Abstract
Chandipura virus (CHPV) (Vesiculovirus: Rhabdoviridae) garnered global attention as an emerging neurotropic pathogen inflicting high mortality in children within 24 h of commencement of symptoms. The 2003-2004 outbreaks in Central India witnessed case fatality rates ranging from 56-75 per cent in Andhra Pradesh and Gujarat with typical encephalitic symptoms. Due to the acute sickness and rapid deterioration, the precise mechanism of action of the virus is still unknown. Recent studies have shown increased expression of CHPV phosphoprotein upto 6 h post infection (PI) demonstrating CHPV replication in neuronal cells and the rapid destruction of the cells by apoptosis shed light on the probable mechanism of rapid death in children.
Phlebotomine sandflies are implicated as vectors due to their predominance in endemic areas, repeated virus isolations and their ability to transmit the virus by transovarial and venereal routes. Significant contributions have been made in the development of diagnostics and prophylactics, vaccines and antivirals. Two candidate vaccines, viz. a recombinant vaccine and a killed vaccine and siRNAs targeting P and M proteins have been developed and are awaiting clinical trials. Rhabdomyosarcoma and Phlebotomus papatasi cell lines as well as embryonated chicken eggs have been found useful in virus isolation and propagation. Despite these advancements, CHPV has been a major concern in Central India and warrants immediate attention from virologists, neurologists, paediatricians and the government for containing the virus.
Keywords: Antivirals, candidate vaccines, Chandipura virus, Phlebotomus papatasi, real time RT-PCR, sandflies, siRNA
Introduction
Chandipura virus (CHPV), an arbovirus belonging to genus Vesiculovirus in the family Rhabdoviridae has gained global attention as an encephalitis causing virus after the 2003-2004 outbreaks in central India1,2,3,4,5. A total of 322 child deaths; 183 in Andhra Pradesh (AP), 115 in Maharashtra and 24 in Gujarat were reported during the outbreaks. Case fatality rates (CFR) in AP and Gujarat were 56 to 75 per cent respectively. In majority of the cases, mortality was reported within 24 h of commencement of symptoms. The disease was characterized by sudden onset of high fever followed by seizures, altered sensorium, diarrhoea and vomiting followed by death in majority of the cases4,5. The rapid deterioration and death among the patients could not be explained satisfactorily to date though several hypotheses have been postulated6,7,8. The cause of death was interpreted as encephalitis, acute catastrophic event in the brain, spasm or transient obstruction due to vasculitis. However, none of these could be confirmed scientifically3. The presence of CHPV in the brain biopsy specimens as detected by immunofluorescent antibody technique during the early investigations pointed towards the probable association of CHPV4. But the role of CHPV and the precise mechanism of action could not be explained6,7,8. Increased expression of CHPV phosphoprotein has been demonstrated upto 6 h post-infection (PI) showing the replication of CHPV in neuronal cells7. The investigators reported rapid apoptosis of infected neurons though FAS-associated death domain via an extrinsic pathway following the activation of caspases -8 and -3 as well as prominent cleavage of ADP-ribose polymerase7. They also demonstrated reduction in apoptosis when the pathway was blocked using interfering small RNAs (siRNAs). The disease was predominant in the lower income strata of the population and the affected age group ranged from 2.5 months to 15 yr old. Though the outbreaks were contained, sporadic cases were reported from Warangal district of Andhra Pradesh (now Telangana) and Vidarbha region of Maharashtra with a few case fatalities9,10,11.
Family Rhabdoviridae of Order Mononegavirales comprises negative sense, single stranded viruses with a bullet shaped virions of approximately 11kb. Amongst the 10 genera, genus Lyssavirus and genus Vesiculovirus are of public health importance. Rabies virus, the prototype virus of genus Lyssavirus, is the most important pathogen of Rhabdoviridae with a worldwide distribution. Genus Vesiculovirus, comprises viruses of human and veterinary importance; the prototype specimen being vesicular stomatitis Indiana virus, which infects cattle, horses, pigs, etc. causing mild flu-like symptoms2. Majority of the viruses in the genus are transmitted by Phlebotomine sandflies. Among the Vesiculoviruses discovered so far, CHPV is considered to be the most significant pathogen of public health importance due to the high CFR2.
Though CHPV was first isolated in 1965, it was considered as an orphan or concomitant virus due to low pathogenicity to cause infections in man and domestic animals1. No efforts were, therefore, made to develop diagnostics or prophylactics. However, post-2003 outbreak in central India, CHPV garnered global attention as a human pathogen of public health importance and significant advances were made in basic understanding of the virus as well as in the development of diagnostics and vaccines. The present review is focused on the studies conducted since 2004 on virus vector interactions and development of diagnostics and prophylactics with a special mention on the changing clinical scenario observed during the recent outbreaks. No attempt is made to review the studies conducted at the molecular level though significant contributions have been reported3,6,12,13.
