Abstract
Introduction
Neurotransmitters are chemical messengers that enable communication between the neurons in the synaptic cleft. Inborn errors of neurotransmitter biosynthesis, breakdown and transport are a group of very rare neurometabolic diseases resulting in neurological impairment at any age from newborn to adulthood.
Methods and results
The International Working Group on Neurotransmitter related Disorders (iNTD) is the first international network focusing on the study of primary and secondary neurotransmitter disorders. It was founded with the aim to foster exchange and improve knowledge in the field of these rare diseases. The newly established iNTD patient registry for neurotransmitter related diseases collects longitudinal data on the natural disease course, approach to diagnosis, therapeutic strategies, and quality of life of affected patients. The registry forms the evidence base for the development of consensus guidelines for patients with neurotransmitter related disorders.
Conclusion
The iNTD network and registry will improve knowledge and strengthen research capacities in the field of inborn neurotransmitter disorders. The evidence-based guidelines will facilitate standardized diagnostic procedures and treatment approaches.
Abbreviations: AADC, aromatic l-amino acid decarboxylase; AR/ADGTPCH, autosomal recessive/dominant GTP-cyclohydrolase deficiency; BH4, tetrahydrobiopterin; DAT, dopamine transporter; DßH, dopamine β-hydroxylase; DHFR, dihydrofolate reductase deficiency; DHPR, dihydropteridine reductase; FOLR1, folate receptor alpha; GABA, gamma aminobutyric acid; MAOA, monoamine oxidase A; 5-MTHF, 5-methyltetrahydrofolate; NKH, nonketotic hyperglycinemia; NOS, nitric oxide synthase; PAH, phenylalanine hydroxylase; 3-PGDH, 3-phosphoglycerat dehydrogenase; 3-PGH, 3-phosphoglycerat dehydrogenase; PSAT, phosphoserine aminotransferase; 3-PSP, 3-phosphoserine phosphatase; PTPS, 6-pyruvoyl-tetrahydropterin synthase; SR, sepiapterin reductase; SSADH, succinic semialdehyde dehydrogenase; TH, tyrosine hydroxylase; TPH, tryptophan hydroxylase; VMAT, vesicular monoamine transporter
Keywords: Neurotransmitter, Network, Database, Dopamine, Serotonin, Glycine, GABA, Serine, Guideline, Patient registry
1. Introduction
Neurotransmitters are a group of chemical messengers that enable communication between the neurons in the synaptic cleft. With regard to chemical properties, neurotransmitters can be grouped into amino acids (including glutamate, glycine, serine and gamma aminobutyric acid), peptides, purines, and monoamines or biogenic amines (including acetylcholine, epinephrine, norepinephrine, dopamine and serotonin). Most neurotransmitters have either excitatory or inhibitory effects, but only a few can exert both depending upon the type of receptors that are present [13]. After biosynthesis neurotransmitters are stored within synaptic vesicles and secreted in response to the appropriate nerve impulse [13]. Peptides have higher molecular weight than biogenic amines and amino acids, and are produced and released by neurons through the regulated secretory route. Interestingly, many peptides exhibit neurotransmitter activity as well as possess hormonal function.
Inborn errors of neurotransmitter biosynthesis, breakdown or transport are a group of very rare neurometabolic diseases. The incidence of the combined neurotransmitter diseases can only be estimated, but so far around 1500 patients have been published worldwide [3], [12], [19], [21], [28]. Clinical symptoms can appear at any age from newborn to adulthood. In the following the different groups of neurotransmitter disorders and their main features are described:
Disorders of monoamines and tetrahydrobiopterin metabolism.
Tetrahydrobiopterin (BH4) is known to be the natural cofactor for phenylalanine hydroxylase (PAH), tyrosine hydroxylase (TH), and tryptophan hydroxylase (TPH) as well as all three isoforms of nitric oxide synthase (NOS) [27]. The BH4 dependent enzymes TH and TPH are together with the aromatic amino acid decarboxylase (AADC) the key enzymes in the biosynthesis of the neurotransmitters dopamine and serotonin [1]. Accordingly, disorders of BH4 metabolism result in deficiency of biogenic amines. In addition to enzyme deficiencies, two transporter defects are known to cause disorders of biogenic amine metabolism [16], [23]. The relevant enzymes and transporters are listed in Table 1. The clinical presentation is determined by the type and severity of the underlying disorder [18] and ranges from intermittent focal dystonia and dystonia-parkinsonism to severe, lethal infantile encephalopathies. In adulthood these diseases may cause behavioral and mood disorders [11], [20], [25]. Patients with disorders of BH4 metabolism can with two exceptions be identified by detection of hyperphenylalaninemia on newborn screening (PKU), thereby allowing early diagnosis and initiation of treatment in asymptomatic individuals.
