Abstract
Background & objectives:
Insulin resistance (IR) is a major confounding factor in polycystic ovarian syndrome (PCOS) irrespective of obesity. Its exact mechanism remains elusive till now. C/T polymorphism in the -34 promoter region of the CYP17 gene is inconsistently attributed to elucidate the mechanism of IR and its link to hyperandrogenemia in obese PCOS patients. In the present study we aimed to evaluate any association of this polymorphism with IR in non-obese women with PCOS.
Methods:
Polymorphism study was performed by restriction fragment length polymorphism (RFLP) analysis of the Msp A1 digest of the PCR product of the target gene in 75 PCOS cases against 73 age and BMI matched control women. Serum testosterone, BMI and HOMA-IR (homeostatic model of assessment-insulin resistance) were analyzed by standard techniques. A realistic cut-off value for the HOMA-IR was obtained through receiver operating characteristic (ROC) curve for exploring any possible link between IR and T/C polymorphism in the case group.
Results:
Significant increases in serum testosterone and HOMA-IR values were observed among the case group (P<0.001) without any significant elevation in BMI and FBG compared to controls. Cut-off value for IR in the PCOS patients was 1.40 against a maximum sensitivity of 0.83 and a minimum false positivity of 0.13. The analysis revealed an inconclusive link between the C/T polymorphic distribution and insulin resistant case subjects.
Interpretation & conclusions:
The results showed that CYP17A1 gene was not conclusively linked to either IR or its associated increased androgen secretion in non-obese women with PCOS. We propose that an increased sensitivity of insulin on the ovarian cells may be the predominant reason for the clinical effects and symptoms of androgen excess observed in non-obese PCOS patients in our region.
Keywords: C/T polymorphism, CYP 17 α hydroxylase, hyperandrogenemia, insulin resistance, polycystic, ovarian syndrome
Almost 15-20 per cent women worldwide are affected by polycystic ovarian syndrome (PCOS) in their reproductive age group1. With the characteristic features of hyperandrogenemia, high LH : FSH (luteinizing hormone : follicle stimulating hormone) ratio (>2/3:1) and polycystic ovaries, it remains a major cause of anovulatory infertility2. Among several aetiological factors for its pathogenesis, insulin resistance (IR) is a major metabolic and endocrinological factor. Insulin resistance is generally associated with PCOS and is prevalent in about 50-70 per cent of these women irrespective of obesity3. Obesity, when present, acts as a confounding factor for insulin resistance. It is estimated that 44 per cent of women with PCOS suffer from obesity4 that might influence significantly the concurrent insulin resistance observed in them. The insulin resistance with hyperinsulinemia initiates hyperandrogenism through an increase in ovarian androgen biosynthesis5. In addition, insulin resistance and the resultant hyperinsulinemia increase the risk of long-term metabolic disorders, such as impaired glucose tolerance, type 2 diabetes mellitus and finally, cardiovascular diseases in these patients.
Although different mechanisms for insulin resistance in PCOS have been suggested, yet the precise mechanisms of IR and insulin induced hyperandrogenemia in the ovarian tissues remain inconclusive6,7. The paradoxical action of insulin is perhaps, most evidently expressed in the ovaries, which, in spite of a generalized insulin resistant state, retains the ability to produce androgen to its response5,8. However, as PCOS itself is associated with multiple genetic polymorphisms, studies have been undertaken throughout the world to underscore any possible linkage of these polymorphisms with the insulin resistance.
Polymorphisms of several genes like FSHR, CYP17, CYP1A1, CAPN10, INSR, SERPINE, IL-1β and SHBG have been found to be associated with hyperandrognemia and infertility found in PCOS9,10. Variable associations between the single nucleotide polymorphism (SNP) of genes involved in steroid biosynthesis and insulin resistance have been reported. Significant associations of SNPs from the genes encoding aromatase (CYP 19) and insulin sensitivity were observed in African, American, Caucasian, and Japanese women11. A C/T SNP at His 1058 in exon 17 of insulin receptor gene has been found closely linked to the insulin resistance and hyperandrogenemia among lean PCOS women rather than the obese ones12. Homozygosity of the A2/A2 type of C/T polymorphism due to T-->C substitution (-34 bp) in the gene of CYP17 promoter site in the Greek PCOS patients has been suggested to influence steroid biosynthesis and thereby induce the hyperandrogenemia found in this disorder13. Echiburu et al14 reported a significant association of C/T polymorphism in the promoter region of CYP17 gene in women with increased body weight and significant insulin resistance and recommended it as a major confounding factor in development of abdominal obesity and metabolic derangements in the PCOS patients. Variable associations of VNTR (variable number tandem repeats) at the upstream region of insulin gene have been reported with insulin resistance in PCOS15. Thus, the importance of the link relating insulin resistance and body weight regulatory mechanisms with several types of genetic polymorphisms including that for the cytochrome P450 in the PCOS patients has been observed16. We hypothesized that there might be an association between the C/T polymorphism in the -34 promoter region of the CYP17 gene with insulin resistance in non-obese women with PCOS and undertook the present study to evaluate it in an Eastern Indian subpopulation.
