retrospective cohort analysis of data from the NLST, a randomised, multicentre screening trial comparing three annual low-dose CT assessments with three annual chest radiographs for the early detection of lung cancer in high-risk, eligible individuals (aged 55–74 years with at least a 30 pack-year history of cigarette smoking, and, if a former smoker, had quit within the past 15 years), recruited from US medical centres between Aug 5, 2002, and April 26, 2004. Participants were followed up for up to 5 years after their last annual screen. For the purposes of this analysis, our cohort consisted of all NLST participants who had received a low-dose CT prevalence (T0) screen. We determined the frequency, stage, histology, study year of diagnosis, and incidence of lung cancer, as well as overall and lung cancer-specific mortality, and whether lung cancers were detected as a result of screening or within 1 year of a negative screen. We also estimated the effect on mortality if the first annual (T1) screen in participants with a negative T0 screen had not been done. The NLST is registered with ClinicalTrials.gov, number NCT00047385.
Findings
Our cohort consisted of 26 231 participants assigned to the low-dose CT screening group who had undergone their T0 screen. The 19 066 participants with a negative T0 screen had a lower incidence of lung cancer than did all 26 231 T0-screened participants (371·88 [95% CI 337·97–408·26] per 100 000 person-years vs 661·23 [622·07–702·21]) and had lower lung cancer-related mortality (185·82 [95% CI 162·17–211·93] per 100 000 person-years vs 277·20 [252·28–303·90]). The yield of lung cancer at the T1 screen among participants with a negative T0 screen was 0·34% (62 screen-detected cancers out of 18 121 screened participants), compared with a yield at the T0 screen among all T0-screened participants of 1·0% (267 of 26 231). We estimated that if the T1 screen had not been done in the T0 negative group, at most, an additional 28 participants in the T0 negative group would have died from lung cancer (a rise in mortality from 185·82 [95% CI 162·17–211·93] per 100 000 person-years to 212·14 [186·80–239·96]) over the course of the trial.
Interpretation
Participants with a negative low-dose CT prevalence screen had a lower incidence of lung cancer and lung cancer-specific mortality than did all participants who underwent a prevalence screen. Because overly frequent screening has associated harms, increasing the interval between screens in participants with a negative low-dose CT prevalence screen might be warranted.
Introduction
Results of the National Lung Screening Trial (NLST) showed that annual low-dose CT screening reduced lung cancer mortality in high-risk individuals followed up for up to 5 years after their last annual screen by about 15–20% relative to a control group receiving chest radiographs.1,2 On the basis of these data and results from other screening programmes,3–6 annual low-dose CT screening has been advocated in these high-risk individuals. However, low-dose CT is not without risk and substantial health-care costs, and refinements in screening protocols should be considered to improve the efficiency and cost-effectiveness of population-based screening programmes.7
Cancer screening is an inherently inefficient process, mainly due to the low prevalence of disease. Even in high-risk groups such as cigarette smokers, only a small proportion of individuals will be diagnosed with lung cancer. Additionally, some individuals will have a false-positive screen, necessitating further assessment: 39% of all NLST trial participants who underwent low-dose CT had at least one false-positive screen, although the proportion of false-positive screens varied among other screening trials depending on the criteria used to define a positive screen. Therefore, distinguishing the vast majority of individuals with benign indeterminate nodules from those with lung cancer remains a diagnostic dilemma.8–10
Just as importantly, whether annual low-dose CT studies are needed in all high-risk individuals is not clear, because no biological justification exists to show that annual screening is the optimal screening interval. Increasing the time between screens might be warranted if it does not substantially affect mortality, and at the same time reduces the number of screens, unnecessary work-ups, invasive procedures, overall radiation dose during screening period, and cost. Although several reports have explored the effect of increased screening intervals,6,8,11 the aim of our study was to focus on the subset of NLST participants who had a negative prevalence (initial) screen on low-dose CT to determine the subsequent incidence and mortality of lung cancer in this group, and to estimate the effect on mortality if the first annual screen had not been done. Identifying a population in whom less frequent screening produces a more favourable benefit-to-harm ratio will inform risk prediction models, might reduce the need for annual low-dose CT screens in some individuals, and might lead to a more efficient lung cancer screening programme.11–13
Methods
NLST design and participants
This study was a retrospective analysis of data from the NLST, a randomised, multicentre screening trial that recruited participants who were at high risk for lung cancer from 33 US medical centres. The design, methods, and initial results of the NLST have been previously reported.14,15 Briefly, eligible participants were those aged 55–74 years with at least a 30 pack-year history of cigarette smoking, and if a former smoker, had quit less than 15 years before study entry. Participants were randomly assigned to receive either three annual low-dose CT assessments or three annual single-view posterior-anterior chest radiographs, and were then followed up for up to an additional 5 years. The NLST was approved at each of the 33 sites by the local institutional review board, and all participants gave written, informed consent.
The primary objective of the NLST was to determine if low-dose CT screening, as compared with chest radiograph screening, would reduce lung cancer mortality. General recommendations for the management of participants with a positive screen were detailed in the trial protocol,1,2 although the referring clinician ultimately determined further assessment. Trial participants with any negative screen (either prevalence or subsequent annual screen) were recommended to return for their next annual screen.14–16
Lung cancer diagnoses were established mainly through the review of standardised data forms administered to study participants. Medical abstractors confirmed all lung cancer cases using patient medical records, including pathology reports. An independent death review committee masked to study group adjudicated whether deaths were due to lung cancer (or attributable to the investigation of suspected lung cancer) to compare lung cancer mortality between the two screening groups.