Historical perspective
A new aetiological agent causing febrile illness in man was discovered during an investigation of dengue/chikungunya outbreak in Nagpur district, Maharashtra, India in 196514. Characterization of the agent subsequently revealed it as a new virus. It was named after the place of isolation and placed under the VSV group, genus Vesiculovirus, family Rhabdoviridae15. The term Rhabdo, meaning ‘rod shaped’ in Greek has been assigned due to the bullet shaped morphology of the viruses belonging to the family. CHPV was characteristic with its unique pattern of pathogenesis as it killed infant mice within 10 h of inoculation through intracerebral route as well as produced cytopathic effect (CPE) in vertebrate cell lines within 3-4 h of inoculation, a probable reason for missing the agent on earlier occasions14. The virus was subsequently isolated from Phlebotomine sandflies, the incriminated vector of CHPV, collected from Aurangabad district, Maharashtra, India, during 1967-196916.
After its discovery in 1965 and subsequent isolation from sandflies, no cases of human involvement or any outbreak of public health importance were reported from the area or elsewhere for approximately two decades. Seroprevalence studies carried out in retrospective samples collected since 1955 from different parts of the country demonstrated prevalence of neutralizing (N) antibodies in humans (Table I). N-antibodies were also detected in animals1,14.
Table I.
Seroprevalence of CHPV in human serum samples collected during 1955-1966
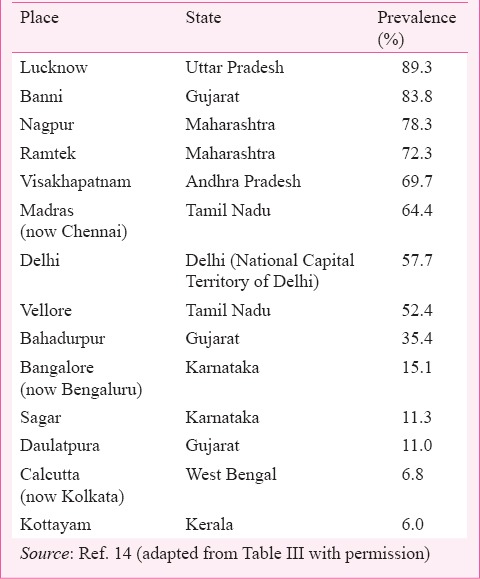
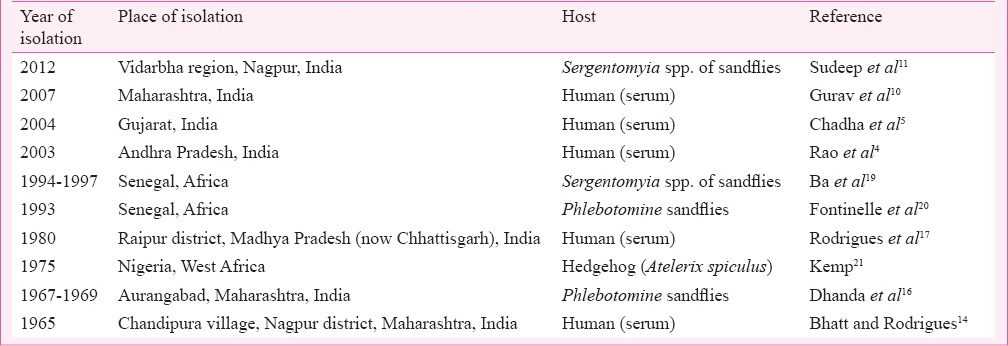
Potential of CHPV to cause mortality in humans was detected when the virus was held solely responsible for the death of an 11 yr old child in Raipur district, Madhya Pradesh (now in Chhattisgarh State). The child developed complications and died within 24 h of admission due to CHPV induced encephalopathy17. Two decades after the episode, CHPV caused explosive outbreaks in Maharashtra, and AP killing over 300 children with a CFR exceeding 50 per cent1,4. The rapid progression and the fatality rates were so confusing that the outbreak was referred to as killer brain disease or mystery disease18. In the following year, a focal outbreak with similar aetiology was reported from Gujarat with CFR exceeding 75 per cent5. Though no outbreak with high CFR was reported since 2004, recurring sporadic cases were reported from Warangal district of Andhra Pradesh (now in Telangana) and Vidarbha region of Maharashtra9,10. Table II lists isolation of CHPV obtained during outbreaks and non-outbreak periods.
Table II.
Chandipura virus (CHPV) isolations during outbreaks and non-outbreak periods
Geographic distribution
The epicenter of CHPV activity has been restricted to Central India comprising parts of Gujarat, Madhya Pradesh, AP and Maharashtra since 1965. However, reports of similar aetiology have been documented from Nagpur in Maharashtra, Muzaffarpur in Bihar, Warangal in AP (now Telangana) and Vadodara in Gujarat prior to 19531. Presence of neutralizing antibodies to CHPV in human and animal samples collected since 1955 substantiates this observation and points towards the circulation of CHPV or a closely related virus across the country1,14. Apart from India, CHPV activity was prevalent in West Africa since 1975 as the virus has been isolated from a hedgehog and wild caught phlebotomine sandflies from Nigeria and Senegal, respectively19,21. CHPV activity was also reported from Sri Lanka as CHPV antibodies were detected in monkeys22.
Serological evidence of natural infections
Retrospective studies with human serum samples collected from 1955 to 1966 from different parts of the country showed prevalence of N-antibodies in humans and domestic animals across the country except in Kashmir and Arunachal Pradesh14. N-antibody prevalence ranged from a minimum of 6 per cent in Kerala to a maximum of 89 per cent in Uttar Pradesh while other places showed varying percentages (Table I). N-antibodies to CHPV were also detected in animals serum samples including domestic animals, camels, Rhesus monkeys, etc.14. Serological studies also demonstrated presence of N-antibodies in pigs and other domestic animals in the affected areas1. However, none of these animals exhibited sickness. Low seroprevalence to CHPV in wild macaques (Macaca sinica) was reported from Sri Lanka without any documented evidence of CHPV infection22.
