Abstract
Background
Many animal experimental studies have been performed to investigate the efficacy of acupuncture in Parkinson’s disease (PD). Sex differences are a major issue in all diseases including PD. However, to our knowledge, there have been no reviews investigating sex differences on the effectiveness of acupuncture treatment for animal PD models. The current study aimed to summarize and analyze past studies in order to evaluate these possible differences.
Method
Each of 7 databases (MEDLINE, EMBASE, the Cochrane Library, 3 Korean medical databases, and the China National Knowledge Infrastructure) was searched from its inception through March 2015 without language restrictions.
Results
We included studies of the use of acupuncture treatment in animal models of PD. A total of 810 potentially relevant articles were identified, 57 of which met our inclusion criteria. C57/BL6 mice were used most frequently (42 %) in animal PD models. Most of the studies were carried out using only male animals (67 %); only 1 study (2 %) was performed using solely females. The further 31 % of the studies used a male/female mix or did not specify the sex.
Conclusions
The results of our review suggest that acupuncture is an effective treatment for animal PD models, but there is insufficient evidence to determine whether sex differences exist. Future studies of acupuncture treatment for PD should use female animal models because they reflect the physiological characteristics of both males and females to fully evaluate the effect and the safety of the treatment for each sex.
Electronic supplementary material
The online version of this article (doi:10.1186/s12906-016-1405-5) contains supplementary material, which is available to authorized users.
Keywords: Electro-acupuncture, Manual acupuncture, Bee-venom acupuncture, C57/BL6, Acupuncture point
Background
Parkinson’s disease (PD) is a progressive neurodegenerative disease caused by the loss of dopaminergic neurons in the substantia nigra [1]. PD usually occurs in individuals over 50 years of age, and its incidence and prevalence increases among individuals approximately 60 years of age and older. PD has become more common due to the rapid aging of human populations around the world [2]. Epidemiological studies have reported that the incidence of PD is 1.5–2 times higher in men than in women, and the onset of symptoms may occur later in women due to the neuroprotective effects of estrogen [3]. For the disease manifestations of PD, women have higher Unified Parkinson’s Disease Rating Scale (UPDRS) motor scores, but present with dyskinesia, tremor, and PD-related complications more often than men [4].
Because the FDA reported that eight out of ten new drugs that had been sold on the market were discontinued because they resulted in far more detrimental side effects in women, the sex perspective began to be discussed in many other fields as well [5]. Adverse drug reactions can be caused by the physiological difference between men and women, and women can be more vulnerable to a particular drug [6]. Because sex is often not considered an important variable in animal research with the exception of research related to features of a particular sex, such as reproduction and endocrine secretion, the overwhelming majority of experimental research uses only males and many studies do not even disclose the sex of the experimental animals. Basic research studies using cells in culture also often fail to present the sex of the organism from which the cell strain originated, but the results of such basic research has been applied generally to humans. Because medical research studies are performed primarily by male researchers [7–9], the research subjects are also mostly males [10–12], and there has been a tendency to be careless of females [13], which can aggravate treatment problems related to the physiological differences between men and women. The National Institutes of Health (NIH) requires applicants to report their cell and animal inclusion plans as part of the preclinical experimental design [14]. Therefore, studies are being performed to determine what sex differences need to be accounted for in preclinical and clinical stages, and the importance of the applying these principles is being highlighted [15].
PD treatment options include pharmacological treatment, non-pharmacological treatment, surgical therapy, and dopaminergic cell transplantation [15]. Acupuncture has long been employed for numerous disorders, and it has been traditionally used to relieve PD-related symptoms and to delay the clinical progression of PD symptoms [16]. We have reported that acupuncture exerts increased neuroprotective effects in regions including the substantia nigra, caudate, thalamus, and putamen in animal models of PD [17–20]. Acupuncture was also found to inhibit microglial activation, inflammation, and iron-related oxidative damage in PD [21].
Sex differences have emerged recently as an important issue, but sufficient efficacy tests for sex differences in acupuncture, as in preclinical studies for drug development, have not yet been performed. It is necessary to clarify efficacy differences according to sex in order to more effectively utilize acupuncture in clinical practice. Therefore, we carried out the present study to identify whether adequate research has been conducted so far to determine the sex differences in the efficacy of acupuncture. Specifically, we analyzed past studies of acupuncture treatment conducted in animal PD models, and determined whether the body of data was sufficient to determine the effects of sex differences on the effectiveness of acupuncture treatment. This review provides the basis for establishing whether future animal model studies are necessary to determine possible sex-related differences in the efficacy of acupuncture for PD.
Methods
Search methods for the identification of studies
The search was performed without restrictions on language or year of publication. We searched Medline, EMBASE, and the Cochrane Central Register of Controlled Trials from the inception of each database through March 2015. For Korean publications, we searched three Korean medical databases (Research Information Service System, National Discovery for Science Leaders, and OASIS). For Chinese articles, we searched the China National Knowledge Infrastructure. The keywords used for the search were the following: “Parkinson’s disease” OR “Parkinson” AND “acupuncture” OR “acupoints” OR “electroacupuncture” OR “electro-acupuncture” OR “auriculotherapy” OR “auriculoacupuncture” OR “bee venom acupuncture” in each database language. The search strategy was adjusted for each database.
Inclusion/exclusion criteria
We included studies of the use of acupuncture treatment in animal PD models. Trials were excluded if the study designs did not evaluate the effectiveness of acupuncture in animal PD models, or if they reported insufficient data. No search restrictions on language or publication forms were imposed. During the first stage of selection/exclusion, titles and abstracts were analyzed, and literature that had no relevance to our study was excluded. The second stage of selection/exclusion involved analyzing the full text of particular studies, because it was impossible to determine the relevance of the studies based solely on the abstracts.