As described in the NLST protocol, a positive low-dose CT scan was defined as one or more non-calcified nodules at least 4 mm in longest diameter.15 A positive scan also included CT findings of a mediastinal mass, pleural disease, or atelectasis in one or more lobes. Other radiographic findings, including the presence of emphysema on CT, were also recorded by the interpreting radiologist on the appropriate data sheet. The participants were also given a questionnaire at the entrance of the study that included several questions related to their past medical history, including a history of chronic obstructive pulmonary disease (COPD) and family history of cancer. Details of the NLST protocol outlining these features have been previously reported.14–16
Procedures
For the purposes of this analysis, we retrospectively reviewed the NLST database to identify all lung cancers diagnosed during the trial and lung cancer-related deaths during study follow-up in participants in the low-dose CT group of the NLST. We excluded participants assigned to the low-dose CT group who had not undergone a prevalence (T0) screen from our analysis. We recorded the number of lung cancer cases according to study year of diagnosis, stage, histology, and lung cancer-free survival and determined the number of lung cancer deaths and deaths from all causes. We also determined the number of lung cancers detected as a result of screening and the number diagnosed within 1 year of a negative screen. A lung cancer was deemed to be screen-detected if diagnosis occurred within 1 year of a positive screen and before the next screen (if any), or if diagnosis occurred more than 1 year after a positive screen during which time the participant had no further screening but did have a sequence of diagnostic procedures prompted by the positive screen and less than a 1-year gap between procedures. We used the term interval lung cancer to define any lung cancer diagnosed within 1 year of a negative screen.
Statistical analysis
Using descriptive statistics, we compared the number of participants who were diagnosed with lung cancer at each screening stage. We also compared the yield of the T0 screen—ie, the number of screen-detected cancers divided by the total number of participants screened—in all participants, with the yield of the first annual low-dose CT screen (T1) in the T0 negative subset of participants.
We determined the incidence of lung cancer and lung cancer-specific mortality during the trial in all eligible participants who underwent a T0 screen in the NLST compared with participants who had a negative T0 screen. We calculated lung cancer incidence and mortality per 100 000 person-years, defined as the ratio of the number of events to the person-years at risk for the event. For the incidence of lung cancer, person-years were measured from the time of the T0 screen to the date of diagnosis of lung cancer, death, or censoring (whichever came first); for mortality, person-years were measured from the time of the T0 screen to the date of death from lung cancer, death from another cause, or censoring (whichever came first); participants who died from causes other than lung cancer were censored at the time of death when estimating lung cancer mortality. Those with missing lung cancer data or vital status were censored at the date that they were last known to be cancer free or alive, respectively. We also calculated lung cancer incidence and mortality per person-years measured from the time of the second annual (T2) screen to the date of first event (ie, lung cancer diagnosis for incidence and lung cancer death for mortality), death, or censoring (whichever came first). We estimated the relative risks of lung cancer incidence and mortality by diagnosis year and screening status, and derived 95% CIs using the bootstrap method.
We estimated the upper bound of the potential increase in mortality that would be expected if all participants with a negative T0 screen were screened again in 2 years instead of 1 year, under the assumption that the T1 screen was not done in participants with a negative T0 screen, and that all T1-screen-detected cancers were reclassified as a lung cancer-specific death, irrespective of the true outcome.
We compared the time to lung cancer diagnosis, incidence of lung cancer, and lung cancer-specific mortality of participants with a negative T0 screen with those with a positive T0 screen. For those with a negative T0 screen, we also grouped participants with respect to the status of their subsequent T1 and T2 screens and compared those groups (eg, T1 negative vs T1 positive).
We used Cox proportional hazards regression to investigate time to lung cancer diagnosis (defined as time from T0 screen to lung cancer diagnosis, death or censoring; participants who died with no lung cancer diagnosis were censored at the time of death) by screening status. We used univariate and multivariable models for each comparison. The full model was adjusted for age, sex, race, smoking history, emphysema finding on the T0 screen, family history of lung cancer, and self-reported history of COPD.
We examined demographic and clinical features (age, sex, race, smoking history, emphysema on T0 screen, history of COPD, and family history of lung cancer) in two Cox regression models, to explore factors associated with lung cancer diagnosis after a negative T0 screen and in all participants who completed a T0 screen (positive and negative screens). We used Kaplan-Meier curves to compare lung cancer-free survival stratified according to the following criteria: age (<65 years vs ≥65 years); smoking history (<45 pack-years vs ≥45 pack-years); age younger than 65 years and smoking history (<45 pack-years vs ≥45 pack-years); and history of COPD, age 65 years or older, and smoking history of 45 or more pack-years.
We completed all analyses using SAS statistical software (version 9.4) and Stata statistical software (release 14), and used the statistical package R (2014) to create the Kaplan-Meier plots.
The NLST is registered with ClinicalTrials.gov, number NCT00047385.
Role of the funding source
This analysis had no funding support. The National Institutes of Health supported the NLST but had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. The corresponding author (EFP) had full access to all the data in the study and had final responsibility for the decision to submit for publication. EG, CG, and PP also had access to the data.
Results
Participants were enrolled into the NLST between Aug 5, 2002, and April 26, 2004. Annual screening was complete by Sept 6, 2007, and individuals were followed up until Dec 31, 2009. 26 722 participants were randomly assigned to receive three annual low-dose CT assessments; 95 participants were subsequently considered ineligible for the trial and an additional 396 participants were excluded from our retrospective analysis for having no T0 screen, leaving 26 231 eligible participants for our analysis (appendix). 78 (82%) of the 95 participants who were ineligible had a T0 screen; they were found to be ineligible after enrolment and randomisation. 19 066 (73%) of 26 231 low-dose CT participants had a negative T0 screen and 7165 (27%) had a positive T0 screen (appendix).