CHIP transmission by sandflies
Experimental studies with Phlebotomus papatasi showed their potential not only to replicate the virus but also to transmit the virus through vertical, venereal and horizontal routes23,24. The potential of P. papatasi to transmit the virus vertically and venereally points towards maintenance of the virus in nature during non-epidemic periods. This mechanism could have helped the virus to remain dormant for prolonged periods and initiate outbreaks when sandfly population increased under favourable conditions. P. argentipus, a zoophilic species has also been found competent to transmit the virus horizontally to infant mice25. However, their role as vector is still not confirmed.
Initially, members of the genus Phlebotomus was indicated as the vector of CHPV as all the isolations were made only from this genus in India though CHPV isolation from Sergentomyia spp. were reported from Africa2. However, the role of Sergentomyia spp. in CHPV transmission was realized when CHPV RNA was detected in Sergentomyia spp. collected from Karimnagar and Vidarbha region during epidemic periods10,26. It was further confirmed when CHPV was isolated from Sergentomyia spp. collected during an outbreak of acute encephalitis syndrome in Vidarbha region of Maharashtra in 201211. Members of genus Sergentomyia are predominantly peridomestic in nature and seldom come in contact with humans unlike Phlebotomus sandflies. Studies in Vidarbha region showed a reverse trend in which Phlebotomus sandflies were being replaced by Sergentomyia spp. in domestic environments10,11. This was in contrast to that recorded during 1960s and 1970s from the area when the former was predominant16. Detection and isolation of CHPV from Sergentomyia spp. also demonstrates their anthropophagic nature. However, more systematic studies on the bionomics of Sergentomyia spp. are needed for confirming the vector status of the species.
Mosquitoes were not found to be involved in the transmission of CHPV though several species of mosquitoes replicated and transmitted the virus experimentally1. Among the different mosquito species studied, Aedes aegypti was found to be highly susceptible and could transmit the virus more efficiently than others through vertical and venereal routes under laboratory conditions27. The probability of Ae. aegypti as a vector of CHPV could not be justified as no isolation of the virus from the mosquito has been reported so far despite processing several thousand pools of the mosquito collected from outbreak areas in Warangal district (NIV unpublished data).
Experimental studies in cell cultures
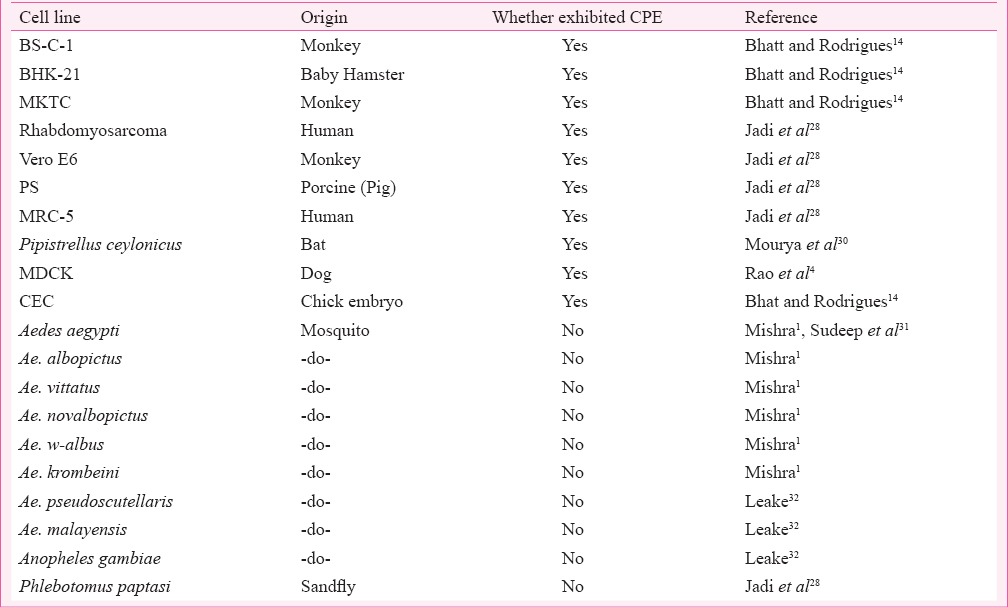
A large number of vertebrate and insect cell lines replicated CHPV giving high yields. Vertebrate cell lines showed distinct cytopathic effects (CPE) while insect cell lines did not. CPE was distinct and characterized by rounding of cells followed by detachment and rapid deterioration. BS-C-1 cell line was used initially to isolate and characterize CHPV and found to be the most sensitive as CPE was exhibited at 3 h post-infection (PI)14. Rhabdomyosarcoma and Vero E6 cell lines were also found highly susceptible and exhibited CPE at 4 and 6 h PI28. Chick embryo was also found susceptible to CHPV and yielded high titre29. Cell lines that were found to be susceptible to CHPV are listed in Table III.
Table III.
