Abstract
Background & objectives:
Age related macular degeneration (ARMD) is a leading cause of blindness, particularly in persons above 60 yr of age. Homocysteine is implicated in many ocular diseases including ARMD. This study was undertaken to assess the status and relationship between plasma homocysteine, homocysteine - thiolactone, homocysteinylated protein and copper levels in patients with ARMD.
Methods:
A total of 16 patients with ARMD and 16 age-matched controls were recruited for the study. Plasma glutathione, homocysteine, homocysteine - thiolactone and extent of homocysteine conjugation with proteins, copper and thiobarbituric acid reactive substances were measured.
Results:
Homocysteine levels were elevated with increase in homocysteine-thiolactone, thiobarbituric acid reactive substances and a decrease of glutathione. The levels of homocysteinylated protein were elevated in ARMD. The elevated homocysteine, homocysteine-thiolactone correlated with the decrease in copper level.
Interpretation & conclusions:
Elevated homocysteine and its metabolite homocysteine-thiolactone and decreased levels of copper may play an important role in the pathogenesis of ARMD.
Keywords: Age-related macular degeneration, copper, glutathione, homocysteine, Homocysteine-thiolactone, oxidative stress
Age-related macular degeneration (ARMD) is a leading cause for blindness in the western world in persons older than 60 yr of age1. It is a complex multifactorial disease that affects the central region of the retina. It has two main presentations, atrophic and exudative. Exudative ARMD is characterized by the presence of choroidal neovascularization with disciform scar2. Although the cause of ARMD remains unknown, a number of risk factors associated with ARMD have been identified. These factors include age1,3, heredity4, high blood pressure, high levels of serum cholesterol and obesity4,5. In addition, exposure to cigarette smoke has been shown to be associated with the disease6. Increased oxidative stress and decreased nitric oxide levels have been implicated in choroidal perfusion and drusen formation in ARMD patients7. Increased plasma levels of vascular endothelial growth factors (VEGF), von Willebrand factors, and endothelial cell damage have also been reported in ARMD8. These findings suggest an association of markers of angiogenesis, haemostasis, oxidative stress and endothelial dysfunction with ARMD8.
Homocysteine (Hcy) is a sulphur-containing non-protein amino acid endogenously produced during the metabolic conversion of methionine to cysteine9. Metabolites of homocysteine, namely mixed disulphides and homocysteine-thiolactone (HcyTL) are toxic, since these alter the structure and function of proteins10. Besides, increased level of Hcy results in abnormal chelation of the trace element copper, leading to the possible loss of this element, a leading cause for cardiac diseases11. Elevated Hcy levels have been shown to induce vascular injury, aiding in atherothrombogenesis and this has been considered as an independent risk factor for the development of vascular diseases12.
It has been hypothesized that ARMD may also be associated with elevated plasma levels of Hcy, HcyTL and oxidative stress. To further understand this relationship, we undertook this study to investigate the plasma levels of Hcy, HcyTL, protein homocysteinylation, thiobarbituric acid reactive substances (TBARS), glutathione (GSH) and copper in patients with ARMD.
Material & Methods
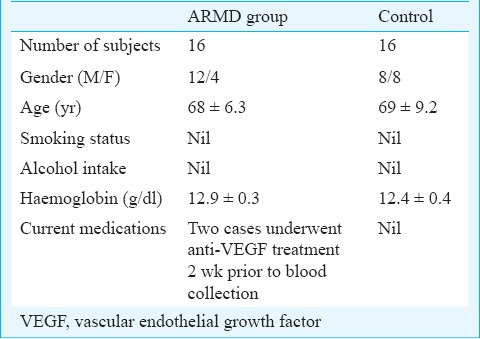
This pilot study was conducted in the department of Biochemistry and Cell Biology, Vision Research Foundation, Chennai, India. The patient recruitment was done at Sankara Nethralaya, Chennai, between July 2009 - August 2011. Consecutive patients diagnosed to have ARMD visiting the vitreoretinal services of Sankara Nethralaya over a period of two years were included in the study. A total of 16 patients with ARMD were included and equal member of controls (volunteers from hospital staff) matched for age were included (Table I). Patients with ARMD were on vegetarian diets at the time when their samples were collected. The grading of exudative ARMD was based on the Age-Related Eye Disease Study group (AREDS) recommendations13. Those (patients and controls) with history of diabetes mellitus, renal disease, hypertension, cardiovascular disease, smoking, alcohol consumption and those on antioxidant supplements were excluded. Any systemic/ophthalmic diseases were ruled out from the control subjects after a detailed examination. Informed written consent was obtained from all the participants in the study. Institutional Research and Ethical Committee approved the study protocol.