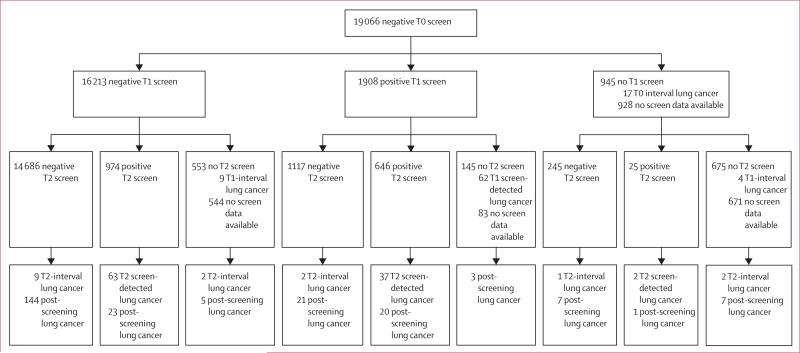
Demographic characteristics according to T0 screening status are shown in table 1. 14 686 participants had negative scans at T0, T1, and T2, and 2762 had a T0 negative scan and a positive scan at either T1 or T2 (this number does not include those that did not have a T1 scan but then went on to have a negative T2 scan; figure 1).
Table 1.
Demographic information by baseline screening status in participants from the National Lung Cancer Trial who had low-dose CT screening
| Negative T0 screen (n=19 066) | Positive T0 screen (n=7165) | |
|---|---|---|
| Age (years) | 60 (55-74) | 61 (55-74) |
| Sex | ||
| Male | 11 320 (59%) | 4184 (58%) |
| Female | 7746 (41%) | 2981 (42%) |
| Race | ||
| White | 17 250 (91%) | 6683 (93%) |
| Black or African-American | 936 (5%) | 224 (3%) |
| Asian | 422 (2%) | 123 (2%) |
| American Indian or Alaskan Native | 68 (<1%) | 21 (<1%) |
| Native Hawaiian or other Pacific Islander | 67 (<1%) | 16 (<1%) |
| More than one race | 242 (1%) | 83 (1%) |
| Participant refused to answer | 44 (<1%) | 6 (<1%) |
| Unknown | 37 (<1%) | 9 (<1%) |
| Ethnic origin | ||
| Hispanic or Latino | 355 (2%) | 107 (1%) |
| Neither Hispanic nor Latino | 18 649 (98%) | 7016 (98%) |
| Participant refused to answer | 3 (<1%) | 2 (<1%) |
| Unknown | 59 (<1%) | 40 (1%) |
| Results of T1 scan | ||
| Negative T1 | 16 213/18 121 (90%) | NA |
| Positive T1 | 1908/18 121 (11%) | NA |
| Results of T2 scan | ||
| Negative T1 and T2 | 14 686/17 448 (84%) | NA |
| Subsequent positive (T1 or T2)* | 2762/17 448 (16%) | NA |
Data are median (range) or n (%). T0=prevalence screen. T1=first annual screen. T2=second annual screen.
NA=not applicable.
Any participant who had at least one positive screen at T1 or T2.
Figure 1. Flow diagram of screen results in participants with a negative T0 screen.
Interval and screen-detected lung cancers are defined as occurring within 12 months of the previous screen—eg, T2-interval lung cancers are those diagnosed in the 12 months after a negative or missed T2 screen. NLST=National Lung Screening Trial. T0=prevalence screen. T1=first annual screen. T2=second annual screen.
Of 26 231 eligible participants, 1063 (4%) had been diagnosed with lung cancer at the time of the last available follow-up. Median time from T0 screen to diagnosis for these participants was 2·2 years (95% CI 2·1–2·3). Of the 19 066 participants with a negative T0 screen, 441 (2%) had been diagnosed with lung cancer at the time of the last available follow-up; the median follow-up time from T0 to diagnosis for these patients was 3·3 years (95% CI 3·0–3·6). In the first year after a negative T0 screen and before the scheduled T1 screen, 17 (<1%) of 19 066 individuals were diagnosed with lung cancer (interval lung cancers). Additionally, 75 (<1%) individuals were diagnosed with lung cancer between the T1 and T2 screens (62 screen-detected and 13 interval cancers), 118 (1%) individuals had lung cancer detected at or within 1 year after the T2 screen (102 screen-detected and 16 interval cancers), and 231 (1%) individuals were diagnosed during the follow-up phase of the trial (between 3 and 7 years after their prevalence screen). Lung cancer stage and histology by time of lung cancer detection is shown in table 2.
Table 2.