Material & Methods
This cross-sectional study was carried out in the departments of Biochemistry and Gynaecology of Burdwan Medical College and Hospital and Biochemistry department, Calcutta National Medical College, Kolkata, West Bengal, India, during 2010-2014. Non-obese cases and controls with a body mass index (BMI) less than 30 kg/m2 were considered. A total of 135 unrelated age matched women were screened for PCOS during this period. Seventy five women aged between 19 and 29 yr were recruited in the case group following the revised Rotterdam criteria17. At least two of the following criteria were present in the patients for their selection as cases: (i) oligomenorrhoea and/or anovulation, (ii) hyperandrogenism, clinical or biochemical, and (iii) polycystic ovaries with exclusion of other aetiologies17. Patients suffering from other causes of hyperandrogenism, thyroid function abnormalities, diabetes mellitus, hyperprolactinemia, hypertension and other cardiovascular diseases were excluded. Seventy three age and BMI matched healthy women with normal menstrual cycle, no clinical or biochemical signs for hyperandrogenism, and ultrasound exclusion of any polycystic ovary were selected in the control group. Women having a history of taking oral contraceptives or any other drugs during last three months having a potential effect on the lipid profile, insulin level, or carbohydrate metabolism were excluded from the control group. Informed written consents were obtained from all participants and the study was approved by the institutional ethical committee.
As studies from India18,19 suggested a cut-off value of 23 kg/m2 BMI for Asian women for an increased risk of development of type 2 diabetes mellitus, we classified both our case and control groups into two subgroups each according to a BMI of > and < 23 kg/m2.
All parameters were assayed from 12 h fasting blood samples (5 ml) obtained from cases and controls. The samples were divided into two aliquots. The first one was collected in fluoride-oxalated vials for obtaining plasma and the other in plain dry vials for obtaining serum.
Measurement of fasting blood glucose (FBG): Plasma glucose was assayed by glucose oxidase peroxidase method (GOD-POD)20. Assay was carried out in the autoanalyzer XL-600 from ERBA, USA with the coefficient of variance (CV) within 3 per cent.
Measurement of insulin and testosterone levels: The serum was separated at 4°C and was stored at -20°C till assay was done. Assays were done within one week from the date of collection. Serum insulin and testosterone were assayed by the ELISA kit AccuBind from Monobind Inc, USA. The inter- and intra-assay coefficients of variation (CV) for serum testosterone measurement were 3.4 and 3.6, respectively, and those for the serum insulin assay were 5.9 and 7.2, respectively.
Calculation of HOMA-IR and determination of the cut-off value of HOMA-IR: HOMA-IR was computed with the formula: fasting plasma glucose (mmol/l) times fasting serum insulin (mU/l) divided by 22.5, with the help of HOMA2 Calculator v2.2 (http://www.dtu.ox.ac.uk/homa). The cut-off value of the HOMA-IR for insulin resistance was calculated in women with PCOS with the help of receiver operating characteristic (ROC) curve. A cut-off value for the HOMA-IR in the PCOS patients was derived depending on the maximum possible level of sensitivity, minimum false positivity and maximum possible area under the curve.
Methodology of PCR and RFLP (restriction fragment length polymorphism)
DNA isolation from the case and control groups: Peripheral blood from healthy women were collected in EDTA coated vials and stored in -20°C. The genomic DNA was isolated from the blood by the method of Blin and Stafford using standard phenol-chloroform method21. The concentration of the DNA was obtained spectrophotometrically. Extracted DNA (5 ml) was diluted in 1000 ml of autoclaved distilled water and absorbency of the DNA was measured at 260 nm wavelengths and the concentration was calculated by the following formula: Concentration = absorbance × 50(μg/ml). Final concentration of the isolated DNA was kept at about 50 μg/ml. The quality of the DNA was verified by ratio of the absorbency at 260 and 280 nm. Integrity of the genomic DNA was assayed by electrophoresing the extracted DNA in one per cent agarose gel.