Table 1.
Overview on inborn errors of neurotransmitter metabolism included in the iNTD patient registry, including acronym and OMIM number (Online Mendelian Inheritance in Man).
| Disease name | Acronym | Gene name | OMIM# |
|---|---|---|---|
| Aromatic l-amino acid decarboxylase deficiency | AADCD | DDC | #608643 |
| Tyrosine hydroxylase deficiency | THD | TH | #191290 |
| Dopamine β-hydroxylase deficiency | DßHD | DßH | #223360 |
| Monoamine oxidase A deficiency | MOAAD | MAO-A | # 309850 |
| Dopamine transporter deficiency | DATD | SLC6A3 | #126455 |
| Vesicular monoamine transporter deficiency | VMATD | SLC18A2 | #193001 |
| Autosomal recessive GTP-cyclohydrolase deficiency | ARGTPCHD | GCH1 | #233910 |
| Autosomal dominant GTP-cyclohydrolase deficiency | ADGTPCHD | GCH1 | #600225 |
| 6-Pyruvoyl-tetrahydropterin synthase deficiency | PTPSD | PTS | #261640 |
| Dihydropteridine reductase deficiency | DHPRD | QDPR | #261630 |
| Sepiapterin reductase deficiency | SRD | SPR | #182125 |
| Folate receptor alpha deficiency | FOLR1D | FOLR1 | # 613068 |
| Dihydrofolate reductase deficiency | DHFRD | DHFR | # 613839 |
| 3-Phosphoglycerat dehydrogenase deficiency | 3-PGDHD | PHGDH | #606879 |
| 3-Phosphoserine phosphatase deficiency | 3-PSPD | PSPH | #172480 |
| Phosphoserine aminotransferase deficiency | PSATD | PSAT1 | #610936 |
| Nonketotic hyperglycinemia | NKH |
AMT GLDC GCSH |
T#238310 P#238300 H#238330 |
| GABA-transaminase deficiency | GABATD | ABAT | #137150 |
| Succinate semialdehyde dehydroxylase deficiency | SSADHD | ALDH5A1 | #271980 |
1.1. Folates
Folates play an essential role in central one-carbon methyl transfer reactions, mediating several biological processes including synthesis of neurotransmitters. 5-MTHF is the widely distributed form in the bloodstream. The autosomal recessive inherited folate receptor alpha (FOLR1) deficiency leads to impaired transport of folate to the CNS resulting in psychomotor decline, progressive movement disturbance, white matter disease, and epilepsy [5], [24]. Patients with dihydrofolate reductase deficiency (DHFR) present with megaloblastic anemia, cerebral folate deficiency and a variety of neurological manifestations, which respond at least partly to treatment with folinic acid [6].
1.2. Disorders of GABA metabolism
Two disorders of GABA catabolism are known: Succinic semialdehyde dehydrogenase (SSADH) and GABA-transaminase deficiency. Most patients with SSADH deficiency develop symptoms in the first 2 years of life and present with prominent deficits in expressive language, motor delay, hypotonia, non-progressive ataxia, epilepsy and neuropsychiatric symptoms [10], [22]. Two families with GABA-transaminase deficiency have been reported displaying developmental delay and hypotonia in early childhood and severe expressive language impairment and obsessive-compulsive disorder in adolescence and adulthood as well as ataxia and hyporeflexia [14], [26].