Lung cancer stage, histology, and survival data in participants with a negative T0 screen who developed lung cancer, by time of lung cancer detection
| T0 interval lung cancer (n=17) | T1-detected or interval lung cancer (n=75) | T2-detected or interval lung cancer (n=118) | Post-screening-detected lung cancer* (n=231) | |
|---|---|---|---|---|
| Lung cancer stage | ||||
| Stage IA | 1 (6%) | 29 (39%) | 61 (52%) | 43 (19%) |
| Stage IB | 1 (6%) | 6 (8%) | 10 (8%) | 21 (9%) |
| Stage IIA | 2 (12%) | 6 (8%) | 5 (4%) | 5 (2%) |
| Stage IIB | 1 (6%) | 2 (3%) | 2 (2%) | 11 (5%) |
| Stage IIIA | 2 (12%) | 7 (9%) | 7 (6%) | 21 (9%) |
| Stage IIIB | 7 (41%) | 9 (12%) | 12 (10%) | 37 (16%) |
| Stage IV | 3 (18%) | 14 (19%) | 17 (14%) | 92 (40%) |
| Occult carcinoma | 0 | 0 | 1 (1%) | 0 |
| Not assessable | 0 | 2 (3%) | 3 (3%) | 1 (<1%) |
| Histology | ||||
| Small-cell carcinoma | 4 (24%) | 17 (23%) | 14 (12%) | 51 (22%) |
| Squamous-cell carcinoma | 6 (35%) | 20 (27%) | 38 (32%) | 63 (27%) |
| Adenocarcinoma | 3 (18%) | 17 (23%) | 34 (29%) | 66 (29%) |
| Bronchiolo-alveolar carcinoma | 0 | 6 (8%) | 10 (8%) | 8 (3%) |
| Large-cell carcinoma | 3 (18%) | 5 (7%) | 3 (3%) | 3 (1%) |
| Adenosquamous carcinoma | 0 | 1 (1%) | 4 (3%) | 0 |
| Pleomorphic/sarcomatoid | 0 | 0 | 0 | 1 (<1%) |
| Carcinoid tumour | 0 | 0 | 1 (1%) | 1 (<1%) |
| Unclassified carcinoma | 1 (6%) | 9 (12%) | 12 (10%) | 34 (15%) |
| Carcinosarcoma | 0 | 0 | 0 | 1 (<1%) |
| Unknown | 0 | 0 | 2 (2%) | 3 (1%) |
| Overall survival | ||||
| Alive at end of study | 3 (18%) | 28 (37%) | 66 (56%) | 115 (50%) |
| All deaths | 14 (82%) | 47 (63%) | 52 (44%) | 116 (50%) |
| Lung cancer-specific survival | ||||
| Alive at end of study or non-lung-cancer death | 4 (24%) | 34 (45%) | 71 (60%) | 116 (50%) |
| Lung cancer death | 13 (76%) | 40 (53%) | 47 (40%) | 115 (50%) |
| Missing information | 0 | 1 (1%) | 0 | 0 |
Data are number (%) of participants with a negative T0 screen who had a subsequent lung cancer diagnosis. Data are presented according to when the lung cancer was first confirmed (either at screening [screen-detected] or within 12 months of screening [interval]). T0=baseline prevalence screen. T1=first annual screen. T2=second annual screen.
Lung cancers detected during the follow-up phase of the trial (3-7 years after prevalence screen).
The yield of lung cancer at the T1 screen among participants with a negative T0 screen was 0·34% (62 screen-detected cancers out of 18 121 screened participants), compared with a yield at the T0 screen among all eligible participants of 1·0% (267 of 26 231); the ratio of these yields is 0·34 (95% CI 0·26–0·43).
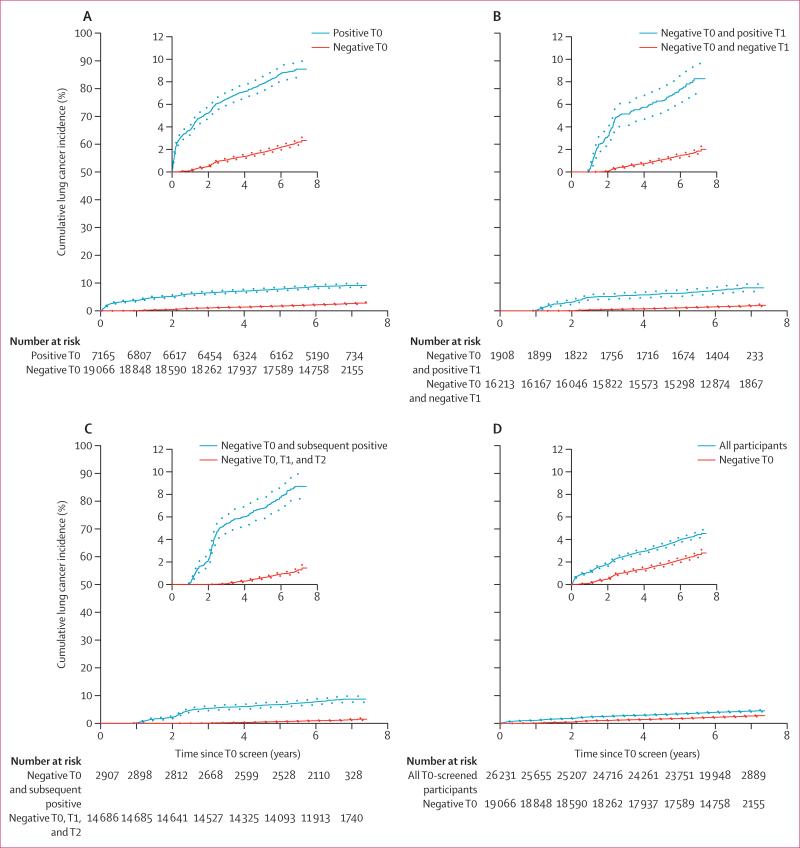
The median follow-up for all participants who had a T0 screen was 6·4 years (IQR 6·1–6·8) and for participants with a negative T0 screen it was 6·4 years (6·1–6·8). The overall incidence of lung cancer in participants with a negative T0 screen was lower than that for all participants who underwent a T0 screen (table 3). The overall incidence of lung cancer per 100 000 person-years by screen result is shown in table 3 and the cumulative incidence of lung cancer according to screen result is shown in figure 2. The incidence rate of lung cancer after three negative screens (T0, T1, and T2), measured from the T2 screen to lung cancer diagnosis, death, or data cutoff, was reduced compared with that of all participants who had a T0 screen, measured from the T0 screen to lung cancer diagnosis, death, or data cutoff (rate ratio [RR] 0·36 [bootstrap 95% CI 0·31–0·42]), and compared with participants who had a negative T0 screen (0·64 [0·57–0·72]; table 3).
Table 3.