Susceptibility and exhibition of cytopathic effect (CPE) of certain cell lines to CHPV
Animal model
An animal model for studying the pathogenesis was developed using Swiss albino mice by Jadi33. He demonstrated infant mice mortality when inoculated with CHPV by intracranial (IC), intraperitoneal, subcutaneous, intradermal, nasal and oral routes. Adult mice showed age related susceptibility as adults above the age of 17 days survived CHPV infection through IC route, but those below 17 days succumbed to infection. The infected mice showed convulsions followed by paralysis of front or hind limbs. Ruffled fur, hunched posture, rapid running movements when stimulated were other symptoms. Urine retention was also observed in certain cases. Histopathological analysis showed moderate perivascular cuffing in brain, mild congestion and collapsed areas in lungs, increased intracellular spaces in heart and focal degenerative changes in liver. Blood brain barrier breakage and virus replication in central nervous system was observed when inoculated through IC or intravenous routes34.
Changing clinical manifestations in CHPV disease
RNA viruses are known for mutation as it provides evolutionary advantages over their host organisms in survival. CHPV has also shown a dramatic change in virulence from a virus causing febrile illness in man to an encephalopathy/encephalitis causing agent with fatal outcomes as observed in 1980 and later in 2003-2004. The first indication of enhanced virulence of the virus was observed in 198017. An 11 yr old child was presented with high grade fever, vomiting and loose motion and developed unusual complications and collapsed within 1 h of admission. The patient experienced convulsions lasting for 2-5 min at an interval of 15-20 min and had generalized hypertonia of the limbs, hyper-reflexia, bilateral extensor plantar response with sudden drop in blood pressure. The investigators felt it a case of encephalitic syndrome and needed further studies to confirm the role of CHPV.
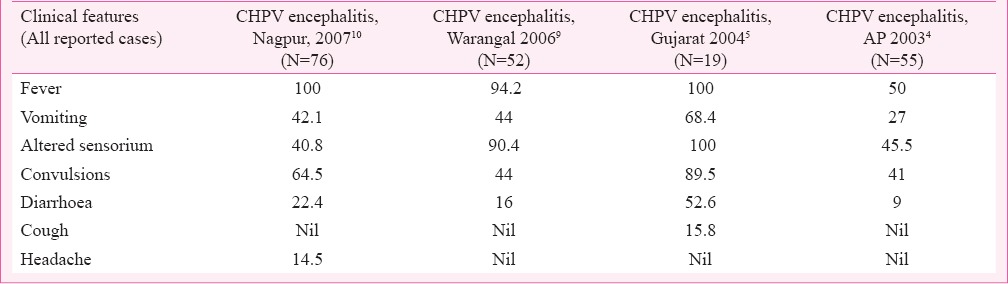
The 2003-2004 outbreaks in Andhra Pradesh (AP), Maharashtra and Gujarat were explosive due to a large number of cases and deaths. Another characteristic was that only children below 15 were involved in the outbreaks. The outbreaks reported the death of 322 children; 183 in AP, 114 in Maharashtra and 24 in Gujarat. CFR in AP and Gujarat were 56 and 76 per cent, respectively4,5. In the AP outbreak, the patients (n=28) had high fever followed by vomiting, diarrhoea and convulsions. Eighty nine per cent of patients had altered sensorium and a few showed neurological deficit (14%) and meningeal irritation (7%). In AP also, the clinical progression of the disease was similar to that of the index case.
Considering the high case fatality in the 2003-2004 outbreaks, it was presumed that the virus genome might have mutated to produce enhanced virulence. However, sequence analysis of N, P and G proteins of the prototype strain (1965) and the recent isolates showed no significant change at the amino acid level36. Elevated levels of interleukin (IL)-2, IL-6, interferon (IFN)-γ and tumour necrosis factor (TNF)α were observed in CHPV infected children in comparison to control population35.
Vesicular eruptions were seen in a few cases during Gujarat outbreak, which developed to hyperpigmentation on healing5. Another clinical manifestation observed was bilateral crepitations on auscultation of lungs in a majority of patients. Tachycardia, another typical clinical manifestation of CHPV was also present among the patients. Palpable liver and elevated levels of alanine aminotransferase and aspartate aminotransferase were also detected in a few cases. Subsequent outbreaks reported from Warangal district (2006)9 and Nagpur, Maharashtra (2007)10 also had identical clinical manifestations. However, a low CFR was observed. A comparative analysis of clinical presentations with the important CHPV outbreaks is depicted in Table IV.
Table IV.
Clinical manifestations (in %) observed in patients at different CHPV outbreaks
Advancements in diagnostics
Tremendous progress has been made in the development of diagnostics and prophylactics37. The significant findings are reviewed here for ready reference:
Immunofluorescent antibody technique (IFA) and virus isolation using cell lines: IFA was successfully employed to detect the presence of CHPV in brain tissues during the 2003 outbreak in Andhra Pradesh1. Subsequently, IFA was standardized to detect the presence of CHPV in cell cultures inoculated with field samples. Jadi et al28 demonstrated the application of sandfly and mosquito cell lines for early detection of CHPV as the virus antigen could be detected within 2 h of inoculation using IFA. The application of cell culture systems has also been successfully employed to detect and isolate the virus as CHPV produced characteristic CPE in vertebrate cell lines4,11,14.