PCR reaction: PCR reaction was carried out using the reagents of the GeNei™ DNA amplification core kit from Bengaluru Genei, India. The CYP17 amplification assay was done as described previously22. PCR fragment containing the target site was generated using the following primers: (i) forward primer: 5’-CATTCGCACCTCTGGAGTC-3’, and (ii) reverse primer: 5’-GGCTCTTGGGGTACTTG-3’. The master mix was prepared by mixing the 10x DNA amplification buffer (500 mM KCl, 100 mM Tris HCl, pH 8.5, 15 mM MgCl2,) 2.5 mM deoxynucleotide triphosphates (dNTP) mix, 50 pmole forward primer, 50 pmole reverse primer, Millipore water, and Taq DNA polymerase. PCR reactions were carried out in 25 μl aliquots containing about 50 ηg of genomic DNA, 50 pmol of each primer, 1x reaction buffer, 0.25 mM dNTP, and 1 unit of Taq polymerase. The amplification was continued for 30 cycles with denaturation at 94°C for one min, annealing at 57°C for one min, and extension at 72°C for one min. An initial denaturation step of 5 min at 94°C and a final extension at 72°C for 5 min were used.
RFLP analysis: The PCR products were digested for 3 h at 37°C using MspAI (from Genei, Bengaluru, India) and separated by gel electrophoresis on 2 per cent agarose (DNA grade from Hi-media, Mumbai, India) followed by staining with ethidium bromide (SRL, Ranbaxy) to identify the base pair change. In addition, all gels were re-read blindly by three persons without any change, and 15 per cent of the analyses were randomly repeated.
Statistical analysis: For analysing the association of C/T allele distribution among PCOS patients and their insulin resistance, chi square tests and odds ratio (OR) ranges at 95% confidence interval (CI) were performed. The significance of differences in the values of FBG, HOMA-IR and serum testosterone between the case and control groups were assessed by independent t test. The two subgroups of case and control groups based on BMI cut-off were compared by independent t test for fasting blood glucose (FBG), serum testosterone and HOMA-IR (homeostatic model of assessment-insulin resistance). Distribution of the allelic polymorphism was compared between these two groups, with the help of chi square test and Fisher's exact P value. Further, the effect of BMI cut-off value of 23 was assessed on HOMA-IR, FBG and serum testosterone taken together, by analysis of covariance through a general linear model of multivariate analysis. The BMI was included as a covariate and HOMA-IR, FBG and testosterone were included as dependent factors for both the case and control groups. All tests were performed with the help of SPSS software version 17.0 for Windows (SPSS, Inc., Chicago, USA).
Results
HOMA-IR, the marker of insulin resistance was significantly (P<0.001) higher in the case group in spite of a non-significant change in their FBG values. Serum testosterone level was found to be significantly (P<0.001) higher in the PCOS cases indicating for hyperandrogenaemia (Table I). Fig. 1 revealed the cut-off value of HOMA -IR for the case group to be 1.40 (leftmost corner of ROC curve, marked by a circle) having maximum sensitivity of 0.83 against a minimum false positivity (1 - specificity) of 0.13 with an area under the curve 0.890.
Table I.
Distribution of study parameters between PCOS cases and normal controls
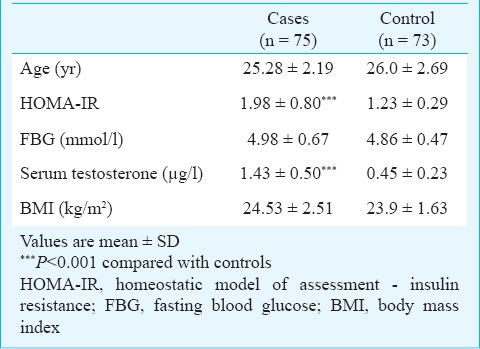
Fig. 1.