Incidence and mortality of lung cancer in participants who had a T0 screen, by screen results
| N | Lung cancer incidence rate (95% CI)* | Lung cancer mortality rate (95% CI)† | |
|---|---|---|---|
| All participants who had a T0 screen | 26 231 | 661·23 (622·07–702·21) | 277·20 (252·28–303·90) |
| Positive T0 screen | 7165 | 1474·87 (1361·23–1595·48) | 523·58 (458·37–595·47) |
| Negative T0 screen | 19 066 | 371·88 (337·97–408·26) | 185·82 (162·17–211·93) |
| Negative T0 and positive T1 screens | 1908 | 1259·61 (1062·98–1482·19) | 521·86 (400·09–668·98) |
| Negative T0 and T1 screens | 16 213 | 248·65 (219·06–281·12) | 129·17 (108·15–153·08) |
| Negative T0 and subsequent positive | 2907 | 1333·49 (1168·09–1515·74) | 528·95 (428·46–645·94) |
| Negative T0, T1, and T2 screens (from T0) | 14 686 | 162·97 (138·17–190·93) | 93·57 (75·04–115·28) |
| Negative T0, T1, and T2 screens (from T2)‡ | 14 686 | 238·35 (202·08–279·25) | 136·68 (109·62–168·39) |
Individuals can appear in more than one row. NA=not applicable. T0=prevalence screen. T1=first annual screen. T2=second annual screen.
Defined as all observed lung cancers from T0 screen to Dec 31, 2009 (data cutoff), per 100 000 observed person-years from the T0 screen to lung cancer diagnosis, death, or data cutoff (whichever came first), unless otherwise specified.
Defined as all observed lung cancer deaths from T0 screen to Dec 31, 2009 (data cutoff), per 100 000 observed person-years from T0 screen to lung cancer death, other death, or data cutoff (whichever came first), unless otherwise specified.
Recalculation of incidence and mortality in participants with a negative T0, T1, and T2 screen per 100 000 observed person-years from the T2 screen.
Figure 2. Cumulative incidence of lung cancer diagnosis, by screening result.
Dotted lines show 95% CIs. T0=prevalence screen. T1=first annual screen. T2=second annual screen.
Overall survival and lung cancer-specific survival in participants with lung cancer diagnoses is shown in table 2. Cause of death in the mortality analysis is shown in the appendix. The mortality rate due to lung cancer during the trial was lower in participants with a negative T0 screen compared with that in all participants who had a T0 screen (table 3). The mortality rate of lung cancer after three negative screens, measured from the T2 screen, was reduced compared with that of all participants who had a T0 screen, measured from the T0 screen (RR 0·49 [bootstrap 95% CI 0·40–0·59]), and when compared with participants who had a negative T0 screen (0·74 [0·62–0·85]).
In our hypothetical analysis, which assumes that if the T1 screen had not been done for any participants with a negative T0 screen, all such participants with a T1-screen-detected lung cancer (n=62) would have died of lung cancer, we found that not having a T1 scan would potentially increase the lung cancer mortality rate in the negative T0 screen group from 185·82 (95% CI 162·17–211·93) per 100 000 person-years to 212·14 (186·80–239·96) per 100 000 person-years. This result implies that an additional 28 participants would have died from lung cancer if the T1 scan was not done in the T0 negative group. This estimated hypothetical mortality rate is less than the lung cancer mortality rate in all participants who had a T0 screen (table 3). The comparison of risk of lung cancer diagnosis by screen result is shown in table 4.
Table 4.
Comparative risk of lung cancer diagnosis by screen result
| Univariate model* |
Multivariate model† |
|||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Negative T0 vs positive T0 | 0·25 (0·22–0·29) | <0·0001 | 0·27 (0·24–0·31) | <0·0001 |
| Negative T0 and T1 vs negative T0 and positive T1 | 0·20 (0·16–0·24) | <0·0001 | 0·22 (0·18–0·27) | <0·0001 |
| Negative T0, T1, and T2 vs negative T0 and subsequent positive | 0·12 (0·10–0·15) | <0·0001 | 0·13 (0·11–0·17) | <0·0001 |
Cox proportional hazards model predicting time from T0 screen to lung cancer diagnosis.
Cox proportional hazards model predicting time from T0 screen to lung cancer diagnosis that includes the following additional baseline parameters: age, sex, race, smoking pack-years, emphysema on T0 screen, history of COPD, and family history of lung cancer. HR=hazard ratio. T0=baseline prevalence screen. T1=first annual screen. T2=second annual screen.
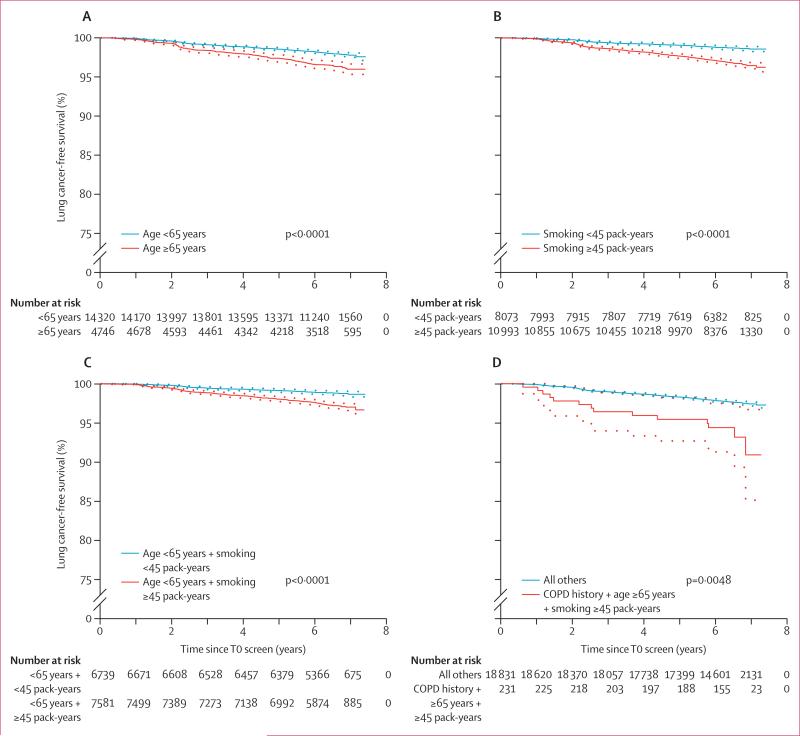
Age, smoking history, emphysema on T0 screen, and a history of COPD were significant predictors of risk of lung cancer diagnosis in participants with a negative T0 screen and in all participants with a T0 screen; however, sex and race were not predictors of risk in either population (table 5). A family history of lung cancer was a significant predictor of risk of lung cancer diagnosis in all participants with a T0 screen, but not in the subgroup of participants with a negative T0 screen. Lung cancer-free survival according to risk group based on these risk predictors is shown in figure 3.