Data extraction
Two reviewers (LSH and KJY) independently reviewed the data extracted from each article using a standardized data extraction form and reached consensus on all items. The extracted data included the type of animal PD models, the sex of the animal PD models, the methods used to induce PD, the types of acupuncture, the acupuncture points, and the effectiveness of the treatment.
Results
Study description
We identified 810 publications, 57 of which met the eligibility criteria (Fig. 1). The 57 articles were published from 1996 to 2014. The characteristics of the studies are summarized in Table 1 [7–12, 18, 19, 21–69].
Fig. 1.
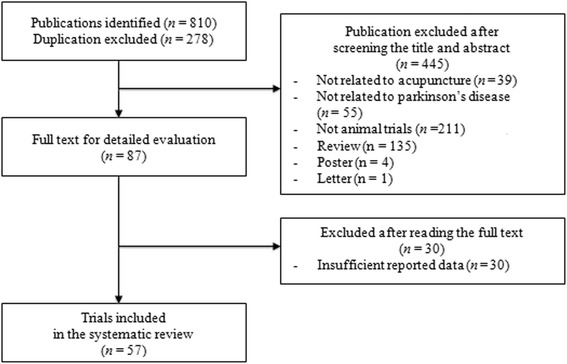
Flowchart of the study selection process
Table 1.
Summary of acupuncture for animal PD models
| First author (year) | Type of animal PD models | Sex of animal PD models | Drugs used to induce PD | Types of acupuncture | Types of acupuncture points | Evaluation of the treatment effectiveness |
|---|---|---|---|---|---|---|
| Bai (2014a) [22] | Undefined | Undefined | 6-OHDA | EA | GV20, EX-HN5 | DA |
| Bai (2014b) [8] | Undefined | Male | 6-OHDA | EA | GV20, EX-HN5 | Caspase-3 |
| Feng (2014) [10] | C57BL/6 | Male | MPTP | MA | Undefined | Pole-climbing test, BDNF, TH, DA |
| Yeo (2013) [19] | C57BL/6 | Male | MPTP | MA | GB34, LR3 | TH, gene expression |
| Alvarez-Fischer (2013) [7] | C57BL/6 | Male | MPTP | BV | Undefined | DA, DOPAC, IL-1β, IL-6, TNF-α, HVA, TH, rotational test |
| Ding (2013) [11] | SD | Male | 6-OHDA | EA | LI4, LR3 | nNOS, GFAP |
| Wang (2013a) [9] | SD | Male | Rotenone | EA | GV16, LR3 | TH, COX-2 |
| Wang (2013b) [23] | SD | Male | Rotenone | EA | GV16, LR3 | TH, p-p38 MARK, COX-2 |
| Wang (2013c) [12] | SD | Male | Rotenone | EA | GV16, LR3 | TH, SOD, GSH, CAT, MDA |
| Wang (2013d) [24] | SD | Male | Rotenone | EA | GV16, LR3 | UCH-L1, UBE1, Parkin, TH, α-synuclein |
| Ding (2012) [25] | SD | Male | 6-OHDA | EA | LI4, LR3 | TH, GFAP, PCNA |
| Huang (2012) [26] | ICR | Male | MPTP | EA | GB34 | Lamp 1, α-synuclein |
| Lu (2012) [27] | C57BL/6 | Male | MPTP | EA | GV20, GV16, GB34 | Locomotor counts, swimming test, pole-climbing test |
| Guo (2012) [28] | SD | Male | 6-OHDA | EA | GV20, GV16, GB34 | GSH, SOD, MDA, GSH-Px |
| Yang (2011) [29] | C57BL/6 | Male | MPTP | EA | PC7 | Pole-climbing test, TH, DA, DOPAC, HVA |
| Choi (2011) [18] | C57/BL6 | Male | MPTP | MA | GB34, LR3 | TH, DAT, gene expression |
| Kim (2011) [30] | C57BL/6 | Male | MPTP | BV | ST36 | MAC-1, iNOS, TH |
| Du (2011) [31] | SD | Male | 6-OHDA | EA | GV20, GV14 | GABA, rotational test |
| Wang (2011) [32] | C57BL/6 | Male | MPTP | EA | ST36, SP6 | TH, DA, DOPAC, HVA, SOD, GSH, GSH-Px |
| Doo (2010) [33] | C57BL/6 | Male | MPTP | BV | GB34 | TH |
| Hong (2010) [34] | C57BL/6 | Male | MPTP | MA | GB34 | Gene expression |
| Jun (2010) [35] | C57BL/6 | Male | MPTP | BV | BL23 | TH, caspase-3, iNOS |
| Kim (2010) [36] | C57BL/6 | Male | MPTP | EA | GB34, GB39 | DA |
| Park (2010) [37] | C57BL/6 | Male | MPTP | BV | GB39, LI11, BL23 | TH, MAC-1, HSP70 |
| Sun (2010) [38] | C57BL/6 | Male | MPTP | MA | GV20, GV14 | Pole-climbing test, TH, DA, DOPAC |
| Wang (2010a) [39] | Wistar | Undefined | 6-OHDA | EA | GV16, LR3 | TH, DA |
| Wang (2010b) [40] | Wistar | Undefined | 6-OHDA | EA | GV16, LR3,CV4, ST36 | GDNF |
| Wang (2010c) [41] | C57/BL6 | Male | MPTP | MA | GV20, GV14 | Pole-climbing test, TH, DA, NA, DOPAC, 5HIAA, 5HT |
| Yu (2010) [42] | Wistar | Male | 6-OHDA | MA | GB34, LR3, ST36, SP10 | Rotational test, SOD, GSH-Px, CAT, GSH, MDA |
| Huang (2010) [43] | Wistar | Male | 6-OHDA | EA | LI4, LR3 | Rotational test, BDNF, TrKB |
| Choi (2009) [21] | C57/BL6 | Male | MPTP | MA | LR3, GB34 | TH, DAT |
| Kim (2009) [44] | C57BL/6 | Male | MPTP | BV | BL23 | TH, MAC-1, HSP70 |
| Wang (2009a) [45] | Wistar | Male, Female | 6-OHDA | EA | GV20, EX-NH5 | TH, BDNF |