Table I.
General characteristic of the patients and controls
Blood samples (10 ml) were drawn from participants after overnight fasting into EDTA containing tubes. Haemoglobin (Hb), TBARS and GSH levels were estimated on the same day of the sample collection; plasma was separated immediately by centrifugation at 3000 g at 25°C for 10 min and stored at -80°C up to two weeks for other investigations. Hcy, HcyTL, protein homocysteinylation and copper level estimations on samples from test and control subjects were performed simultaneously.
Chemicals: All fine chemicals used in the study were purchased from Sigma Chemical Company (St Louis, MO, USA) unless otherwise specified. All other high grade reagents were obtained from E-Merck chemicals (Mumbai, India). Water used in this study was purified through Millipore Water Purification System (Millipore Co., India).
Biochemical analysis: TBARS determination in erythrocytes was performed by the method described by Devasagayam et al14. The amount of TBARS in erythrocytes was expressed in nmoles malondialdehyde (MDA)/g Hb. Plasma GSH level was determined by following the method of Hu15 and the amount of plasma GSH was expressed in μM. Fasting plasma total Hcy analysis was done using a commercial ELISA kit (Bio-Rad, CA, USA). The plasma level of Hcy was expressed in μM, and a level greater than 15 μM was considered to be hyperhomocysteinemia.
Measurement of protein homocysteinylation was performed by the method as described by Bharathselvi et al16. The plasma levels of protein-Cysteine bound Hcy and protein-Lysine bound Hcy were expressed in μM of Hcy. Before analysis, the system was calibrated with authentic DL-Hcy standards in the range of 25 to 100 ng. The Hcy was eluted at a 12.3 min retention time. Plasma HcyTL was determined by HPLC using a cation-exchange Poly SULFOETHYL aspartamide column17,18. The detection limit was 10 pmol. The levels of plasma HcyTL were expressed in nM. Sample preparation for the determination of copper in plasma was done as described previously19 and the amount of copper was estimated by atomic absorption method20. The level of plasma copper was expressed in μg/dl.
Plasma methionine was estimated as described by Huesgen21, with slight modification. Plasma samples were deproteinized by adding equal volume of 10 per cent TCA (trichloro acetic acid) and centrifuged at 700 g for 10 min and the supernatants were used for amino acids analysis. Free amino acids were derivitized using ortho-phthaldehyde (OPA) and resolved in C18 column, (150 × 4.6 mm internal diameter, 5 μm pore size) (ODS; phenomenex, Torrance, CA). The elution procedure was performed with a linear gradient using sodium acetate buffer (pH 7.2) and 40 per cent acetonitrile in methanol. Methionine detection was done using UV monitor at 338 nm. The level of methionine was expressed in μM.
Serum vitamin B12 levels were estimated using an automated chemiluminescence analyser with a kit from Roche diagnostics, GmbH, Mannheim, USA. The serum level of vitamin B12 was expressed in pg/ml.
Statistical analysis: Patients and control groups were compared using student t test. Pearson's correlation test was employed to assess the relationship between the Hcy, HcyTL, TBARS, protein-Cys bound Hcy, protein-Lys bound Hcy with GSH using SPSS software (version 16.0) (SPSS, Inc., Chicago, USA). Multiple testing corrections (Bonferroni corrections) were done for HcyTL, protein-Cys bound Hcy and protein-Lys bound Hcy to eliminate the false positive results.
Results & Discussion
The plasma levels of Hcy, HcyTL, protein-Cys bound Hcy, protein-Lys bound Hcy, GSH, copper and TBARS in erythrocytes in patients and control subjects are given in Table II.