Table 5.
Cox regression comparing results from two models on the risk of lung cancer diagnosis
| Model 1: negative T0 screen (n=19 066) |
Model 2: positive or negative T0 screen (n=26 231) |
|||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age (per 1 year increase) | 1·05 (1·03–1·07) | <0·0001 | 1·07 (1·06–1·08) | <0·0001 |
| Sex (female vs male) | 0·93 (0·77–1·14) | 0·50 | 1·07 (0·95–1·22) | 0·27 |
| Race (white vs other) | 0·88 (0·57–1·37) | 0·57 | 1·07 (0·78–1·47) | 0·66 |
| Race (black or African-American vs other) | 1·13 (0·62–2·06) | 0·68 | 1·36 (0·89–2·07) | 0·16 |
| Smoking pack-years (per 1 pack-year increase) | 1·01 (1·01–1·01) | <0·0001 | 1·01 (1·01–1·01) | <0·0001 |
| Emphysema on T0 CT (yes vs no) | 1·96 (1·62–2·38) | <0·0001 | 1·67 (1·48–1·89) | <0·0001 |
| Self-reported history of COPD (yes vs no) | 1·94 (1·43–2·64) | <0·0001 | 1·75 (1·43–2·14) | <0·0001 |
| Family history of lung cancer (yes vs no) | 1·09 (0·88–1·36) | 0·42 | 1·19 (1·04–1·37) | 0·014 |
Two models were fit for risk for lung cancer diagnosis including the above variables. Model 1 only contained participants with a negative T0 screen. Model 2 contained all participants with a T0 screen (negative and positive). T0=prevalence screen.
Figure 3. Lung cancer-free survival according to risk group.
Kaplan-Meier curves (ie, time from T0 screen to date of diagnosis of lung cancer, death, or censoring, whichever came first) in participants with a negative T0 screen, stratified according to specific criteria: (A) age (<65 years vs ≥65 years); (B) smoking history (<45 pack-years vs ≥45 pack-years); (C) age younger than 65 years and smoking history (<45 pack-years vs ≥45 pack-years); and (D) history of chronic obstructive pulmonary disorder (COPD), age 65 years or older, and smoking history of 45 or more pack-years versus all other eligible participants. Dotted lines show 95% CIs.
Discussion
The incidence of lung cancer in the group of high-risk participants enrolled in the NLST who had a negative low-dose CT prevalence screen was low in the year following this initial screen, relative to those with a positive low-dose CT prevalence screen, suggesting that annual screening after a negative screen might be unnecessary. Yearly screening for lung cancer has been proposed for decades as a method to improve patient outcomes, and the results from several screening trials show that low-dose CT screening in high-risk individuals increased detection of early-stage disease and reduced lung cancer mortality.2–6,16,17 These data have been met with much enthusiasm and will lead to changes in public policy, particularly since most treatment trials in lung cancer, even with targeted therapy, have shown only slight effects on progression-free survival.
However, screening is not without challenges and limitations. Some individuals will have unnecessary procedures with inherent complications and cost. Overdiagnosis and the need for continued surveillance are key issues.18 Additionally, low-dose CT screening has only been shown to be effective in individuals who fulfilled the NLST criteria, which represents an estimated 26% of all patients diagnosed with lung cancer;19 most cases of lung cancer would still be detected outside the high-risk screening programme, and no direct evidence shows how effective annual screening would be in other groups. Thus, the ultimate effect on lung cancer mortality in the best-case scenario, if every individual eligible for screening is enrolled, is estimated to be about a 5% overall reduction in lung cancer deaths.
Although this is a large number of averted deaths, advances are still needed in several problematic areas, including more accurate identification of the highest-risk individuals, a reduction in false-positive screen results, prediction of which tumours are aggressive and which are more indolent, and determination of the optimal appropriate screening interval to achieve the best possible balance of benefit versus harm. Ongoing assessment of the screening trial data continues to explore these issues in an attempt to understand and elucidate all of the risks and benefits, and to use prediction models to optimise imaging utilisation while reducing cost.
Whether our findings of a relatively low risk of lung cancer death in participants with no baseline T0 examination abnormalities are because participants with this screen result had very slow growing tumours (ie, a direct effect of indolent biological behaviour) or are due to a more indirect effect (eg, some participants might not have developed the same degree of tobacco-induced lung injury) cannot be clearly discerned from our results and is an area for future study.
One of the fundamental limitations of low-dose CT screening is the occurrence of false-positive screen results. The number of individuals that require further assessment varies among screening trials, and typically depends on the criteria used to define a positive CT screen. In the NLST, about 25% of all low-dose CT group participants had an abnormality on their prevalence screen that required further assessment, although in the NELSON trial,4 tumour volume and doubling times were effectively used to reduce the number of positive scans. However, any positive screen necessitates further assessment, potential invasive procedures, radiation, and cost. To address this issue, imaging improvements and peripheral specimen biomarkers have been suggested, which could be integrated into the diagnostic work-up.20 However, improving imaging and identifying effective biomarkers will not be simple, and much effort has been made to improve efficiency and eliminate unnecessary intervention.