1.3. Disorders of serine metabolism
Defects of serine metabolism encompass phosphoglycerate dehydrogenase deficiency, phosphoserine aminotransferase deficiency, and 3-phosphoserine phosphatase deficiency. Patients with phosphoglycerate dehydrogenase or phosphoserine aminotransferase manifest with severe intellectual disability, spastic tetraparesis, severe microcephaly and epilepsy [8]. A deficiency of 3-phosphoserine phosphatase was identified in one patient with moderate intellectual disability who also had Williams's syndrome [15] and in another patient with intrauterine growth restriction, intellectual disability, childhood onset epilepsy, and borderline microcephaly who developed progressive lower extremity hypertonia, axonal neuropathy, and hand contractures in adulthood [4].
1.4. Disorders of glycine breakdown
A deficiency of the activity of the glycine cleavage enzyme system leads to nonketotic hyperglycinemia (NKH) due to an accumulation of glycine in tissues and the central nervous system. Based on the amount of residual activity resulting from the particular mutation, clinical presentation of NKH ranges from severe neonatal hypotonia, failure to thrive and burst suppression pattern on EEG in the severe form, to mild mental retardation, learning disabilities or even normal intelligence in the mild from [7], [12].
All neurotransmitter related disorders are rare, and consequently, patients are scattered around the world and frequently do not have access to medical care at centers of expertise. Furthermore, evidence base of current diagnostic and therapeutic approaches is extremely limited and current diagnostic and treatment strategies vary enormously between centers, resulting in sub-optimal care for individual patients. It can be expected that these inequalities have a negative impact on health outcome and on socio-economics in analogy to other rare diseases [2], [17].
The major aims of the International Working Group on Neurotransmitter related Disorders (iNTD) are to establish the first network and patient registry for neurotransmitter related disorders. The patient registry will enable detailed analysis of the natural courses of the diseases, the diagnostic approaches and the current therapy strategies as well as the quality of life of the affected patients and possible genotype/phenotype-correlations. Besides expanding knowledge about these diseases, iNTD will enable the development of the first evidence-based consensus guidelines for patients with various neurotransmitter related disorders.
This paper describes the establishment of iNTD and major achievements within the first 2 years of the project.
2. Methods
2.1. The iNTD network
In 2013 the initiative “International Working Group on Neurotransmitter Related Disorders (iNTD)” was founded by three medical experts from the University Hospital Heidelberg (Germany), the Hospital Sant Joan de Déu in Barcelona (Spain) and the Great Ormond Street Hospital in London (United Kingdom) with the aim to foster scientific and clinical networking on an international level and, ultimately, to promote health care for patients with neurotransmitter related disorders. Since then the network has continued to grow and today iNTD consists of 43 project partners from 24 countries worldwide (Fig. 1 and supplement 1). iNTD launched a network website (www.intd-online.org), where network information and activities are announced. Furthermore, the website provides disease related information for families and caretakers as well as health professionals. The steering committee consists of Thomas Opladen (University Hospital Heidelberg), Àngels Garcia-Cazorla (Hospital Sant Joan de Déu Barcelona) and Manju Kurian (Great Ormond Street Hospital London). The steering committee has the overall responsibility to ensure satisfactory progress of the network. It specifies and defines the organization, management, responsibilities and tasks of the network. All iNTD network members that are contributing to the registry have signed a consortium agreement.
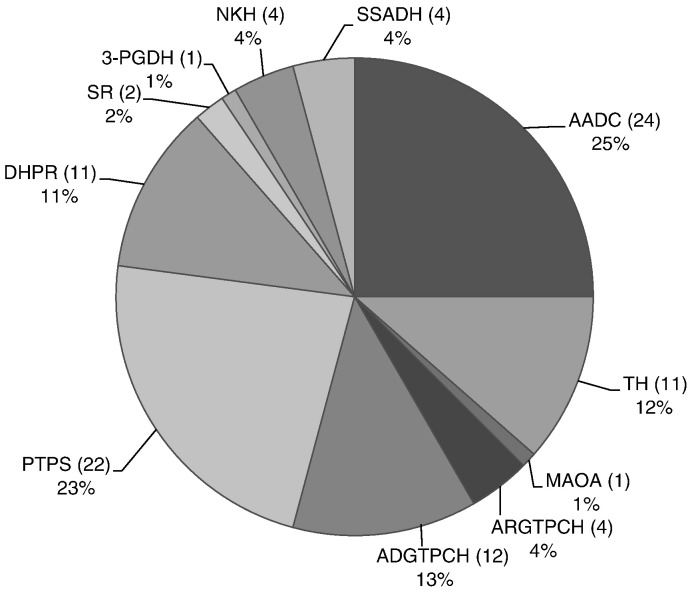
Fig. 1.