Table II.
Plasma levels of homocysteine, homocysteinethiolactone, protein-Cys bound Hcy, protein-Lys bound Hcy, glutathione, copper, methionine and TBARS levels in erythrocytes in controls and patients with age-related macular degeneration
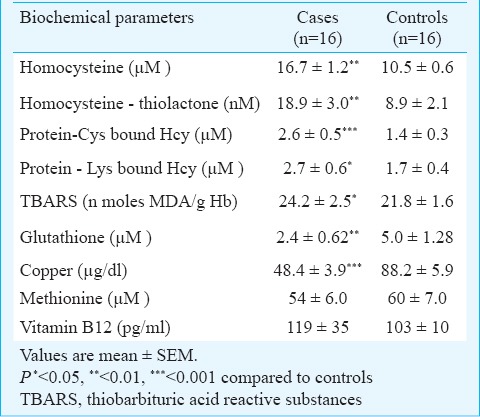
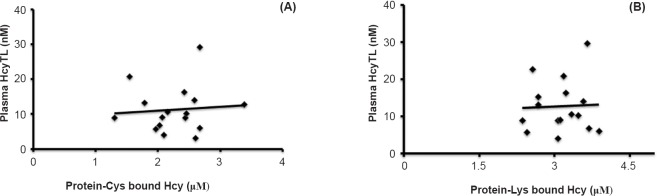
There was a significant increase in the level of plasma Hcy and its metabolite HcyTL, protein homocysteinylation via lysine and cysteine residues and TBARS observed in patients with ARMD when compared with controls. There was a significant positive correlation between plasma HcyTL and protein-Cys bound Hcy (r=0.1711, P<0.001) and protein-Lys bound Hcy (r=0.0674, P<0.01) in patients with ARMD (Fig. 1).
Fig. 1.
Correlation between plasma homocysteine-thiolactone (HcyTL), protein-Cys bound Hcy, protein-Lys bound Hcy in patients with age-related macular degeneration (ARMD). (A) Positive correlation between plasma HcyTL and protein-Cys bound Hcy in patients with ARMD (r=0.1711, P<0.001). (B) Positive correlation between plasma HcyTL and protein-Lys bound Hcy in patients with ARMD (r=0.0674, P<0.01).
Hcy has been shown to undergo autoxidation to generate reactive oxygen intermediates (ROI), such as the superoxide anion (O2–) and hydroxyl radical (OH-) thus initiating the oxidation of LDL, and this has been shown to induce vascular dysfunction10. HcyTL is a product of an error-editing reaction in protein biosynthesis which forms when Hcy is mistakenly selected by methionyl-tRNA synthetase10. The thioester chemistry of HcyTL underlines its ability to form isopeptide bonds with protein lysine and cysteine residues, which impair or alter the protein's function10. The present study shows elevated protein-Cys bound Hcy, protein-Lys bound Hcy, HcyTL and diminished GSH in patients with ARMD, implying altered protein function playing an important role in the disease. Increased plasma Hcy levels in both the exudative and dry subtypes of ARMD were observed by Kamburoglu et al22. Another study also revealed this relationship to be significantly associated with exudative ARMD than in dry form23. In the present study, majority of patients had wet ARMD, while only three among 16 had features of dry form. Surprisingly, these three cases individually had Hcy level (10.0 ± 0.03 μM) comparable to controls (10.5 ± 0.6 μM). To confirm this finding we need to analyse Hcy in a large number of dry ARMD patients.