Diagnosis with molecular tools: Since the progression of disease in children is rapid causing death within 24-48 h of commencement of symptoms, the need for rapid diagnosis was felt. A reverse transcriptase PCR (RT-PCR) and real time RT-PCR have been standardized for routine diagnosis35,38. A highly sensitive diagnostic RT-PCR targeting N gene (527 bp) with a detection limit of 10-100 plaque forming units (pfu) has been developed and is being used for routine diagnosis of human and sandfly samples10,37. Development and standardization of a one-step real time RT-PCR using Taqman technology targeting P gene of CHPV has become a major achievement for detection and quantitation of CHPV from field samples38. Optimization of the assay was done using in vitro transcribed (IVT) RNA. Standard curve analysis showed linear relationship for a wide range (r2=0.99) with maximum coefficient of variation (5.91%) for IVT RNA. Detection limit was found at par with nested RT-PCR. It was found more superior in sensitivity than conventional systems viz. infant mice, embryonated eggs and cell culture.
Current status of CHPV vaccine
Considering the rapid progression of the disease resulting in case fatalities, vaccination of the population in endemic areas seems to be the choice to prevent outbreaks. This led to the development of a recombinant and an inactivated vaccine. Both the vaccine candidates induced high immunogenicity in mice and appeared to be promising.
Recombinant vaccine: Venkateshwarlu and Arankalle39 reported the efficiency of a recombinant vaccine using the complete G gene of CHPV isolated from a patient during the 2003 outbreak in AP. The G gene was expressed in a baculovirus expression system and used as an immunogen in mice. A three dose schedule four weeks apart produced 90 per cent seroconversion and protected mice from live virus challenge with 2 log10 TCID50 /ml. Neutralizing and ELISA antibody titres were 1:320 and 1:1200, respectively. The immunized mice also showed 60 per cent T-cell proliferation. The vaccine developed by the recombinant G-protein were found to induce both cell mediated and humoral immune response making it an ideal vaccine candidate against CHPV. Antibody response was found dose dependant and neutralizing antibodies were detected as early as two weeks after the 1st dose39. The vaccine study was completed in 2008, however, no clinical trials in human has been done so far.
Subsequent to the laboratory study, the same investigators tested the efficiency of the vaccine candidate as a combination vaccine with commercially available DPT vaccine40. The combination vaccine (DPT+CHPV) produced increased antibody response to CHPV in comparison to CHPV vaccine alone yielding 90-100 per cent seroconversion and produced ELISA titres in the range of 1:1200 to 1:2400. Antibody titres of individual components persisted for six months without significant drop. The immunized mice also survived intracerebral virus challenge, making it an ideal candidate to protect children of the endemic areas through national vaccination programme.
Killed virus vaccine: A beta propio lactone (BPL) inactivated tissue culture based vaccine has also been developed and evaluated for immunogenicity in mice41. CHPV produced in Vero E6 cell line was purified by differential centrifugation and inactivated with BPL at a concentration of 1:3500, and was used as immunogen. The vaccine produced 100 per cent seroconversion after the third dose in mice (Fig. 1). The neutralizing antibody titres after the 3rd dose ranged from 1:80 to 1:320. Challenge studies have shown that all the immunized mice having 1:20 antibody titre, survived live virus challenge with CHPV intracerebrally (Fig. 2). Even a two dose vaccine yielded 100 per cent protection in seroconverted mice. Though the vaccine candidate has been found to be promising this vaccine awaits clinical trials in humans.
Fig. 1.
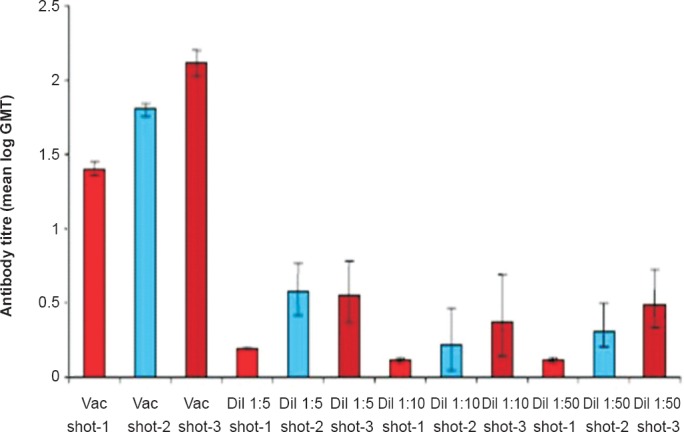
Comparison of antibody titres at different doses and dilutions of vaccine. (Source: Ref. 41, reproduced with permission).
Fig. 2.
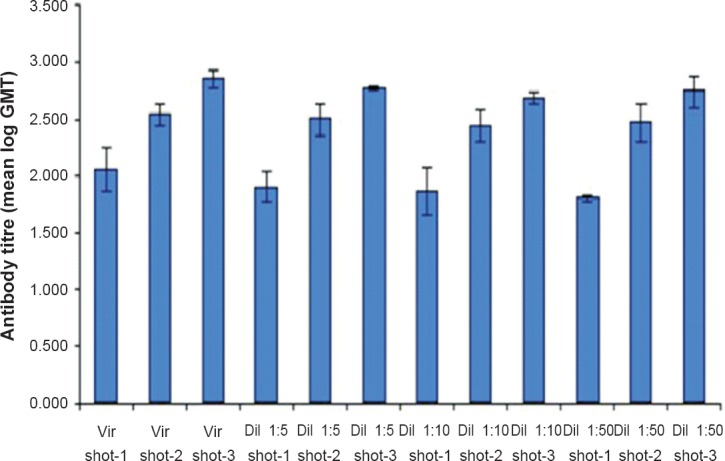
Geometric mean of reciprocal anti CHPV antibody titres obtained with different concentration of purified virus. (Source: Ref. 41, reproduced with permission).