Total number, frequency and distribution of neurotransmitter deficiencies in the iNTD patient registry.
2.2. The patient registry
Within one working group iNTD set up the first international, longitudinal patient registry for neurotransmitter related disorders. The patient registry is web-based and password-protected (https://intd-registry.org). It was approved by the local ethics committee of the University Hospital Heidelberg (coordinating center, application number S-471/2014) on 22th December 2014. The data are managed on a secure server, hosted by University Hospital Heidelberg, the site responsible for data storage and processing. The previously existing, stand-alone registries for BH4 and AADC deficiencies, BIODEF and JAKE, established and hosted at the University Children's Hospital in Zürich, Switzerland by Prof. Nenad Blau, have ceased collection of new patient data. To preserve the highly valuable historical data contained in BIODEF and JAKE, the iNTD registry will collaborate with both registries to transfer existing data into the iNTD registry after obtaining informed consent of the respective patients, parents or legal representatives.
Patients' data is collected after written informed consent is obtained by physicians at each participating iNTD center. After a baseline visit, longitudinal follow-up visits are performed annually. Initially only disorders of biogenic amine metabolism, BH4 deficiencies and cerebral folate deficiencies were included. In January 2016 the registry was extended to amino acid neurotransmitter diseases including the serine synthesis deficiencies, disorders of glycine metabolism and GABA related disorders (listed in Table 1). At the same time we also began to register patients with abnormal CSF results suggesting a neurotransmitter disorder but without known diagnosis, for further research purposes.
3. Results
iNTD aims for worldwide coverage. At the time of publication, the iNTD network included 43 partners from 3 continents and 24 different countries (for details see list of iNTD network partners in the supplement). At present, 21 clinical partners have met all necessary legal requirements and are contributing data to the registry. The approval of further study centers is in process and will follow. Since data collection began in January 2015, the number of patients is steadily growing. As of 30 June 2015, 95 patients with a confirmed diagnosis of neurotransmitter related disorders have been registered. In total 95 baseline visits and 33 follow up visits have been documented in the registry so far.
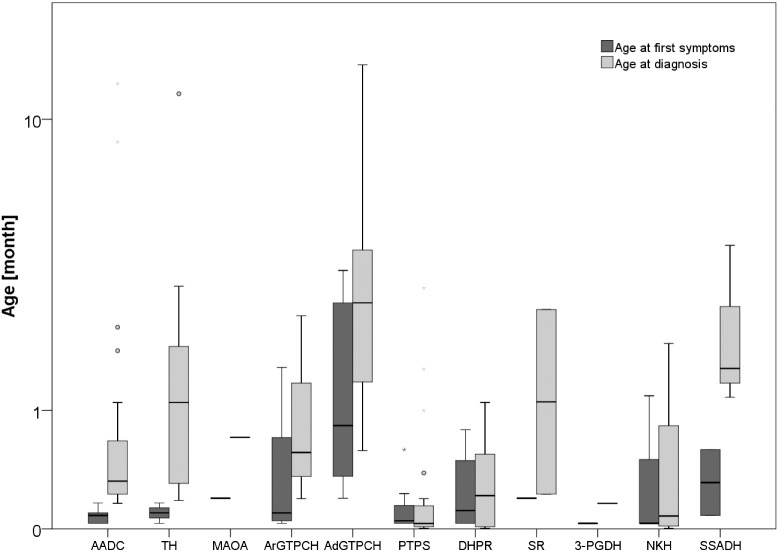
Patients with AADC deficiency are so far the most frequent population in the registry (n = 24, 25%), followed by PTPS deficiency (n = 22, 23%) and autosomal dominant GTPCH deficiency (n = 12, 13%; details in Fig. 1). The predominance of AADC deficiency is partly explained by the support of the AADC parent organization (AADC Research Trust; www.aadcresearch.org) which promoted the study within their membership. There are few patients with disorders of amino acid neurotransmitter metabolism as a result of their very recent inclusion in the registry. At present there are no patients with folate disorders included. The median age of patients in the database is 10.3 years (range: 3 months to 42.5 years). Of note, 25 patients (26.3%) in the registry are older than 16 years (Table 2). It is obvious that with a growing adult cohort, new medical and social care aspects including behavioral as well as psychological problems, puberty and integration into the social life and work systems will arise. First epidemiological results confirm the discrepancy between age of symptom onset and age at diagnosis for some disorders (Fig. 2).