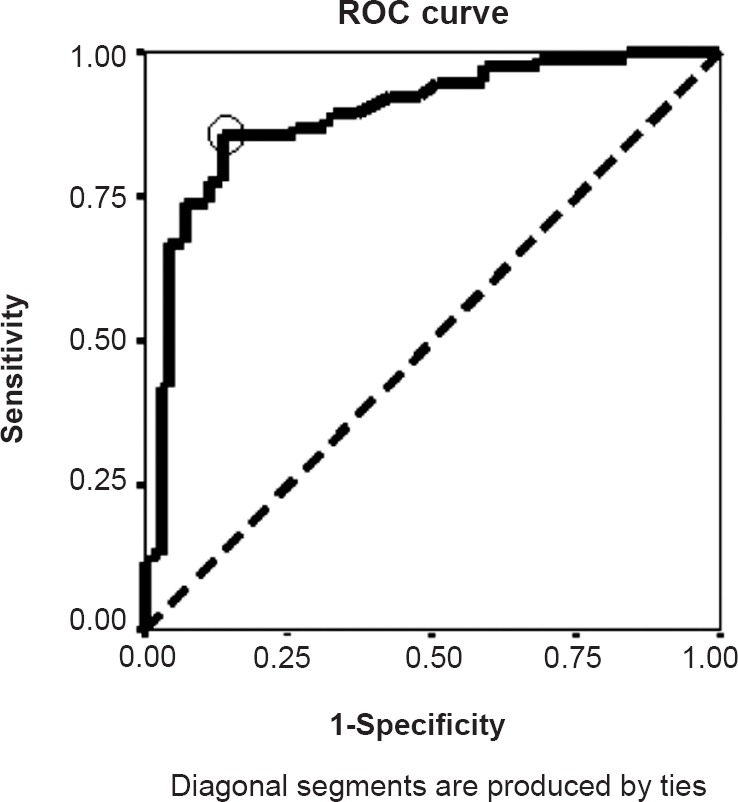
ROC curve for obtaining the HOMA-IR cut-off value.
There was no significant difference in the distribution of CC, TT and TC genotypic forms between the two case groups having HOMA-IR indices above and below the cut-off value of 1.40. No significant difference was observed in the exclusive distribution of C and T allele between these two groups of PCOS cases. Similarly, no such difference was observed in control group (Table II). The overall frequency distribution of these alleles in their genotypic and individual forms in both case and control groups is shown in Table III. Chi square test and Fisher's exact P value did not show any significant difference between the distribution patterns of these alleles in these groups. An odds ratio of 0.945 suggested no significant difference in the allelic distribution between these two groups.
Table II.
Distribution of different alleles according to HOMA-IR cut-off value in PCOS cases
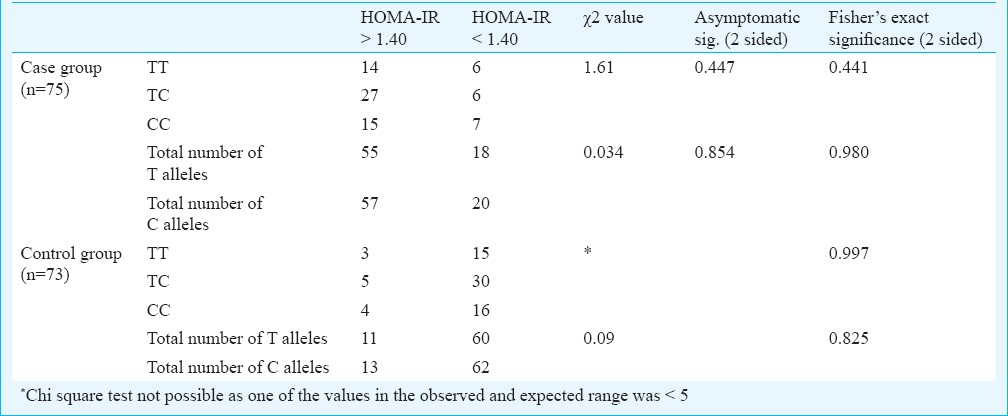
Table III.
Distribution of different alleles in the PCOS cases and normal control subjects
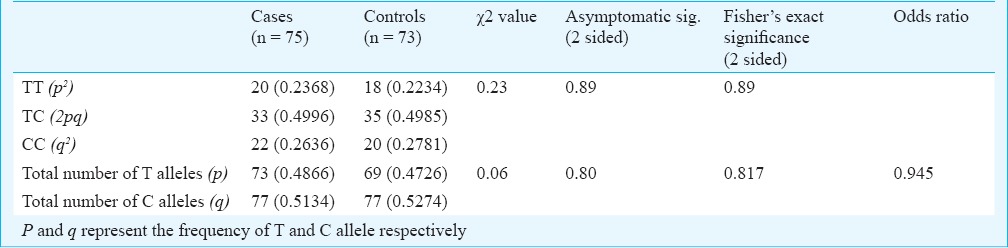
No significant difference was observed between the insulin resistance parameter HOMA-IR, FBG and serum testosterone values between cases and controls having a BMI value < and > 23 kg/m2 (Table IV). Similarly, there was no significant difference between the allelic distribution between these two groups for both cases and controls.