| Wang (2009b) [46] | Wistar | Male, Female | 6-OHDA | EA | GV20, EX-NH5 | TH, DAT |
| Kim (2008) [47] | C57BL/6 | Male | MPTP | MA | GB34 | TH |
| Guan (2008) [48] | C57BL/6 | Male | MPTP | EA | GV20 | Fn |
| Wang (2008) [49] | Wistar | Male, Female | 6-OHDA | EA | GV20, EX-NH5 | TH |
| Jeon (2008) [50] | C57BL/6 | Male | MPTP | EA | GB34, SI3, BL62, ST36 | Pole-climbing test, TH, DA, BDNF |
| Xie (2007) [51] | Wistar | Undefined | 6-OHDA | MA | GV20 | Rotational test, MDA, NO, SOD |
| Kang (2007) [52] | C57BL/6 | Male | MPTP | MA | GB34, LR3 | TH, COX-2, iNOS, DA, DOPAC, HVA |
| Huang (2007) [53] | SD | Male | 6-OHDA | MA | GB34, LR3 | TH |
| Luo (2007) [54] | Wistar | Male, Female | 6-OHDA | EA | GV20, EX-NH5 | NOS |
| Wang (2007) [55] | SD | Male, Female | 6-OHDA | MA | GV20, GV16, GB34 | Rotational test, DA |
| Jin (2006a) [56] | Wistar | Male, Female | 6-OHDA | EA | Undefined | GSH, GSH-Px,SOD, MDA, NOS |
| Jin (2006b) [57] | Wistar | Male, Female | 6-OHDA | EA | Undefined | DA, HVA, DOPAC |
| Ma (2006) [58] | Wistar | Male, Female | 6-OHDA | EA | GV16, LR3 | Rotational test, DA |
| Tang (2006) [59] | C57BL/6 | Male | MPTP | EA | LI4, LR3 | BDNF |
| Wang (2006) [60] | SD | Male, Female | 6-OHDA | EA | GV16, LR6 | Glutamic acid |
| Kim (2006) [61] | C57BL/6 | Male | MPTP | MA | LR8, LR4, LR2 | TH |
| Kim (2005) [62] | SD | Undefined | 6-OHDA | MA | ST36 | Rotational test, TH |
| Ma (2005) [63] | Wistar | Male, Female | 6-OHDA | EA | GV16, LR3 | Rotational test, SOD, GSH, GSH-Px |
| Wang (2005) [64] | Wistar | Undefined | 6-OHDA | MA | GV16, LR3, CV4, ST36 | TH |
| Park (2003) [65] | SD | Male | 6-OHDA | MA | GB34, LR3, LI4, LI11 | Rotational test, TH, TrkB |
| Liang (2002) [66] | Wistar | Female | MFB transection | EA | GV14, GV21 | TH, BDNF |
| Lin (2000) [67] | SD | Male, Female | 6-OHDA | EA | LR3, SP6, ST36, GB34 | DA, HVA, DOPAC |
| He (1998) [68] | SD | Male, Female | 6-OHDA | EA | GV20, GV14 | DA, NA, 5HT |
| Zhu (1996) [69] | C57BL/6 | Male | MPTP | MA | GV20 | DA, DOPAC |
Abbreviations: BDNF Brain-derived neurotrophic factor, BV Bee-venom acupuncture, CAT Catalase, Caspase-3: caspase protein, COX-2 Cyclooxygenase-2, DA Dopamine, DAT Dopamine active transporter, DOPAC Dihydroxyphenyl acetic acid, EA Electro-acupuncture, Fn Ferritin, GABA gamma-aminobutyric acid, GDNF Glial cell-derived neurotrophic factor, GFAP Glial fibrillary acidic protein, GSH Glutathione, GSHpx Glutathione peroxidase, HSP70 70 kilo Dalton heat shock proteins, HVA Homovanillic acid, IL-1β Interleukin-1 beta, IL-6 Interleukin-6, iNOS Inducible nitric oxide synthase, Lamp 1 Lysosomal-associated membrane protein 1, MA Manual acupuncture, MAC-1 Macrophage-1 antigen, MDA Malondialdehyde, NO Nitric oxide, nNos Neuronal nitric oxide synthase, MFB Medial forebrain bundle, MPTP 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, p-p38 MARK Phospho-p38 MAPK, PCNA Proliferating cell nuclear antigen, SD Sprague–Dawley, SOD Superoxide dismutase, TH Tyrosine hydroxylase, TNF-α Tumor necrosis factor alpha, TrkB Tropomyosin receptor kinase B, UBE1 Ubiquitin-like Modifier Activating Enzyme 1, UCH-L1 Ubiquitin C-terminal hydrolase, 5HIAA 5-Hydroxyindoleacetic acid, 5HT 5-hydroxytryptamine, 6-OHDA 6-hydroxydopamine
Animals of PD models
The animals of PD models included mice (C57/BL6 and ICR) and rats (Sprague–Dawley, and Wistar) (Fig. 2). The most frequently used animal PD model was C57/BL6, which was used in 24 articles, followed by SD and Wistar, each of which were used in 15 articles, and ICR and undefined animals, which were used in one article each. All of the studies using C57/BL6 animals used only males. Of the studies using SD animals, ten used males only, four used a male/female mix, and one used animals with undefined sex. Of the studies using Wistar animals, nine used a male/female mix, two used males only, three used animals with undefined sex, and one study used females only.
Fig. 2.