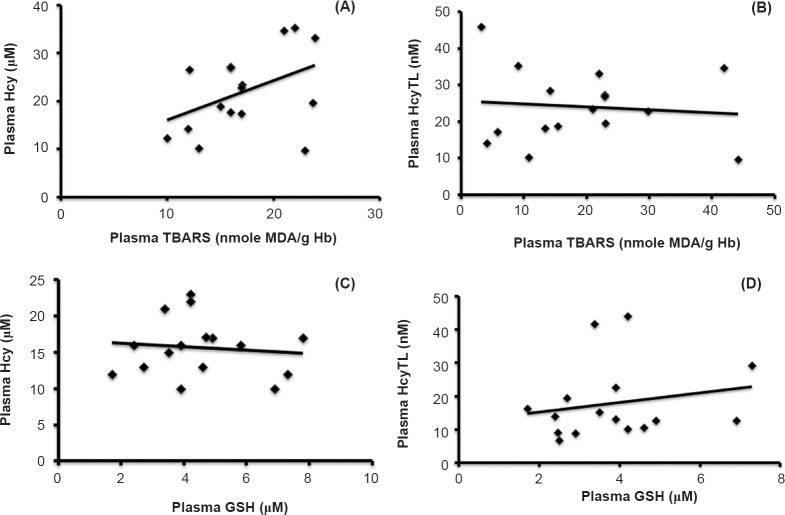
There was a significant increase in the observed levels of erythrocytes TBARS in the ARMD group as compared with the control group (Table II). Increased levels of oxidative stress led to increased plasma Hcy and its metabolite HcyTL and a decrease in the levels of antioxidants GSH in ARMD group as compared with controls. There was a significant positive correlation between plasma Hcy, plasma HcyTL and TBARS in ARMD and a corresponding significant negative correlation between plasma Hcy, plasma HcyTL and plasma GSH in ARMD groups (Fig. 2).
Fig. 2.
Correlations between plasma homocysteine (Hcy), plasma homocysteine-thiolactone (HcyTL), thiobarbituric acid reactive substances (TBARS) and plasma glutathione (GSH). (A) Positive correlation between plasma Hcy and TBARS in patients with ARMD (r=0.4450, P<0.001). (B) Positive correlation between plasma HcyTL and TBARS in patients with ARMD (r=0.4764, P<0.01). (C) Significant negative correlation between plasma Hcy and plasma GSH in patients with ARMD (r=- 0.1007, P<0.001). (D) Significant negative correlation between plasma HcyTL and plasma GSH in patients with ARMD (r=- 0.2240, P<0.001).
Apart from the damage through Hcy another probable candidate to join hands in this is HcyTL, since it is known to be more cytotoxic and pro-inflammatory than Hcy itself24. It is hypothesized that HcyTL also inactivates enzymes such as decreased lysyl oxidase, a copper dependent enzyme activity25. Protein homocysteinylation also affects the normal function of low density lipoprotein (LDL). It is interesting to note that multiple retinal degeneration is caused by injecting HcyTL in an animal eye26.
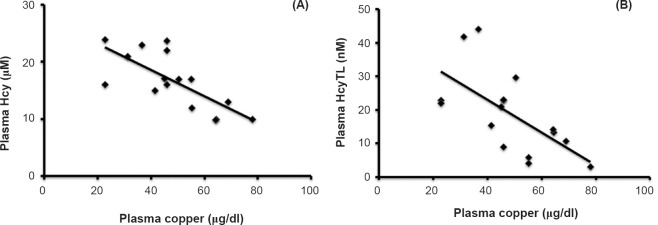
Increase of Hcy and HcyTL negatively correlated with plasma copper in ARMD patients (Fig. 3). To verify that increased Hcy and HcyTL was due to the deficiency of vitamin B12 and methionine, the study was extended to look for altered levels of methionine and vitamin B12 in patients suffering from ARMD. No significant changes in the levels of methionine and vitamin B12 in ARMD group were noticed when compared with control subjects. The methionine level was high in both ARMD and control groups (54 ± 6 and 60 ± 7 μM) compared to normal adult methionine reference interval 6 - 40 μM27. However, vitamin B12 levels were low in both ARMD and control groups at 119 ± 35 and 103 ±10 pg/ml with a reference interval of 200 - 900 pg/ml, respectively. These results indicate that both high levels of methionine and low levels of vitamin B12 may be a common factor for this age group in India.
Fig. 3.
Correlation between plasma levels of copper, homocysteine (Hcy) and homocysteine-thiolactone (HcyTL) in patients with ARMD. (A) Negative correlation between plasma copper and plasma Hcy in patients with ARMD (r=-0.671, P<0.001). (B) Negative correlation between plasma copper and plasma HcyTL in patients with ARMD (r=-0.405, P<0.001).