Antivirals
In the field of antivirals, siRNAs have been found to be promising as inhibition of virus replication was observed both in vitro and in vivo42. The P and M proteins were targeted due to their importance in virus life cycle. Two log reduction in virus titre was obtained in the siRNA treated Vero cells in comparison to controls. In mice experiments, the investigators observed delayed mortality in mice treated with siRNAs administered in whatever combinations than control mice. They also demonstrated a drop of 4 log virus in siRNA treated mice in comparison to mice inoculated with virus alone. In the survived mice, anti-CHPV-IgG antibodies could not be detected on 7, 14 and 21 days PI demonstrating total clearance of the virus42. Despite the therapeutic potential, no clinical trials have been carried out for use in humans.
Vector control
CHPV is transmitted by Phlebotomine sandflies as evidenced by repeated isolations and their ability to transmit the virus by transovarial and venereal routes23,24,25. Spraying of insecticides could bring down the population drastically. However, control of vectors in endemic areas is difficult as they breed in damp places inside crevices of stone used for construction where insecticide spraying is generally not possible. Cow dung smearing of the floor and walls of houses is another practice of the inhabitants in the endemic areas and they accumulate dried cow dung sheets inside the houses as fodder. It has been reported that cow dung serves as the feed for sandfly larvae43.
Conclusion
CHPV has gained importance as a major public health problem in Central India after the death of >300 children during the 2003-2004 outbreak despite controversies regarding the role of CHPV to cause outbreaks of encephalitis/encephalopathy or acute brain attack. However, presence of CHPV in the brain in at least a few cases confirmed the role of CHPV in the epidemic brain attack (EBA), causing encephalitis like symptoms and rapid death among the patients. In vitro studies have shown increased expression of CHPV phosphoprotein in neuronal cells and their death subsequently by apoptosis. However, more comprehensive studies are needed to confirm the precise mechanism. In the field of diagnostics, antivirals and prophylactics, significant progress has been made. The development of molecular tools, viz. RT-PCR, qRT-PCR has been noteworthy as these are not only highly sensitive but also rapid to detect and quantitate viral RNA from clinical samples. The potential of siRNAs as an antiviral agent has been demonstrated both in vivo and in vitro and would find application for treatment of patients at least during outbreaks. Development of a G-protein based recombinant vaccine and a BPL inactivated vaccine were found to be highly immunogenic. Both the vaccines induced seroconversion in mice and protected the immunized mice from live virus challenge. A combination of the former with commercially available DPT vaccine induced high antibody titres for both the vaccines demonstrating its potential application in the national programme of vaccination.
Despite the advancements made in understanding the virus and development of diagnostics, antivirals and prophylactics, CHPV remained a major concern in certain parts of Maharashtra and Andhra Pradesh. Case fatality, though reduced, still continues to occur in these areas. The natural factors contributing towards the amplification of the virus leading to outbreaks are still not understood. Similarly, the host/genetic factors that contribute to high case fatality are also not clear. A licensed vaccine, which is the need of the hour, for vaccination of children at least in the endemic areas is still not available despite the availability of the technology. Combined efforts from virologists, neurologists, paediatricians and the government are warranted to address this important issue of public health importance for minimizing the recurrence of the disease.
Footnotes
Conflicts of Interest: None.