Table IV.
Distribution of different parameters among PCOS cases and normal control group according to BMI (in kg/m2)
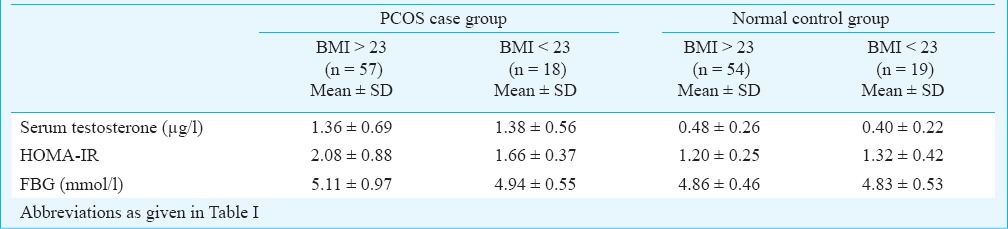
Multivariate general linear model test for analysis of covariance was done to assess the effect of BMI on study parameters by analysing them simultaneously. Results (Table V) showed that there was no significant difference in the FBG, HOMA-IR and serum testosterone values between subjects having a BMI greater than 23 and those with BMI lesser than 23 kg/m2 for both cases and controls.
Table V.
Analysis of covariance by general linear model, multivariate analysis for showing the effect of BMI on other study parameters in PCOS cases (n = 75) and normal control subjects (n = 73)
Fig. 2 shows the distribution of allelic patterns of CYP17A1 in terms of the C/T polymorphism. It was observed that the gene containing both T alleles was not digested and showed a single band at 459 bp, whereas the gene having an SNP of C > T was digested to show two bands for CC homozygotes at 335 and 124 bp and three banks for heterozygotes at 459 bp (for one TT allele) and 335 and 124 bp (for another CC allele).
Fig. 2.
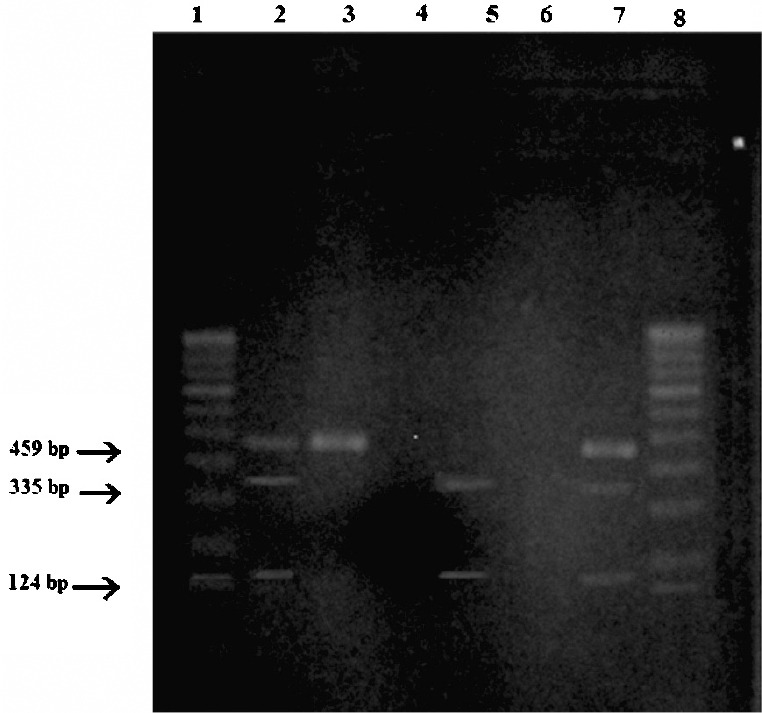
Restriction fragment length polymorphism (RFLP) pattern of MspA1 digest of the CYP17A1 gene. Lanes 1 and 8 : 100 bp DNA ladder, Lane 2: TC heterozygote, Lane 3: TT homozygote, Lane 4: No DNA sample, Lane 5: CC homozygote, Lane 6: No DNA sample, Lane 7: TC heterozygote.