Table 2.
Current number, gender distribution, age range, age at first symptoms and age at diagnosis of 95 patients included in the iNTD registry. m = male; f = female.
| Disease name | Number and gender of patients | Age range | Age range at first symptoms (median) | Age range at diagnosis (median) |
|---|---|---|---|---|
| AADC deficiency | 24 (5 m/19f) | 8.0 months–36.0 years | 1st month of life–5.0 months (2.5 months) |
4.9 months–32.0 years (9.8 months) |
| TH deficiency | 11 (3 m/8f) | 3.0 years–35.6 years | 1st month of life–5.0 months (3.0 months) |
5.5 months–30.0 years (2.8 years) |
| MAOA deficiency | 1 (1 m/0f) | 4.5 years | 6.0 months | 21.6 months |
| ARGTPCH deficiency | 4 (4 m/0f) | 4.0 years–32.7 years | 1st month of life–48.0 years (13.8 months) |
5.9 months–6.0 years (1.4 years) |
| ADGTPCH deficiency | 12 (3 m/9f) | 3.8 years–41.8 years | 6.0 months–9.0 years (44.1 months) |
1.5 years–36.0 years (7.0 years) |
| PTPS deficiency | 22 (9 m/13f) | 2.0 years–42.5 years | 1st month of life–1.5 years (3.8 months) |
0.1 months–7.8 years (1.0 month) |
| DHPR deficiency | 11 (5 m/6f) | 3.0 months–26.3 years | 1st month of life–2.0 years (8.0 months) |
0.1 months–2.8 years (6.9 month) |
| SR deficiency | 2 (1 m/1f) | 3.7 years–8.6 years | 6.0 months (6.0 months) |
6.9 months −.6 years (3.6 years) |
| 3-PGDH deficiency | 1 (1 m/0f) | 8.9 years | 1st month of life | 4.9 months |
| NKH | 4 (2 m/2f) | 5.2 years–17.9 years | 1st month of life–3.0 years (9.8 months) |
0.1 months–5.0 years (2.4 months) |
| SSADH deficiency | 4 (2 m/2f) | 1.2 years–10.5 years | 2.0 months–.5 years (10.3 months) |
2.9 years–10.8 years (4.0 years) |
| Total | 95 (36 m/59f) | 3.0 months–42.5 years | 1st month of life–9.0 years (3.0 months) |
0.1 months–36.0 years (1.1 years) |
Fig. 2.
Age at first symptoms versus age at diagnosis in 95 patients with different neurotransmitter disorders (Boxplot with median, quartiles, outliers (cases with values between 1.5 and 3 box lengths from the upper or lower edge of the interquartile range) and extremes (cases with values more than 3 box lengths from the upper or lower edge of the interquartile range).
3.1. Guideline development
In neurotransmitter related disorders clinical symptoms can be non-specific and diagnosis is often delayed depending significantly on the personal experience of the treating physician. As a consequence, patients and their parents often traverse a stressful odyssey from doctor to doctor with frequent misdiagnoses, such as cerebral palsy, myasthenia gravis or seizure disorders [9], [19] before the correct diagnosis is finally reached. Misdiagnosis or delayed diagnosis may lead to irreversible worsening of the patient's condition and result in significant costs for the health care system. Since evidence-based guidelines for diagnosis and treatment do not exist for any of these disorders, one working group of iNTD is focused upon the development of evidence based guidelines for the most frequent neurotransmitter disorders. The evidence base will be derived from a systematic literature search and review, in addition to data from the patient registry. Relevant publications will be identified and evaluated using a standardized procedure in analogy to the methodology of SIGN (Scottish Intercollegiate Guideline Network; www.sign.ac.uk) and GRADE (Grading of Recommendations Assessment, Development and Evaluation Working Group). In the following, evidence-based recommendations will be formulated for diagnostic procedures, therapeutic strategies, and follow-up monitoring. The guidelines will be published in peer reviewed journals.