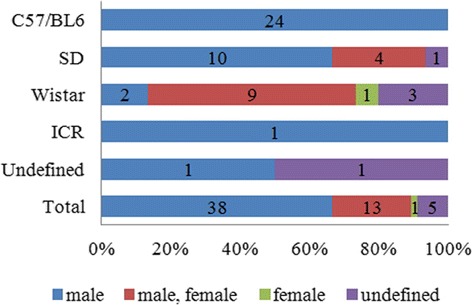
Sex differences according to the types of animal used as PD model
Methods used to induce PD
The drugs 6-hydroxydopamine (6-OHDA), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), and rotenone, as well as medial forebrain bundle (MFB) transection, were used to induce PD in the animal models (Fig. 3). 6-OHDA was used in 47 % (27) of the studies, MPTP was used in 44 % (25) of the studies, and rotenone was used in 7 % (4) of the studies. MFB transection was used in 2 % (1) of the studies. Of the studies using 6-OHDA, 13 used a male/female mix, nine used only males, and five used animals with undefined sex. All of the studies using MPTP or Rotenone used only male animals. The study using MFB transection used only females. Therefore, three out of the four PD induction models studied were only used in animals of a single sex. Only the results of 6-OHDA induced animal PD models could potentially be compared between the sexes.
Fig. 3.
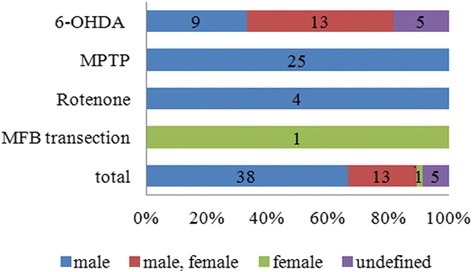
Sex differences according to the method used to induce PD
Types of acupuncture
Electro-acupuncture (EA) was used in 54 % (38) of the studies, manual acupuncture (MA) was used in 30 % (18) of the studies, and bee-venom (BV) acupuncture was used in 11 % (6) of the studies. Of the studies using EA, 18 used only males, 11 used a male/female mix, three used animals of undefined sex, and one used only females. Of the studies using MA, 14 used only males, two used a male/female mix, and two used animals with undefined sex. All of the studies using BV acupuncture used only males (Fig. 4).
Fig. 4.
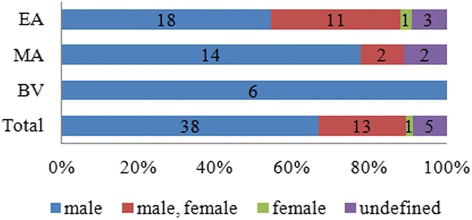
Sex differences according to the type of acupuncture performed
Acupuncture points
Regardless of the type of acupuncture, the acupuncture points used consisted mainly of LR3, GB34, GV20, GV16, and ST36 (Additional file 1). LR3 was used in 35 % (20) of the studies, and GV34 and GV20 were each used in 26 % (16) of the studies. Of the studies using LR3, 14 used only males, three used a male/female mix, and three used animals with undefined sex. Of the studies using GB34, 14 used only males, and two used a male/female mix. Of the studies using GV20, eight used only males, seven used a male/female mix, and one used animals with undefined sex. Of the studies using GV16, seven used only males, three used a male/female mix, and three used animals with undefined sex. Of the studies using ST36, four used only males, two used animals with undefined sex, and one used a male/female mix.
Behavioral test
Behavioral analyses were carried out using the rotational behavior test, the pole-climbing test, the swimming test, and locomotor counts (Additional file 2). The rotational behavior test was used in 56 % (10) of the studies, the pole-climbing test was used in 22 % (6) of the studies, and the swimming test, and locomotor counts were each used in 6 % (1) of the studies. The rotational behavior test was mainly used in conjunction with 6-OHDA (8 studies), the pole-climbing test was used in conjunction with MPTP (6 studies), and the swimming test and locomotor counts were each used in conjunction with MPTP (Additional file 3). The rotational behavior test was used in five studies with only males, four studies with a male/female mix, and one study with animals with undefined sex. The studies using the pole-climbing test, the swimming test, and locomotor counts were each conducted with males only. Of all studies including behavioral analyses, 72 % (13) of the studies used only male animals, 22 % (4) used a male/female mix, and 6 % (1) used animals with undefined sex. In these studies, PD was induced using MPTP in 53 % (9) of the studies and 6-OHDA in 47 % (8).
Evaluation of treatment effectiveness
The effectiveness of the treatment on PD was evaluated by levels of tyrosine hydroxylase (TH), dopamine (DA), dihydroxyphenyl acetic acid (DOPAC), homovanillic acid (HVA), superoxide dismutase (SOD), glutathione (GSH), and brain-derived neurotrophic factor (BDNF) (Additional file 4). TH was the most frequently used method to determine the effectiveness of the treatment on PD (56 % [32] of the studies). Of the studies using TH, 26 used only males, two used a male/female mix, three used animals with undefined sex, and one used only females. Of the studies using DA, ten used only males, five used a male/female mix, and two used animals with undefined sex. Of the studies using DOPAC, seven used only males, and two used a male/female mix. Of the studies using HVA and GSH, respectively, four of each used only males, and two of each used a male/female mix. Of the studies using SOD, four used only males, and two used a male/female mix. Of the studies using BDNF, four used only males, one used a male/female mix, and one used only females.
Discussion
We analyzed sex differences among previous studies that used animal PD models of acupuncture treatment. A total of 810 potentially relevant articles were identified, 57 of which met our inclusion criteria. C57/BL6 mice were the most frequently used (42 %) animal PD models. Most of the studies evaluating the effectiveness of acupuncture treatment for PD were performed using only male animals (67 %); only one study (2 %) was performed using female animals.