Discussion
The major target in the present study was to explore the relationship of insulin resistance with CYP17A1 gene polymorphism in our region by examining any possible link between insulin resistance and C/T polymorphism at the -34 region in the promoter site of this gene. To find out the status of this polymorphism we performed Hardy-Weinberg equilibrium analysis which revealed χ2 for the case and control groups to be, 1.36 and 0.31, respectively (data not shown in Tables). These values are less than the critical value of 3.84 for 1 degree of freedom that signifies that this polymorphism is stabilized in this population according to the Hardy-Weinberg equilibrium rule. These findings were similar to the observations in different regions of the world that reported a steady state of this polymorphism among PCOS cases9. However, in the present study these stabilized allelic distribution failed to show any significant relationship with the group having increased HOMA-IR values. Although there was a modest increase in total number of C alleles against their T counterparts, it failed to show any significant relationship with the insulin resistance parameter HOMA-IR in the patient group. These observations indicated that the elevated insulin resistance in our PCOS case group did not have any link to the C/T polymorphism at the -34 region in the promoter site of the CYP17 gene. Insulin has been found to increase the expression of steroidogenic genes like StAR and CYP1723. CYP17 gene codes for the protein, P450c17, which is a single cytochrome P450 enzyme that mediates both 17α-hydroxylase and 17, 20 lyase activity in the biosynthetic pathway of steroid hormones14. Importantly, a significant association of C/T polymorphism in the promoter region of CYP17 gene with insulin resistance has been reported14, but not found in our study group.
In patients suffering from PCOS, insulin resistance has been reported to be linked to several variable factors like body weight, some hormones like adiponectin and atrial natriuretic peptide24 and genetic polymorphism of C/T at the promoter region of CYP17 gene14. Homozygosity of the A2/A2 type of C/T polymorphism at this region is found to influence steroid biosynthesis and thereby cause hyperandrogenemia, and hyperandrogenemia itself is reported to be related to an increased insulin resistance observed in these patients13. The ovarian cells, particularly in PCOS, have been reported to exhibit hypersensitivity to insulin and insulin like growth factor 1 (IGF-1) that may increase ovarian steroid hormone production in absence of this polymorphism25. Furthermore, insulin itself has been found to induce the CYP17 gene expression and augment the 17 α hydroxylase activity via PI-3K pathway that may overstimulate the androgen biosynthetic pathway directly6,26. The consequent hyperandrogenemia plays an augmentative role for producing the state of insulin resistance further13. These observations suggest that hyperinsulinemia and insulin resistance may not be always induced by C/T polymorphism of the CYP17 gene as a cause and effect relationship. Our results indicate that insulin induced increase in the androgen secretion as well as its increased sensitivity on the ovarian cells are, most probably the predominant reasons for the clinical effects and symptoms of androgen excess observed in PCOS patients.
Though modified by body weight and other factors, insulin resistance is an intrinsic part of PCOS and has been reported with different levels of HOMA-IR27. In the present study, we did not observe any significant difference in the BMI between the PCOS patients and controls, indicating that the case group was not obese and was matched with the normal body weights of healthy controls. This might explain the modest increase in the HOMA-IR values against higher values observed in obese PCOS patients in other studies (> 2.0)18,19.
A battery of mechanisms including a reduction in the expression of insulin receptor substrate 1 (IRS-1), its tyrosine posphorylation28, defect in signaling process between insulin receptor and glucose transporter-4 (GLUT-4) proteins, reduced GLUT-4 expression in adipose tissues29 and altered expression of several genes like CYP17, PPAR-γ, retinoid X receptors (RXR) have been suggested as plausible explanations for generating a state of insulin resistance in PCOS cases30. However, in spite of this insulin resistance in the peripheral tissues, the ovarian tissues show a paradoxical response to this hormone5,8. In absence of any link of the CYP17A1 polymorphism with insulin resistance in our study group, we suggest a possible role of any of these other mechanisms, working alone or together, in generating an insulin resistant state in our population that needs further large scale studies.
The major limiting factor of the present study was the relatively small size of non-obese PCOS patients that could be included after a complete diagnosis of the disease within the stipulated time period for obvious reasons. However, two small scale studies conducted earlier reported hyperandrogenemia with A2/A2 phenotype of CYP17 gene31 with 65 patients and a linkage between C/T polymorphism in insulin receptor and PCOS with 61 Japanese patients32. Large multicentric studies involving multiple ethnic groups are required for elaborating the effects of genetic polymorphism on several aspects of PCOS more evidently and conclusively.
Acknowledgment
Authors acknowledge Drs Suparna Roy and Rituparna Maji, Calcutta National Medical College, Kolkata, for logistic and instrumental help.