References
- 1.Mishra AC. Chandipura encephalitis: a newly recognized disease of public health importance in India. In: Scheld WM, Hooper DC, Hughes JM, editors. Emerging infections. Washington DC: ASM Press; 2007. pp. 121–37. [Google Scholar]
- 2.Depaquit J, Grandadam M, Fouque F, Andry PE, Peyrefitte C. Arthropod-borne viruses transmitted by Phlebotomine sandflies in Europe: a review. Euro Surveill. 2010;15:1–8. [PubMed] [Google Scholar]
- 3.Menghani S, Chikhale R, Ravale A, Wadibhasme P, Khedekar P. Chandipura virus: An emerging tropical pathogen. Acta Trop. 2012;124:1–14. doi: 10.1016/j.actatropica.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Rao BL, Basu A, Wairagkar NS, Gore MM, Arankalle VA, Thakare JP, et al. A large outbreak of acute encephalitis with high fatality rate in children in Andhra Pradesh, India, in 2003, associated with Chandipura virus. Lancet. 2004;364:869–74. doi: 10.1016/S0140-6736(04)16982-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chadha MS, Arankalle VA, Jadi RS, Joshi MV, Thakare JP, Mahadev PV, et al. An outbreak of Chandipura virus encephalitis in the eastern districts of Gujarat state, India. Am J Trop Med Hyg. 2005;73:566–70. [PubMed] [Google Scholar]
- 6.Kumar K, Rajasekharan S, Gulati S, Rana J, Gabrani R, Jain CK, et al. Elucidating the interacting domains of chandipura virus nucleocapsid protein. Adv Virol. 2013;2013 doi: 10.1155/2013/594319. article ID 594319 (9 pages) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosh S, Dutta K, Basu A. Chandipura virus induces neuronal death through Fas-mediated extrinsic apoptotic pathway. J Virol. 2013;87:12398–406. doi: 10.1128/JVI.01864-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao PN, Kumar PA, Rao TA, Prasad YA, Rao CJ, Rajyam PL, et al. Role of Chandipura virus in an “epidemic brain attack” in Andhra Pradesh, India. J Pediatr Neurol. 2004;2:131–43. [Google Scholar]
- 9.Tandale BV, Tikute SS, Arankalle VA, Sathe PS, Joshi MV, Ranadive SN, et al. Chandipura virus: a major cause of acute encephalitis in children in North Telangana, Andhra Pradesh, India. J Med Virol. 2008;80:118–24. doi: 10.1002/jmv.21041. [DOI] [PubMed] [Google Scholar]
- 10.Gurav YK, Tandale BV, Jadi RS, Gunjikar RS, Tikute SS, Jamgaonkar AV, et al. Chandipura virus encephalitis outbreak among children in Nagpur division, Maharashtra, 2007. Indian J Med Res. 2010;132:395–9. [PubMed] [Google Scholar]
- 11.Sudeep AB, Bondre VP, Gurav YK, Gokhale MD, Sapkal GN, Mavale MS, et al. Isolation of Chandipura virus (Vesiculovirus: Rhabdoviridae) from Sergentomyia species of sandflies frm Nagpur, Maharashtra, India. Indian J Med Res. 2014;139:769–72. [PMC free article] [PubMed] [Google Scholar]
- 12.Basak S, Mondal A, Polley S, Mukhopadhyay S, Chattopadhyay D. Reviewing Chandipura: A Vesiculovirus in human epidemics. Biosci Rep. 2007;27:275–98. doi: 10.1007/s10540-007-9054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mondal A, Bhattacharya R, Ganguly T, Mukhopadhyay S, Basu A, Basak S, et al. Elucidation of functional domains of Chandipura virus nucleocapsid protein involved in oligomerization and RNA binding: Implication in viral genome encapsidation. Virology. 2010;407:33–42. doi: 10.1016/j.virol.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 14.Bhatt PN, Rodrigues FM. Chandipura: A new arbovirus isolated in India from patients with febrile illness. Indian J Med Res. 1967;55:1295–305. [PubMed] [Google Scholar]
- 15.Rose JK, Whitt MA. Rhabdoviridae: The viruses and their replication. In: Knipe DM, Howley PM, editors. Field's virology. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1221–44. [Google Scholar]
- 16.Dhanda V, Rodrigues FM, Ghosh SN. Isolation of Chandipura virus from Sandflies in Aurangabad. Indian J Med Res. 1970;58:179–80. [PubMed] [Google Scholar]
- 17.Rodrigues JJ, Singh PB, Dave DS, Prasan R, Ayachit V, Shaikh BH, et al. Isolation of Chandipura virus from the blood in acute encephalopathy syndrome. Indian J Med Res. 1983;77:303–7. [PubMed] [Google Scholar]
- 18.John TJ. Outbreak of killer brain disease in children: mystery or missed diagnosis? Indian Pediatr. 2003;40:863–9. [PubMed] [Google Scholar]
- 19.Ba Y, Trouillet J, Thonnon J, Fontenille D. Phlebotomus of Senegal: survey of the fauna in the region of Kedougou. Isolation of arbovirus. Bull Soc Pathol Exot. 1999;92:131–5. [PubMed] [Google Scholar]
- 20.Fontinelle D, Traore-Lamizana M, Trouillet J, Leclerc A, Mondo M, Ba Y, et al. First isolations of arboviruses from Phlebotomine sandflies in West Africa. Am J Trop Med Hyg. 1994;50:570–4. doi: 10.4269/ajtmh.1994.50.570. [DOI] [PubMed] [Google Scholar]
- 21.Kemp GE. Viruses other than arena viruses from West African wild mammals. Factors affecting transmission to man and domestic animals. Bull World Health Organ. 1975;52:615–20. [PMC free article] [PubMed] [Google Scholar]
- 22.Peiris JS, Dittus WP, Ratnayake CB. Seroepidemiology of dengue and other arboviruses in a natural population of toque macaques (Macaca sinica) at Polonnaruwa, Sri Lanka. J Med Primatol. 1993;22:240–5. [PubMed] [Google Scholar]