Many studies have inadvertently excluded females from animal studies of acupuncture treatment for PD. Kang et al. suggested that acupuncture could be used as a neuroprotective intervention for inhibiting microglial activation and inflammatory events in the MPTP-induced male PD model [52]. Yu et al. showed that acupuncture treatment displays antioxidative and/or neuroprotective properties in the 6-OHDA lesioned male rat PD models [3]. Although a few studies were performed using a male/female mix, they could not combine and compare the results from male versus female animals. Only one report used female animals, in which was a study in which different frequencies of chronic EA stimulation were tested in a partially-lesioned female rat model of PD induced by transection of the MFB. This study suggested that long-term high frequency EA is effective in halting the degeneration of dopaminergic neurons in the substantia nigra (SN). Because the studies of male PD models generated using MFB transection are nonexistent, we could not compare the sex differences in this model. Taken together, there is currently insufficient evidence from past studies to determine whether there are sex differences in the effectiveness of acupuncture for animal PD models. In the future, studies should be performed using a male/female mix to minimize performance bias, and ideally should include a comparison of the sex differences.
Animal studies have often focused primarily on males. For the most part, examination of the differences between males and females has been disregarded in biomedical research, leaving gaps in our knowledge [42]. Recently, new drugs have been developed without considering the physiological characteristics of females or sex differences. Women have therefore been frequently exposed to dangerous side effects because the experimental studies and clinical trials had mainly used male subjects [70]. The lack of female participation in drug-development studies affects males as well as females; when side effects not seen in males during the drug safety checks appear in females, the approval of the drugs is delayed, and male patients waiting for the drugs consequently suffer. The NIH requires applicants to report their cell and animal inclusion plans as part of the preclinical experimental design. Despite this NIH policy, numerous scientific publications continue to neglect sex-based considerations and analyses in preclinical and clinical research. A stronger commitment to reporting sex-specific results will strengthen the evidence base [13]. Fortunately, sex differences are increasingly recognized as factors that influence the incidence and disease manifestations of all diseases, including neurodegenerative disorders.
Some gender differences have been documented for PD [3, 4]. Paven et al. suggested gender differences in the epidemiology, clinical features, treatment outcomes (medical and surgical/deep brain stimulation), and social impact among all available PD studies [4]. Wooten et al. performed a meta-analysis of the differences in the incidence of PD between men and women [3]. Smith et al. summarized evidence that estrogen and selective estrogen receptor modulators are neuroprotective in PD, and reviewed sex differences in basal ganglia function and dopaminergic pathways [71, 72]. Consistent with these past studies, if acupuncture research involved both males and females, additional studies of acupuncture for PD would provide a more robust conclusion about sex differences in this treatment.
Review limitations and future areas of research
A number of gaps in the reviewed literature were identified in relation to study quality and findings. Study quality could be improved by using female animal models because they reflect the physiological characteristics of both males and females to fully evaluate the effectiveness and safety of the treatment for each sex, which is largely missing in the literature so far.
Conclusions
The results of our review suggest that acupuncture is an effective treatment for animal PD models, but there is insufficient evidence to determine whether sex differences exist in response to this treatment. Future studies should examine the effects of acupuncture in animal PD models of both sexes, to reflect the physiological characteristics of females as well as males, and to fully evaluate the effect and safety of this treatment.
Acknowledgements
We would like to thank Jong-Yeop Kim for his assistance with the collection of data used for this study.
Funding
This work was supported by the Mid-Career Research Program through an NRF grant funded by the Korean government (No. 2014R1A2A1A11052795).
Availability of data and materials
The data sets supporting the conclusions of this article are included within the article.
Authors’ contributions
SHL and SL created the study background and designed the study; SHL performed data acquisition and analysis, and drafted the article; SL conducted the literature review; MvdN, PB and SL revised the article. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
This information is not relevant.
Ethics approval and consent to participate
Not applicable.
Abbreviations
- BDNF
Brain-derived neurotrophic factor
- BV
Bee-venom acupuncture
- DA
Dopamine
- DOPAC
Dihydroxyphenyl acetic acid
- EA
Electro-acupuncture
- GSH
Glutathione
- HVA
Homovanillic acid
- MA
Manual acupuncture
- MFB
Medial forebrain bundle
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- SD
Sprague-Dawley
- SOD
Superoxide dismutase
- TH
Tyrosine hydroxylase
- 6-OHDA
6-hydroxydopamine
Additional files
Sex differences according to the acupuncture points used. (TIF 781 kb)
Sex differences according to behavioral tests used. (TIF 776 kb)
Behavioral tests performed categorized by the method used to induce PD. (TIF 638 kb)
Sex differences according to the method of evaluation of treatment effectiveness. (TIF 842 kb)
Contributor Information
Sook-Hyun Lee, Email: sh00god@khu.ac.kr.
Maurits van den Noort, Email: info@mauritsvandennoort.com.
Peggy Bosch, Email: p.bosch@donders.ru.nl.
Sabina Lim, Phone: 82-02-961-0324, Email: lims@khu.ac.kr.