Footnotes
Conflicts of Interest: None.
References
- 1.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013;6:1–13. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 3.Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2005;90:3236–42. doi: 10.1210/jc.2004-1843. [DOI] [PubMed] [Google Scholar]
- 4.Dasgupta A, Khan A, Banerjee U, Ghosh M, Pal M, Chowdhury K, et al. Predictors of Insulin resistance and metabolic complications in polycystic ovarian syndrome in an eastern Indian population. Indian J Clin Biochem. 2012;28:169–76. doi: 10.1007/s12291-012-0253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev. 1999;20:535–82. doi: 10.1210/edrv.20.4.0374. [DOI] [PubMed] [Google Scholar]
- 6.Munir I, Yen HW, Geller DH, Torbati D, Bierden RM, Weitsman SR, et al. Insulin augmentation of 17alpha-hydroxylase activity is mediated by phosphatidyl inositol 3-kinase but not extracellular signal-regulated kinase-1/2 in human ovarian theca cells. Endocrinology. 2004;145:175–83. doi: 10.1210/en.2003-0329. [DOI] [PubMed] [Google Scholar]
- 7.Nelson-Degrave VL, Wickenheisser JK, Hendricks KL, Asano T, Fujishiro M, Legro RS, et al. Alterations in mitogen-activated protein kinase kinase and extracellular regulated kinase signaling in theca cells contribute to excessive androgen production in polycystic ovary syndrome. Mol Endocrinol. 2005;19:379–90. doi: 10.1210/me.2004-0178. [DOI] [PubMed] [Google Scholar]
- 8.Ciaraldi TP, Morales AJ, Hickman MG, Odom-Ford R, Yen SS, Olefsky JM. Lack of insulin resistance in fibroblasts from subjects with polycystic ovary syndrome. Metabolism. 1998;47:940–6. doi: 10.1016/s0026-0495(98)90348-1. [DOI] [PubMed] [Google Scholar]
- 9.Unsal T, Konac E, Yesilkaya E, Yilmaz A, Bideci A, Ilke Onen H, et al. Genetic polymorphisms of FSHR, CYP17, CYP1A1, CAPN10, INSR, SERPINE1 genes in adolescent girls with polycystic ovary syndrome. J Assist Reprod Genet. 2009;26:205–16. doi: 10.1007/s10815-009-9308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia YH, Yao L, Zhang ZX. Correlation between IL-1β, IL-1Ra gene polymorphism and occurrence of polycystic ovary syndrome infertility. Asian Pac J Trop Med. 2013;6:232–6. doi: 10.1016/S1995-7645(13)60030-9. [DOI] [PubMed] [Google Scholar]
- 11.Lo JC, Zhao X, Scuteri A, Brockwell S, Sowers MR. The association of genetic polymorphisms in sex hormone biosynthesis and action with insulin sensitivity and diabetes mellitus in women at midlife. Am J Med. 2006;119(9 Suppl 1):S69–78. doi: 10.1016/j.amjmed.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee S, Shaikh N, Khavale S, Shinde G, Meherji P, Shah N, et al. Genetic variation in exon 17 of INSR is associated with insulin resistance and hyperandrogenemia among lean Indian women with polycystic ovary syndrome. Eur J Endocrinol. 2009;160:855–62. doi: 10.1530/EJE-08-0932. [DOI] [PubMed] [Google Scholar]
- 13.Diamanti-Kandarakis E, Bartzis MI, Zapanti ED, Spina GG, Filandra FA, Tsianateli TC, et al. Polymorphism T-->C (-34 bp) of gene CYP17 promoter in Greek patients with polycystic ovary syndrome. Fertil Steril. 1999;71:431–5. doi: 10.1016/s0015-0282(98)00512-3. [DOI] [PubMed] [Google Scholar]
- 14.Echiburu B, Perez-Bravo F, Maliqueo M, Sanchez F, Crisosto N, Sir-Petermann T. Polymorphism T --> C (-34 base pairs) of gene CYP17 promoter in women with polycystic ovary syndrome is associated with increased body weight and insulin resistance: a preliminary study. Metabolism. 2008;57:1765–71. doi: 10.1016/j.metabol.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Waterworth DM, Bennett ST, Gharani N, McCarthy MI, Hague S, Batty S, et al. Linkage and association of insulin gene VNTR regulatory polymorphism with polycystic ovary syndrome. Lancet. 1997;349:986–90. doi: 10.1016/S0140-6736(96)08368-7. [DOI] [PubMed] [Google Scholar]