The first guidelines developed by iNTD were for the diagnosis and treatment of AADC deficiency (Wassenberg T, Orphanet J Rare Dis, 2016, in press). The guideline working group consisted of 13 child neurologists, 5 biochemists and 1 research project manager from several European countries, the USA and Taiwan. All group members are affiliated with iNTD and are experienced in the diagnosis and treatment of AADC deficiency. Furthermore, a representative of the European AADC parental organization participated in guideline development). The development of evidence based guidelines for inborn errors of BH4 disorders will follow in near future as the next iNTD guideline.
4. Discussion
In developed countries, where infant mortality caused by infectious diseases is low, research on rare diseases has a very high priority as stated in the European Commission's Communication on Rare Diseases: “Coordination projects aimed at an optimal use of the limited resources dedicated to research on rare diseases should be encouraged” (Source: Communication from the commission to the European Parliament, the Council, the European Economic and Social Committee of the Regions, 11. November 2008).
Because inherited neurotransmitter related disorders are very rare, significant progress can only be achieved by transnational efforts. For that purpose, an international network and registry for rare neurotransmitter related disorders are indispensable to improve the knowledge base, develop international consensus care guidelines, and foster networking on international level and, ultimately, to promote health care for patients with neurotransmitter related disorders. Additionally, since many of these disorders are amenable to treatment, promoting awareness and knowledge among the medical community is a matter of great importance.
In the last 2 years, iNTD has established the first worldwide network of experts for neurotransmitter related disorders. The iNTD network provides a platform for clinicians and scientists to exchange expertise and to improve international collaborations regarding these rare neurotransmitter related diseases. Currently iNTD includes 43 metabolic centers from 24 countries worldwide and is open for collaboration with additional stakeholders. iNTD seeks to build partnerships between scientists and non-governmental parent organizations with longstanding basic research, diagnostic and clinical activities. The consortium aims to mobilize a critical mass of expertise and patient numbers to enable rapid translation of basic research into clinical studies at the patient's bedside.
The iNTD registry study is the first detailed longitudinal study in the field of neurotransmitter disorders. To enable systematic and standardized collection of data, all patient data entered into the registry are surveyed by medical doctors. The successful initiation of the registry in January 2015 and the constantly growing number of registered patients will enable detailed clinical phenotyping of the diseases for the first time, and interdisciplinary analysis of the natural history, diagnostic approaches and current treatment strategies, genotype/phenotype correlation, as well as the quality of life of affected patients.
4.1. Outlook
Within the last 2 years iNTD has developed a stable framework for exchange in clinical and scientific research. In future iNTD aims to further improve the awareness and knowledge on rare neurotransmitter related disorders by providing clinical and scientific information to patients and health care professionals. The iNTD registry seeks the inclusion of more than 250 patients in the registry by extending cooperation with other expert centers. The long term focus will be on the evaluation of disease natural history, specifically in the context of upcoming new treatment options. As one example Dr. Toni Pearson (Washington University in St Louis, USA) developed a highly detailed parent questionnaire for patients with AADC deficiency which evaluates the natural course, in the context of the upcoming gene therapy trials for AADC deficiency in the USA and England. Thanks to the modular IT system the extension of the iNTD patient registry and the inclusion of any newly described neurotransmitter related disorder are possible. Hereby a standardized clinical and biochemical characterization of the new disorder and a direct comparisons with known disorders will be easily possible.
A competing interest statement
None.
Details of the contributions of individual authors
All authors contributed to conception and design of the study, collected patient data and gave input to data interpretation. The manuscript was prepared and written by Thomas Opladen, Kathrin Jeltsch and Àngels Garcia-Cazorla. All authors revised the article critically to improve intellectual content and approved the final version.
Details of ethics approval and patient consent statement
The study was approved by the local ethics committee of the University Hospital Heidelberg (coordinating center, application number S-471/2014) on 22th December 2014 and in all data contributing local centers. Informed Consent was obtained from all patients and/or legally authorized representatives.