References
- 1.Obeso JA, Rodríguez-Oroz MC, Benitez-Temino B, Blesa FJ, Guridi J, Marin C, Rodriguez M. Functional organization of the basal ganglia: therapeutic implications for Parkinson's disease. Mov Disord. 2008;23(Suppl 3):548–59. doi: 10.1002/mds.22062. [DOI] [PubMed] [Google Scholar]
- 2.Samii A, Nutt JG, Ransom BR. Parkinson's disease. Lancet. 2004;363(9423):1783–93. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- 3.Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson’s disease than women? J Neurol Neurosurg Psychiatry. 2004;75(4):637–9. doi: 10.1136/jnnp.2003.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavon JM, Whitson HE, Okun MS. Parkinson's disease in women: a call for improved clinical studies and for comparative effectiveness research. Maturitas. 2010;65(4):352–8. doi: 10.1016/j.maturitas.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wald C, Wu C. Biomedical research. Of mice and women: the bias in animal models. Science. 2010;327(5973):1571–2. doi: 10.1126/science.327.5973.1571. [DOI] [PubMed] [Google Scholar]
- 6.Haack S, Seeringer A, Thürmann PA, Becker T, Kirchheiner J. Sex-specific differences in side effects of psychotropic drugs: genes or gender? Pharmacogenomics. 2009;10(9):1511–26. doi: 10.2217/pgs.09.102. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez-Fischer DC, Noelker F, Vulinović A, Grünewald C, et al. Bee venom and its component apamin as neuroprotective agents in a Parkinson disease mouse model. Plos One. 2013;8(4):e61700. doi: 10.1371/journal.pone.0061700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan B, Zhigang L, Hu B, et al. Effects of electro-acupuncture on Baihui and Taiyang points on caspase- 3 expression and apoptosis of substantia nigra dopaminergic neurons of rats with Parkinson disease. Chinese J Tradit Med Sci &Tech. 2014;21(5):534–6. [Google Scholar]
- 9.Wang YC, Xu YH, Ma J, et al. Effects of electric acupuncture on the oxidative stress in the rat of Parkinson’s disease abductioned by rotenone. Hubei Univ of TCM. 2013;28(8):2417–9. [Google Scholar]
- 10.Feng J, Sun HM, Wang YY, et al. Influences of needling chorea-tremble controlled zone on expressions of dopaminergic neurons and BDNF in mice with Parkinson’s disease. J Beijing Univ TCM. 2014;37(1):53–7. [Google Scholar]
- 11.Ding YX, Zhao J, Hou LQ. Effects of electroacupuncture on the expressions of neuroal nitric oxide synthase and astrocyte in dentate gyrus of rats with Parkinson's disease. Chin acupunct& mox. 2013;33(6):533–7. [PubMed] [Google Scholar]
- 12.Wang SJ, Fang JQ, Ma J. Effect of electroacupuncture stimulation of ``Fengfu'' (GV 16) and ``Taichong'' (LR 3) on expression of COX-2 and tyrosine hydroxylase in substantia nigra in rats with Parkinson's disease. Acupuncture Research. 2013;38(3):198–201. [PubMed] [Google Scholar]
- 13.Anita H. Gender bias in research: how does it affect evidence based medicine? J R Soc Med. 2007;100(1):2–3. doi: 10.1258/jrsm.100.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clayton JA, Collins FS. NIH to balance sex in cell and animal studies. Nature. 2014;509(7500):282–3. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raz L, Miller VM. Considerations of sex and gender differences in preclinical and clinical trials. Handb Exp Pharmacol. 2012;214:127–47. doi: 10.1007/978-3-642-30726-3_7. [DOI] [PubMed] [Google Scholar]
- 16.Singh N, Pillay V, Choonara YE. Advances in the treatment of Parkinson’s disease. Prog Neurobiol. 2007;81(1):29–44. doi: 10.1016/j.pneurobio.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Walton-Hadlock J. Primary Parkinson's disease: the use of Tuina and acupuncture in accord with an evolving hypothesis of its cause from the perspective of Chinese traditional medicine--part 2. Am J Acupunct. 1999;27(1–2):31–49. [PubMed] [Google Scholar]
- 18.Choi YG, Yeo S, Hong YM, Lim S. Neuroprotective changes of striatal degeneration-related gene expression by acupuncture in an MPTP mouse model of Parkinsonism: microarray analysis. Cell Mol Neurobiol. 2011;31(3):377–91. doi: 10.1007/s10571-010-9629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeo S, Choi YG, Hong YM, Lim S. Neuroprotective changes of thalamic degeneration-related gene expression by acupuncture in an MPTP mouse model of Parkinsonism: microarray analysis. Gene. 2013;515(2):329–38. doi: 10.1016/j.gene.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Yeo S, Lim S, Choe IH, Choi YG, Chung KC, Jahng GH, Kim SH. Acupuncture stimulation on GB34 activates neural responses associated with Parkinson's disease. CNS Neurosci Ther. 2012;18(9):781–90. doi: 10.1111/j.1755-5949.2012.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi YG, Park JH, Lim S. Acupuncture inhibits ferric iron deposition and ferritin-heavy chain reduction in an MPTP-induced Parkinsonism model. Neurosci Lett. 2009;450(2):92–6. doi: 10.1016/j.neulet.2008.11.049. [DOI] [PubMed] [Google Scholar]
- 22.Bai Y, LI ZG, Wang S, et al. Study on protective effect of different electro- acupuncture on Baihui and Taiyang points on dopaminergic neurons of rats with Parkinson Disease. Chin Med Sci Tech. 2014a;21(5):536–8.