Our retrospective analysis explored the incidence and mortality of lung cancer in the 73% of low-dose CT-screened participants who did not have an abnormality on their prevalence screen. We found that both the incidence and mortality of lung cancer over the course of the trial was significantly less in the T0 negative group than in those with a positive initial screen and significantly less than in all low-dose CT-screened participants combined. Moreover, the yield of screen-detected lung cancer at the T1 screen in the negative T0 group was reduced compared with the yield in all T0-screened participants.
Thus, the cost-effectiveness of annual low-dose CT is unclear in this T0 negative group when weighing the potential harms of more intense screening versus the potential benefit in averted lung cancer deaths. In the group of participants with three negative annual low-dose CT screens, the incidence and mortality was even less than that in the T0 negative group. These data not only question the necessity for annual screens following a negative prevalence screen, but also suggest that annual screens after subsequent negative low-dose CT screens might not be needed; no clear biological rationale underlies the selection of yearly examinations. In fact, several studies suggest it takes years for a lung cancer to develop,21 thus the risk of lung cancer in individuals with a negative screen would be low. Screening done at less frequent intervals could lead to a reduction in the number of CT assessments, radiation, and cost. However, some individuals might progress in the interval and be detected with more advanced-stage disease than they would have done if they had undergone yearly screening. In the worst-case scenario, not having a T1 screen could increase the lung cancer-specific mortality in the negative T0 screen group from 185 per 100 000 person-years to 212 per 100 000 person-years, and the full benefit of screening would not be realised.
Risk prediction models will undoubtedly be useful in helping to optimise screening parameters, and in suggesting which individuals should have annual or less frequent assessments.12,13 Our current analysis found that clinical features including age, smoking history, history of COPD, and emphysema were associated with an increased risk for lung cancer. All of these features should be considered in the T0 negative population when developing future guidelines for screening programmes.
We do not present a detailed prediction model in this report, and any model developed solely from the controlled setting of the NLST would have to be regarded as preliminary, given the small number of lung cancer deaths in participants who had a negative T0 examination and a positive T1 examination. Evidence from a larger screened population that include these data and data from other screening programmes will be necessary to develop a fully validated prediction model that avoids overfitting a multivariable model and that is applicable worldwide. Additionally, our analysis did not directly test the effectiveness of biennial screening or screening at longer intervals, so assumptions were required and models will be needed to estimate the ultimate effect on outcomes. Data from ongoing mass screening programmes will be useful in determining the optimal duration and interval for lung cancer screening.
Annual low-dose CT screening detects some lung cancers at an earlier, more treatable stage than no screening at all or annual chest radiograph screening, and will improve outcomes for select patients. However, the magnitude of such an endeavour must be weighed against the risks and costs; resources are not unlimited and society should decide how to implement a responsible screening programme based on evidence-based data. In summary, the results of our retrospective analysis showed a significant reduction in the incidence of lung cancer and lung cancer-specific mortality of low-dose CT-screened participants with a negative prevalence screen, compared with those with a positive prevalence screen, and question the necessity of continuous annual low-dose CT screens for all eligible high-risk individuals. These findings will help inform the ongoing discussions about implementation of effective and efficient low-dose CT screening programmes.
Supplementary Material
Research in context.
Evidence before the study
We searched PubMed for all relevant articles published from Jan 1, 1995, to Dec 31, 2015, on “Low-dose CT screening and lung cancer”, “Risk prediction for low-dose CT”, “Early detection of lung cancer with CT”, and “Biennial CT screening”, without language restrictions. Although we found several related studies about modelling of low-dose CT to identify the highest-risk group and assess the necessity of annual screening, none reported data from a prospective randomised trial on the risk of lung cancer in participants with a negative initial screen or subsequent negative annual screen. Participants with negative screens are a large group of individuals that fulfil the eligibility criteria for screening, but who might not need to be screened every year.
Added value of this study
To our knowledge, this is the first study to explore the risk of lung cancer in National Lung Screening Trial participants who had a negative low-dose CT prevalence (initial) screen, and subsequent annual screens. Our findings suggest that individuals with a negative prevalence screen have a substantially decreased risk of lung cancer compared with those with a positive prevalence screen, and that annual screens might not be warranted in this group. These data will be important to inform ongoing decisions about optimising mass screening programmes in an effort to improve efficiency, while reducing radiation, cost, and morbidity.
Implications of all the available evidence
Future risk prediction and cost-effectiveness models can incorporate our data to optimise screening guidelines. Improving efficiency of low-dose CT screening for lung cancer could substantially reduce the number of annual screens required, which would have a meaningful impact on population-based screening programmes.
Acknowledgments
The National Lung Screening Trial was supported by the National Institutes of Health (grants U01CA079778 and U01 CA080098 and contracts N01-CN-25511, N01-CN-25512, N01-CN-25513, N01-CN-25514, N01-CN-25515, N01-CN-25516, N01-CN-25518, N01-CN-25522, N01-CN-25524, N01-CN-75022, N01-CN-25476, and N02-CN-63300).
Footnotes
Contributors
EFP and PP were responsible for the scientific literature search. All authors contributed to the study design. EFP, EG, CG, PP, and BSK analysed and interpreted the data, and DRA contributed to data interpretation. All authors wrote the report. EG and CG were responsible for the figures and tables.
Funding None.