- 16.Wang CP, Hung WC, Yu TH, Chiu CA, Lu LF, Chung FM, et al. Genetic variation in the G-50T polymorphism of the cytochrome P450 epoxygenase CYP2J2 gene and the risk of younger onset type 2 diabetes among Chinese population: potential interaction with body mass index and family history. Exp Clin Endocrinol Diabetes. 2010;118:346–52. doi: 10.1055/s-0029-1243604. [DOI] [PubMed] [Google Scholar]
- 17.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Ramachandran A, Snehalatha C. Rising burden of obesity in Asia. J Obesity. 2010 doi: 10.1155/2010/868573. pii 868573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pusalkar M, Meherji P, Gokral J, Savardekar L, Chinnaraj S, Maitra A. Obesity and polycystic ovary syndrome: association with androgens, leptin and its genotypes. Gynecol Endocrinol. 2010;26:874–82. doi: 10.3109/09513590.2010.487586. [DOI] [PubMed] [Google Scholar]
- 20.Trinder P. Determination of blood glucose using 4-amino phenazone as oxygen acceptor. J Clin Pathol. 1969;22:246. doi: 10.1136/jcp.22.2.246-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blin N, Stafford DW. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976;3:2303–8. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carey AH, Waterworth D, Patel K, White D, Little J, Novelli P, et al. Polycystic ovaries and premature male pattern baldness are associated with one allele of the steroid metabolism gene CYP17. Hum Mol Genet. 1994;3:1873–6. doi: 10.1093/hmg/3.10.1873. [DOI] [PubMed] [Google Scholar]
- 23.Zhang G, Garmey JC, Veldhuis JD. Interactive stimulation by luteinizing hormone and insulin of the steroidogenic acute regulatory (StAR) protein and 17alpha-hydroxylase/17,20-lyase (CYP17) genes in porcine theca cells. Endocrinology. 2000;141:2735–42. doi: 10.1210/endo.141.8.7595. [DOI] [PubMed] [Google Scholar]
- 24.Lauria PB, Del Puerto HL, Reis AM, Candido AL, Reis FM. Low plasma atrial natriuretic peptide: a new piece in the puzzle of polycystic ovary syndrome. J Clin Endocrinol Metab. 2013;98:4882–9. doi: 10.1210/jc.2013-2141. [DOI] [PubMed] [Google Scholar]
- 25.Pierro E, Andreani CL, Lazzarin N, Cento R, Lanzone A, Caruso A, et al. Further evidence of increased aromatase activity in granulosa luteal cells from polycystic ovary. Hum Reprod. 1997;12:1890–6. doi: 10.1093/humrep/12.9.1890. [DOI] [PubMed] [Google Scholar]
- 26.Nestler JE, Jakubowicz DJ. Decreases in ovarian cytochrome P450c17 alpha activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl J Med. 1996;335:617–23. doi: 10.1056/NEJM199608293350902. [DOI] [PubMed] [Google Scholar]
- 27.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 28.Chu YL, Qiu HY, Sun YY, Li M, Li HF. Detection and significance of phosphatidylinositol 3-kinase in adipose tissue of polycystic ovary syndrome patients with insulin resistance. Zhonghua Fu Chan Ke Za Zhi. 2006;41:455–8. [PubMed] [Google Scholar]
- 29.Ciaraldi TP, Aroda V, Mudaliar S, Chang RJ, Henry RR. Polycystic ovary syndrome is associated with tissue-specific differences in insulin resistance. J Clin Endocrinol Metab. 2009;94:157–63. doi: 10.1210/jc.2008-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wood JR, Dumesic DA, Abbott DH, Strauss JF, 3 rd. Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab. 2007;92:705–13. doi: 10.1210/jc.2006-2123. [DOI] [PubMed] [Google Scholar]
- 31.Perez MS, Cerrone GE, Benencia H, Marquez N, De Piano E, Frechtel GD. Polymorphism in CYP11alpha and CYP17 genes and the etiology of hyperandrogenism in patients with polycystic ovary syndrome. Medicina. 2008;68:129–34. [PubMed] [Google Scholar]
- 32.Kashima K, Yahata T, Fujita K, Tanaka K. Polycystic ovary syndrome: association of a C/T single nucleotide polymorphism at tyrosine kinase domain of insulin receptor gene with pathogenesis among lean Japanese women. Zhonghua Fu Chan Ke Za Zhi. 2013;58:491–6. [PubMed] [Google Scholar]