- 23.Wang SJ, Fang JQ, Ma J, et al. Influence of electroacupuncture on p38-mitogen activated protein kinase in substantia nigra cells of rats with Parkinson disease model. Chin Acupunct Mox. 2013b;33(4):329–33. [PubMed]
- 24.Wang YC, He F, Ma J, et al. impacts of electroacupuncture on ubiquitin-proteasome system in rats with Parkinson’s disease. Hubei Univ TCM. 2013d;33(8):725–9. [PubMed]
- 25.Ding YX, Hou LQ, Xiong KR. Effect of electroacupuncture on expression of proliferating cell nuclear antigen and glial fibrillary acidic protein in subventricular zone of Parkinson's disease rats. Acupunct Res. 2012;37(4):286–290. [PubMed] [Google Scholar]
- 26.Huang Q, Sun Y, Wang L, Zhang Y, et al. Acupuncture improve motor function in a mouse model of Parkinson's disease. Nucl Tech. 2012;35(11):877–880. [Google Scholar]
- 27.Lu ZY, Zhao H, Wang T. Effects of Acupuncture on Behavior and Striatal Apoptosis in Mice with Parkinson Disease. Acupunct Res. 2012;37(3):186–190. [PubMed] [Google Scholar]
- 28.Guo CX, Zhang L, Shao SJ, Hao L, et al. Oxidative stress effects of electro-acupuncture for "Baihui ", "Fengfu", "Yanglingquan " on PD model rat. Yunnan Univ TCM. 2012;35(5):26–29. [Google Scholar]
- 29.Yang JL, Chen JS, Yang YF, et al. Neuroprotection effects of retained acupuncture in neurotoxin-induced Parkinson's disease mice. Brain Behav Immun. 2011;25(7):1452–1459. doi: 10.1016/j.bbi.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Kim JI, Yang EJ, Lee MS, et al. Bee venom reduces neuroinflammation in the MPTP-induced model of Parkinson's disease. Int J Neurosci. 2011;121:209–217. doi: 10.3109/00207454.2010.548613. [DOI] [PubMed] [Google Scholar]
- 31.Du J, Sun ZL, Jia J, Wang X, et al. High-frequency electro-acupuncture stimulation modulates intracerebral γ-aminobutyric acid content in rat model of Parkinson’s disease. Acta Physiol Sinica. 2011;63(4):305–310. [PubMed] [Google Scholar]
- 32.Wang H, Pan Y, Xue B, et al. The Antioxidative Effect of Electro-Acupuncture in a Mouse Model of Parkinson’s Disease. PLoS ONE. 2011;6(5):e19790. doi: 10.1371/journal.pone.0019790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doo AR, Kim ST, Kim SN, et al. Neuroprotective effects of bee venom pharmaceutical acupuncture in acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson's disease. Neurolog Res. 2010;32(Suppl 1):88–91. doi: 10.1179/016164109X12537002794282. [DOI] [PubMed] [Google Scholar]
- 34.Hong MS, Park HK, Yang JS, et al. Gene expression profile of acupuncture treatment in 1-methyl-4-phenyl-1,2, 3,6- tetrahydropyridine-induced Parkinson's disease model. Neurol Res. 2010;32(Suppl 1):S74–S78. doi: 10.1179/016164109X12537002794165. [DOI] [PubMed] [Google Scholar]
- 35.Jun HJ, Kim YS. Dose-dependent Effects of Bee Venom Acupuncture on MPTP-induced Mouse Model of Parkinson's Disease. J Korean Acupunct Mox Med Sci. 2010;27(5):59–68. [Google Scholar]
- 36.Kim ST, Moon W, Chae Y, et al. The effect of electroaucpuncture for 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced proteomic changes in the mouse striatum. J Physiol Sci. 2010;60:27–34. doi: 10.1007/s12576-009-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park W, Kim JK, Kim JI, et al. Neuroprotective and Anti-inflammatory Effects of Bee Venom Acupuncture on MPTP-induced Mouse. J Korean Acupunct Mox Med Sci. 2010;27(3):105–116. [Google Scholar]
- 38.Sun H, Wu H, Xu H, et al. Protective effect of acupuncture in acupoints of Governor Vessel on dopaminergic neurons protection and influence on ultrastructure in mice with Parkinson’s disease. Beijing Univ TCM. 2010;33(4):257–262. [Google Scholar]
- 39.Wang YC, Cheng YH, Ma J, et al. Effect of electroacupuncture on morphological changes and apoptosis of substantia nigra cells in Parkinson's disease rats. Acupunct Res. 2010a;35(6):415–21. [PubMed]
- 40.Wang YC, Cheng YH, Ma J, et al. Effects of electroacupuncture on the expression of GDNF and Ret in Parkinson’s disease model rats. Hubei Univ TCM. 2010b;30(9):739–43. [PubMed]
- 41.Wang YY, Sun HM, He X, et al. The protection effect of acupuncture of baihui and dazhui points on the dopaminergic neurons in PD mice. Prog Anatomic Sci. 2010c;16(1):16–20.
- 42.Yu YP, Ju WP, Li ZG, Wang DZ, Wang YC, Xie AM. Acupuncture inhibits oxidative stress and rotational behavior in 6-hydroxydopamine lesioned rat. Brain Res. 2010;1336:58–65. doi: 10.1016/j.brainres.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 43.Huang PP, Ma J, Wang Y, et al. Effect of electroacupuncture on the expression of BDNF and TrKB in mesencephic substantia nigra of the Parkinson 's disease model rats. J Hubei Univ Chin Med. 2010;12(2):3–5. [Google Scholar]
- 44.Kim CY, Lee JD, Lee SH. Anti-inflammatory Effect of Bee Venom Acupuncture at Sinsu (BL23) in a MPTP Mouse Model of Parkinson Disease. J Korean Acupunct Mox Med Sci. 2009;26(4):49–58. [Google Scholar]
- 45.Wang S, Jiang H, Qu L. Study on the mechanism of electroacupuncture scalp point penetration therapy in action on apoptosis in the Parkinson's disease rat model. Chin Acupunct Mox. 2009a;29(4):309–13. [PubMed]
- 46.Wang S, Qi XJ, Han D. Effect of electroacupuncture scalp point-through-point therapy on the expression of tyrosine hydroxylase and dopamine transporter mRNAs in substantia nigra of Parkinson's disease model rats. Chin Acupunct Mox. 2009b;29(5):391–4. [PubMed]
- 47.Kim YJ, Kim BS, Park HJ. Acupuncture at GB34 modulates laminin expression in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced PD mouse model. J Acupunct Acupoint. 2008;25(1):155–164. [Google Scholar]
- 48.Guan T, Sun H, Zhang L, et al. The effects of electroacupuncture for iron protein and stained cells in the substantia nigra in Parkinson 's disease model mice. Chin J Histochem Cytochem. 2008;17(5):427–431. [Google Scholar]
- 49.Wang S, Qi XJ, Han D. Impacts of penetration therapy with head electrical acupuncture on proliferation of neural stem cells in substantia nigra of rat model of Parkinson. World J Acu-Mox. 2008;18:23–30. [Google Scholar]
- 50.Jeon S, Kim YJ, Kim ST, et al. Proteomic analysis of the neuroprotective mechanisms of acupuncture treatment in a Parkinson's disease mouse model. Proteomics. 2008;8(22):4822–4832. doi: 10.1002/pmic.200700955. [DOI] [PubMed] [Google Scholar]
- 51.Xie XX, Kou ST, Pu ZH, et al. Effects of scalp catgut embedding on SOD, NO, MDA in the rat with Parkinson's disease. Zhongguo Zhen Jiu. 2007;27(10):753–756. [PubMed] [Google Scholar]
- 52.Kang JM, Park HJ, Choi YG, Choe IH, Park JH, Kim YS, Lim S. Acupuncture inhibits microglial activation and inflammatory events in the MPTP-induced mouse model. Brain Res. 2007;1131(1):211–219. doi: 10.1016/j.brainres.2006.10.089. [DOI] [PubMed] [Google Scholar]
- 53.Hwang JY, Choi IH, Park JH, et al. Acupuncture inhibits microglial activation in the rat model of Parkinson's disease. Korean J Meridian Acupoint. 2007;24(1):131–144. [Google Scholar]
- 54.Luo EL, Zhao FZ, Li GZ, et al. Effects of acupuncture on NOS of the medial part of the globus pallidus in rat model of Parkinson's disease. J Clinic Acupunct Mox. 2007;23(1):54–55. [Google Scholar]
- 55.Wang Q, Tang CZ, Chen XH, et al. Effects of acupuncture on dopaminergic neurons in rats with Parkinson Disease. Chin J Basic Med Tradit Chin Med. 2007;13(8):621–622. [Google Scholar]
- 56.Jin Z, Liu TT, Zhou BX. Changes of the anti-oxidation capability of rat model of Parkinson disease after acupuncture at subthalamus. Chin J Clinic Rehabil. 2006a;10(23):188–90.