Acknowledgments
Thomas Opladen and Kathrin Jeltsch were supported by the Dietmar Hopp Foundation, St. Leon-Rot, Germany. Elisenda Cortès-Saladelafont and Àngels Garcia-Cazorla were supported by the FIS-ISCIII grants PI15/01082 and Rio Hortega CM14/00197, respectively. The patient registry was supported in parts by the AADC Research Trust and the Spanish patient association “Proyecto Pol”. Tomas Honzik was supported by program RVO-VFN 64165 from Ministry of Health of the Czech Republic.
We acknowledge the financial support of the Deutsche Forschungsgemeinschaft and Ruprecht-Karls-Universität Heidelberg within the funding programme Open Access Publishing.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ymgmr.2016.09.006.
Appendix A. Supplementary data
Supplementary material.
References
- 1.Blau N., van Spronsen F.J. Disorders of Phenylalanine and Tetrahydrobiopterin Metabolism. In: Blau N., Duran M., KM G., Dionisi-Vici C., editors. Physician's Guide to the Diagnosis, Treatment, and Follow-up of Inherited Metabolic Diseases Heidelberg. Springer; 2014. pp. 3–21. [Google Scholar]
- 2.Brimley C.J., Lopez J., van Haren K. National variation in costs and mortality for leukodystrophy patients in US children's hospitals. Pediatr. Neurol. 2013;49(156–162) doi: 10.1016/j.pediatrneurol.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brun L., Ngu L.H., Keng W.T. Clinical and biochemical features of aromatic l-amino acid decarboxylase deficiency. Neurology. 2010;75:64–71. doi: 10.1212/WNL.0b013e3181e620ae. [DOI] [PubMed] [Google Scholar]
- 4.Byers H.M., Bennett R.L., Malouf E.A. JIMD Reports. 2015. Novel report of phosphoserine phosphatase deficiency in an adult with myeloneuropathy and limb contractures. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cario H., Bode H., Debatin K.M., Opladen T., Schwarz K. Congenital null mutations of the FOLR1 gene: a progressive neurologic disease and its treatment. Neurology. 2009;73:2127–2129. doi: 10.1212/WNL.0b013e3181c679df. [DOI] [PubMed] [Google Scholar]
- 6.Cario H., Smith D.E., Blom H. Dihydrofolate reductase deficiency due to a homozygous DHFR mutation causes megaloblastic anemia and cerebral folate deficiency leading to severe neurologic disease. Am. J. Hum. Genet. 2011;88:226–231. doi: 10.1016/j.ajhg.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinopoulos A., Matsubara Y., Kure S. Atypical variants of nonketotic hyperglycinemia. Mol. Genet. Metab. 2005;86:61–69. doi: 10.1016/j.ymgme.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 8.El-Hattab A.W. Serine biosynthesis and transport defects. Mol. Genet. Metab. 2016;118:153–159. doi: 10.1016/j.ymgme.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Friedman J., Roze E., Abdenur J.E. Sepiapterin reductase deficiency: a treatable mimic of cerebral palsy. Ann. Neurol. 2012;71:520–530. doi: 10.1002/ana.22685. [DOI] [PubMed] [Google Scholar]
- 10.Gibson K.M., Christensen E., Jakobs C. The clinical phenotype of succinic semialdehyde dehydrogenase deficiency (4-hydroxybutyric aciduria): case reports of 23 new patients. Pediatrics. 1997;99:567–574. doi: 10.1542/peds.99.4.567. [DOI] [PubMed] [Google Scholar]
- 11.Gibson K.M., Gupta M., Pearl P.L. Significant behavioral disturbances in succinic semialdehyde dehydrogenase (SSADH) deficiency (gamma-hydroxybutyric aciduria) Biol. Psychiatry. 2003;54:763–768. doi: 10.1016/s0006-3223(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 12.Hennermann J.B., Berger J.M., Grieben U., Scharer G., Van Hove J.L. Prediction of long-term outcome in glycine encephalopathy: a clinical survey. J. Inherit. Metab. Dis. 2012;35:253–261. doi: 10.1007/s10545-011-9398-1. [DOI] [PubMed] [Google Scholar]