Declaration of interests
EFP reports grants from LabCorp, and is a founder of Cue Biologics, LLC, outside the submitted work. EFP also has a patent pending for a therapeutic antibody for cancer. CG reports grants from the National Cancer Institute (NCI) during the conduct of the study, and personal fees from Wilex AG, Genentech, EBG Advisors, and Philips Healthcare, outside the submitted work. PP and BSK are federal employees and are employed by the National Institutes of Health (NIH). DRA reports grants from the American College of Radiology Imaging Network (ACRIN), during the conduct of the study, and travel expenses from the LUNGevity Foundation and Siemens Medical Solutions USA. DRA has received personal fees from the University of Iowa, US Department of Veteran Affairs, Society of Thoracic Radiology (STR), University of Texas Southwestern Medical Center, Vanderbilt-Ingram Cancer Center, American Roentgen Ray Society, Institute of Medicine, California Technology Assessment Forum, Stanford University, New York University, Society of Thoracic Surgeons, 13th International Lung Cancer Congress, European Society of Thoracic Imaging, Glendale Memorial Hospital and Health Center, Colorado Radiological Society, University of Colorado, Denver, International Association for the Study of Lung Cancer, American Association for Cancer Research (AACR)–International Association for the Study of Lung Cancer (IASLC), LUNGevity Award Program Review, Los Angeles Radiological Society, and American Thoracic Society. DRA also reports non-financial support from the National Cancer Advisory Board Meeting, ACRIN, Eastern Cooperative Oncology Group (ECOG)–ACRIN, Institute of Medicine, 3rd World Congress on Thoracic Imaging, NCI, European Congress of Radiology, University of California Dose Retreat, DECAMP Consortium Meeting, AACR, LUNGevity Science Advisory Board Meeting, STR, Cambridge Chest Meeting, Siemens, IASLC, and American Thoracic Society. EG declares no competing interests.
References
- 1.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med. 2013;369:920–31. doi: 10.1056/NEJMoa1208962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker N, Motsch E, Gross ML, et al. Randomized study on early detection of lung cancer with MSCT in Germany: results of the first 3 years of follow-up after randomization. J Thorac Oncol. 2015;10:890–96. doi: 10.1097/JTO.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 4.Horeweg N, Scholten ET, de Jong PA, et al. Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol. 2014;15:1342–50. doi: 10.1016/S1470-2045(14)70387-0. [DOI] [PubMed] [Google Scholar]
- 5.Infante M, Cavuto S, Lutman FR, et al. Long-term follow-up results of the DANTE trial, a randomized study of lung cancer screening with spiral computed tomography. Am J Respir Crit Care Med. 2015;191:1166–75. doi: 10.1164/rccm.201408-1475OC. [DOI] [PubMed] [Google Scholar]
- 6.Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev. 2012;21:308–15. doi: 10.1097/CEJ.0b013e328351e1b6. [DOI] [PubMed] [Google Scholar]
- 7.Black WC, Gareen IF, Soneji SS, et al. Cost-effectiveness of CT screening in the National Lung Screening Trial. N Engl J Med. 2014;371:1793–802. doi: 10.1056/NEJMoa1312547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nair A, Hansell DM. European and North American lung cancer screening experience and implications for pulmonary nodule management. Eur Radiol. 2011;21:2445–54. doi: 10.1007/s00330-011-2219-y. [DOI] [PubMed] [Google Scholar]
- 9.MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237:395–400. doi: 10.1148/radiol.2372041887. [DOI] [PubMed] [Google Scholar]
- 10.Patz EF, Jr, Campa MJ, Gottlin EB, et al. Biomarkers to help guide management of patients with pulmonary nodules. Am J Respir Crit Care Med. 2013;188:461–65. doi: 10.1164/rccm.201210-1760OC. [DOI] [PubMed] [Google Scholar]
- 11.de Koning HJ, Meza R, Plevritis SK, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160:311–20. doi: 10.7326/M13-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tammemagi MC. Application of risk prediction models to lung cancer screening: a review. J Thorac Imaging. 2015;30:88–100. doi: 10.1097/RTI.0000000000000142. [DOI] [PubMed] [Google Scholar]
- 13.Tammemagi MC, Church TR, Hocking WG, et al. Evaluation of the lung cancer risks at which to screen ever- and never-smokers: screening rules applied to the PLCO and NLST cohorts. PLoS Med. 2014;11:e1001764. doi: 10.1371/journal.pmed.1001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aberle DR, Adams AM, Berg CD, et al. for the National Lung Screening Trial Research Team Baseline characteristics of participants in the randomized national lung screening trial. J Natl Cancer Inst. 2010;102:1771–79. doi: 10.1093/jnci/djq434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aberle DR, Berg CD, Black WC, et al. for the National Lung Screening Trial Research Team The National Lung Screening Trial: overview and study design. Radiology. 2011;258:243–53. doi: 10.1148/radiol.10091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aberle DR, Adams AM, Berg CD, et al. for the National Lung Screening Trial Research Team Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duffy SW, Field JK, Allgood PC, Seigneurin A. Translation of research results to simple estimates of the likely effect of a lung cancer screening programme in the United Kingdom. Br J Cancer. 2014;110:1834–40. doi: 10.1038/bjc.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patz EF, Jr, Pinsky P, Gatsonis C, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med. 2014;174:269–74. doi: 10.1001/jamainternmed.2013.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinsky PF, Berg CD. Applying the National Lung Screening Trial eligibility criteria to the US population: what percent of the population and of incident lung cancers would be covered? J Med Screen. 2012;19:154–56. doi: 10.1258/jms.2012.012010. [DOI] [PubMed] [Google Scholar]
- 20.Patz EF, Jr, Caporaso NE, Dubinett SM, et al. National Lung Cancer Screening Trial American College of Radiology Imaging Network Specimen Biorepository originating from the Contemporary Screening for the Detection of Lung Cancer Trial (NLST, ACRIN 6654): design, intent, and availability of specimens for validation of lung cancer biomarkers. J Thorac Oncol. 2010;5:1502–06. doi: 10.1097/JTO.0b013e3181f1c634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–58. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.