- 57.Jin Z, Liu TT, Jiang XC, et al. Effects of electroacupuncture at subthalamus on dopamine homovanillic acid and dihydroxy-phenyl acetic acid of rats with Parkinson disease. Chin J Clinic Rehabil. 2006b;10:128–9.
- 58.Ma J, Wang YC, Gan SY. Effects of electroacupuncture on behaviors and dopaminergic neurons in the rat of Parkinson's disease. Chin Acupunct Mox. 2006;26(9):655–657. [PubMed] [Google Scholar]
- 59.Tang Y, Yu S, Chen J. Effect of Electroacupuncture on the Expression of BDNF and BDNF mRNA in Parkinson's Disease Mice. Acupunct Res. 2006;31(1):38–42. [Google Scholar]
- 60.Wang YC, Ma J, Wang H. Changes of content of glutamic acid in striatum of rats with Parkinson disease after electroacupuncture stimulation at acupoints. Chin J Clinic Rehabil. 2006;10:183–185. [Google Scholar]
- 61.Kim ST, Park HJ, Chae YB, et al. Acupuncture at Liver Meridian Protects the Dopaminergic Neuronal Damage in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s Disease Mouse Model. Korean J Acupunct. 2006;23(4):169–176. [Google Scholar]
- 62.Kim YK, Lim HH, Song YK, et al. Effect of acupuncture on 6-hydroxydopamine-induced nigrostratal dopaminergic neuronal cell death in rats. Neurosci Lett. 2005;384(1-2):133–138. doi: 10.1016/j.neulet.2005.04.068. [DOI] [PubMed] [Google Scholar]
- 63.Ma J, Zhu SX. Effect of electroacupuncture on anti-oxidase in mesencephalic substantia nigra in rats with Parkinson disease. Chin J Clin Rehabil. 2005;9:120–121. [Google Scholar]
- 64.Wang YD, Ma J, Wang H. Experimental study of the protective effect on dopaminergic neurons in substance nigra of the Parkinsonian rats by the acupuncture therapy of 'Shuanggu Yitong'. J Hubei College TCM. 2005;7(3):25–26. [Google Scholar]
- 65.Park HJ, Lim S, Joo WS, et al. Acupuncture prevents 6-hydroxydopamine-induced neuronal death in the nigrostriatal dopaminergic system in the rat Parkinson's disease model. Exp Neurol. 2003;180(1):92–97. doi: 10.1016/S0014-4886(02)00031-6. [DOI] [PubMed] [Google Scholar]
- 66.Liang XB, Liu XY, Li FQ, et al. Long-term high-frequency electro-acupuncture stimulation prevents neuronal degeneration and up-regulates BDNF mRNA in the substantia nigra and ventral tegmental area following medial forebrain bundle axotomy. Brain Res Mol Brain Res. 2002;108:51–59. doi: 10.1016/S0169-328X(02)00513-2. [DOI] [PubMed] [Google Scholar]
- 67.Lin Y, Lin X. Comparative study of D2 receptors and dopamine content in striatum before and after electro-acupuncture treatment in rats. J Chin Med. 2000;113(5):408–411. [PubMed] [Google Scholar]
- 68.He C, Wang L, Dong H, et al. Effects of Acupuncture - Moxibustion on the Contents of Monoamine Transmitters in the Striatum of Rats in Parkinson's Disease. Acupunct res. 1998;1:44–48. [Google Scholar]
- 69.Zhu W, Xi G, Ju J. Effect of acupuncture and Chinese medicine treatment on brain dopamine level of MPTP-lesioned C57BL mice. Acupunct Res. 1996;4:46–9. [PubMed] [Google Scholar]
- 70.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35(3):565–72. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin RM, Biswas PN, Freemantle SN, Pearce GL, Mann RD. Age and sex distribution of suspected adverse drug reactions to newly marketed drugs in general practice in England: analysis of 48 cohort studies. Br J Clin Pharmacol. 1998;46(5):505–11. doi: 10.1046/j.1365-2125.1998.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith KM, Dahodwala N. Sex differences in Parkinson's disease and other movement disorders. Exp Neurol. 2014;259:44–56. doi: 10.1016/j.expneurol.2014.03.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets supporting the conclusions of this article are included within the article.