- 13.Hyman S.E. Neurotransmitters. Curr. Biol. 2005;15:R154–R158. doi: 10.1016/j.cub.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 14.Jaeken J., Casaer P., de Cock P. Gamma-aminobutyric acid-transaminase deficiency: a newly recognized inborn error of neurotransmitter metabolism. Neuropediatrics. 1984;15:165–169. doi: 10.1055/s-2008-1052362. [DOI] [PubMed] [Google Scholar]
- 15.Jaeken J., Detheux M., Fryns J.P., Collet J.F., Alliet P., Van Schaftingen E. Phosphoserine phosphatase deficiency in a patient with Williams syndrome. J. Med. Genet. 1997;34:594–596. doi: 10.1136/jmg.34.7.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurian M.A., Zhen J., Cheng S.Y. Homozygous loss-of-function mutations in the gene encoding the dopamine transporter are associated with infantile parkinsonism-dystonia. J. Clin. Invest. 2009;119:1595–1603. doi: 10.1172/JCI39060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Bastida J., Perestelo-Perez L., Monton-Alvarez F., Serrano-Aguilar P. Social economic costs and health-related quality of life in patients with degenerative cerebellar ataxia in Spain. Mov. Disord. 2008;23:212–217. doi: 10.1002/mds.21798. [DOI] [PubMed] [Google Scholar]
- 18.Opladen T., Hoffmann G.F. Neurotransmitter disorders. In: Blau N., Duran M., KM G., Dionisi-Vici C., editors. Physician's Guide to the Diagnosis, Treatment, and Follow-up of Inherited Metabolic Diseases. Springer; Berlin Heidelberg: 2014. pp. 515–528. [Google Scholar]
- 19.Opladen T., Hoffmann G.F., Blau N. An international survey of patients with tetrahydrobiopterin deficiencies presenting with hyperphenylalaninaemia. J. Inherit. Metab. Dis. 2012;35:963–973. doi: 10.1007/s10545-012-9506-x. [DOI] [PubMed] [Google Scholar]
- 20.Pan L., McKain B.W., Madan-Khetarpal S. BMJ Case Reports 2011. 2011. GTP-cyclohydrolase deficiency responsive to sapropterin and 5-HTP supplementation: relief of treatment-refractory depression and suicidal behaviour. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearl P.L., Capp P.K., Novotny E.J., Gibson K.M. Inherited disorders of neurotransmitters in children and adults. Clin. Biochem. 2005;38:1051–1058. doi: 10.1016/j.clinbiochem.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Pearl P.L., Gibson K.M., Cortez M.A. Succinic semialdehyde dehydrogenase deficiency: lessons from mice and men. J. Inherit. Metab. Dis. 2009;32:343–352. doi: 10.1007/s10545-009-1034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rilstone J.J., Alkhater R.A., Minassian B.A. Brain dopamine-serotonin vesicular transport disease and its treatment. N. Engl. J. Med. 2013;368:543–550. doi: 10.1056/NEJMoa1207281. [DOI] [PubMed] [Google Scholar]
- 24.Steinfeld R., Grapp M., Kraetzner R. Folate receptor alpha defect causes cerebral folate transport deficiency: a treatable neurodegenerative disorder associated with disturbed myelin metabolism. Am. J. Hum. Genet. 2009;85:354–363. doi: 10.1016/j.ajhg.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tadic V., Kasten M., Bruggemann N., Stiller S., Hagenah J., Klein C. Dopa-responsive dystonia revisited: diagnostic delay, residual signs, and Nonmotor signs. Arch. Neurol. 2012:1–5. doi: 10.1001/archneurol.2012.574. [DOI] [PubMed] [Google Scholar]
- 26.Tsuji M., Aida N., Obata T. A new case of GABA transaminase deficiency facilitated by proton MR spectroscopy. J. Inherit. Metab. Dis. 2010;33:85–90. doi: 10.1007/s10545-009-9022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werner E.R., Blau N., Thony B. Tetrahydrobiopterin: biochemistry and pathophysiology. Biochem. J. 2011;438:397–414. doi: 10.1042/BJ20110293. [DOI] [PubMed] [Google Scholar]
- 28.Willemsen M.A., Verbeek M.M., Kamsteeg E.J. Tyrosine hydroxylase deficiency: a treatable disorder of brain catecholamine biosynthesis. Brain. 2010;133:1810–1822. doi: 10.1093/brain/awq087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.