- 23.Tesh RB, Modi GB. Growth and transovarial transmission of Chandipura virus (Rhabdoviridae: Vesiculovirus) in Phlebotomus papatasi. Am J Trop Med Hyg. 1983;32:621–3. doi: 10.4269/ajtmh.1983.32.621. [DOI] [PubMed] [Google Scholar]
- 24.Mavale MS, Fulmali PV, Geevarghese G, Arankalle, VA, Ghodke YS, Kanojia P, et al. Venereal transmission of Chandipura virus by Phlebotomus papatasi (Scopoli) Am J Trop Med Hyg. 2006;75:1151–2. [PubMed] [Google Scholar]
- 25.Mavale MS, Fulmali PV, Ghodke YS, Mishra AC, Kanojia P, Geevarghese G. Experimental transmission of Chandipura virus by Phlebotomus argentipes (Diptera: Psychodidae) Am J Trop Med Hyg. 2007;76:307–9. [PubMed] [Google Scholar]
- 26.Geevarghese G, Arankalle VA, Jadi R, Kanojia PC, Joshi MV, Mishra AC. Detection of Chandipura virus from sandflies in the genus Sergentomia (Diptera: Phlebotomidae) at Karimnagar District, Andhra Pradesh, India. J Med Entomol. 2005;42:495–6. doi: 10.1093/jmedent/42.3.495. [DOI] [PubMed] [Google Scholar]
- 27.Mavale MS, Geevarghese G, Ghodke YS, Fulmali PV, Singh A, Mishra AC. Vertical and venereal transmission of Chandipura virus (Rhabdoviridae) by Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2005;42:909–11. doi: 10.1093/jmedent/42.5.909. [DOI] [PubMed] [Google Scholar]
- 28.Jadi RS, Sudeep AB, Kumar S, Arankalle VA, Mishra AC. Chandipura virus growth kinetics in vertebrate cell lines, insect cell lines & embryonated eggs. Indian J Med Res. 2010;132:155–9. [PubMed] [Google Scholar]
- 29.Pawar SD, Singh A, Gangodkar SV, Rao BL. Propagation of Chandipura virus in chick embryos. Indian J Exp Biol. 2005;43:930–2. [PubMed] [Google Scholar]
- 30.Mourya DT, Lakra RJ, Yadav PD, Tyagi P, Raut CG, Shete AM, et al. Establishment of cell line from embryonic tissue of Pipistrellus ceylonicus bat species from India & its susceptibility to different viruses. Indian J Med Res. 2013;138:224–31. [PMC free article] [PubMed] [Google Scholar]
- 31.Sudeep AB, Parashar D, Jadi RS, Basu A, Mokashi C, Arankalle VA, et al. Establishment and characterization of a new Aedes aegypti (L.) (Diptera: Culicidae) cell line with special emphasis on virus susceptibility. In Vitro Cell Dev Biol (Anim) 2009;45:491–5. doi: 10.1007/s11626-009-9218-1. [DOI] [PubMed] [Google Scholar]
- 32.Leake CJ. London: University of London (England); 1977. Comparative studies on the infection of invertebrate and vertebrate cell lines with some arboviruses, Ph. D. thesis. [Google Scholar]
- 33.Jadi RS. Development of an inactivated candidate vaccine against Chandipura virus, Ph.D thesis. Pune: Pune University; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anukumar B, Mishra AC. Immune response during acute Chandipura viral infection in experimentally infected susceptible mice. Virol J. 2008;5:121. doi: 10.1186/1743-422X-5-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tripathy A, Balaji S, Rao N, Thakare J, Mishra A, Arankalle VA, et al. Cytokine levels in Chandipura virus associated encephalopathy in children. Scand J Infect Dis. 2005;37:590–3. doi: 10.1080/00365540510044067. [DOI] [PubMed] [Google Scholar]
- 36.Arankalle VA, Shrotri SP, Walimbe AM, Hanumaiah, Pawar SD, Mishra AC. G, N, and P Gene-based analysis of Chandipura Viruses, India. Emerg Infect Dis. 2005;11:123–6. doi: 10.3201/eid1101.040602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bondre VP, Sapkal GN. Chandipura virus: the emerging problem in India. In: Arankalle VA, Cecilia D, editors. Golden to Diamond Jubilee: the glorious decade. Pune: Golden Jubilee Publications; 2012. pp. 211–20. [Google Scholar]
- 38.Kumar S, Jadi RS, Anakkathil SB, Tandale BV, Mishra AC, Arankalle VA. Development and evaluation of a real-time one step Reverse-Transcriptase PCR for quantitation of Chandipura Virus. BMC Infect Dis. 2008;8:168. doi: 10.1186/1471-2334-8-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venkateswarlu CH, Arankalle VA. Recombinant glycoprotein based vaccine for Chandipura virus infection. Vaccine. 2009;27:2845–50. doi: 10.1016/j.vaccine.2009.02.089. [DOI] [PubMed] [Google Scholar]
- 40.Venkateswarlu CH, Arankalle VA. Evaluation of the immunogenicity of a recombinant glycoprotein-based Chandipura vaccine in combination with commercially available DPT vaccine. Vaccine. 2010;28:1463–7. doi: 10.1016/j.vaccine.2009.11.072. [DOI] [PubMed] [Google Scholar]
- 41.Jadi RS, Sudeep AB, Barde PV, Arankalle VA, Mishra AC. Development of an inactivated candidate vaccine against Chandipura virus (Rhabdoviridae: Vesiculovirus) Vaccine. 2011;29:4613–7. doi: 10.1016/j.vaccine.2011.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar S, Arankalle VA. Intracranial administration of P gene siRNA protects mice from lethal Chandipura virus encephalitis. PLoS One. 2010;5:e8615. doi: 10.1371/journal.pone.0008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahadev PVM, Shetty PS, Dhanda V. Dung diet of Phlebotomus papatasi larvae: an experimental evaluation. In: Prasad RS, Thomas C, editors. Vector and vector-borne diseases: Proceedings of the second symposium, 1988 February 8-10. Trivandrum, India: pp. 106–9. [Google Scholar]