GSH is the most important endogenous antioxidant in humans. Maintaining the intracellular thiols, such as GSH, in their reduced form, may allow for the maintenance of Hcy and other intracellular thiols in redox states28. It is often accompanied by other endogenous thiols, such as cysteine, cysteinylglycine and even Hcy. These thiols scavenge reactive oxygen species (ROS) and are involved in preserving the pro-oxidant-antioxidant balance in human tissues9. It has been reported that GSH and thiol content may decrease in certain pathological states and on account of the ageing process28. This study confirms the findings of our earlier study with respect to the diminished plasma levels of GSH in ARMD patients29.
Three types of superoxide dismutase (SOD) isoenzymes that catalyze superoxide radical dismutation are present in retina30. Cu, Zn-SOD exists in the cytosol, Mn-SOD in the mitochondrial matrix, and extracellular SOD in the tissue serves as the secretory form31. Because the amount of Cu-Zn SOD is the highest among the three isoenzymes in the human retina, it seemed reasonable to hypothesize that the lack of Cu containing SOD would accelerate age-related pathological changes in the human retina31. Decrease of copper would also weaken the antioxidant potential, through a decrease of ceruloplasmin32, a ferrooxidase which scavenges ferrous ion and mitigates the formation of free hydroxyl radical (OH-).
To conclude, our findings show that increased oxidative stress and Hcy, HcyTL and protein homocysteinylation may play an important role in the pathogenesis of ARMD. Protein damage due to thiolactone and lowered copper levels in this disease are novel findings. The real significance of these findings needs to be understood in a larger patient population based study.
Acknowledgment
Authors acknowledge the Indian Council of Medical Research (ICMR) for financial support. The authors thank Dr S.R. Barathidevi, Shrimati R. Punitham for help, Shri Viswanathan (Biostatistician) for multiple testing corrections and Shriyut M. Arasu Kumar and T. Arokiaswamy (social workers) for recruiting patients and controls.
Footnotes
Conflicts of Interest: None.
References
- 1.Evans JR. Risk factors for age-related macular degeneration. Prog Retin Eye Res. 2001;20:227–53. doi: 10.1016/s1350-9462(00)00023-9. [DOI] [PubMed] [Google Scholar]
- 2.Fine SL, Berger JW, Maguire MG, Ho AC. Age-related macular degeneration. N Engl J Med. 2000;342:483–92. doi: 10.1056/NEJM200002173420707. [DOI] [PubMed] [Google Scholar]
- 3.Smith W, Assink J, Klein R, Mitchell P, Klaver CC, Klein BE, et al. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001;108:697–704. doi: 10.1016/s0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 4.Hammond CJ, Webster AR, Snieder H, Bird AC, Gilbert CE, Spector TD. Genetic influence on early age-related maculopathy: a twin study. Ophthalmology. 2002;109:730–6. doi: 10.1016/s0161-6420(01)01049-1. [DOI] [PubMed] [Google Scholar]
- 5.[No authors listed]. Risk factors for neovascular age-related macular degeneration. The Eye Disease Case-Control Study Group. Arch Ophthalmol. 1992;110:1701–8. doi: 10.1001/archopht.1992.01080240041025. [DOI] [PubMed] [Google Scholar]
- 6.Vingerling JR, Klaver CC, Hofman A, De Jong PT. Epidemiology of age-related maculopathy. Epidemiol Rev. 1995;17:347–60. doi: 10.1093/oxfordjournals.epirev.a036198. [DOI] [PubMed] [Google Scholar]
- 7.Totan Y, Cekic O, Borazan M, Uz E, Sogut S, Akyol O. Plasma malondialdehyde and nitric oxide levels in age related macular degeneration. Br J Ophthalmol. 2001;85:1426–8. doi: 10.1136/bjo.85.12.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lip PL, Blann AD, Hope-Ross M, Gibson JM, Lip GY. Age-related macular degeneration is associated with increased vascular endothelial growth factor, hemorheology and endothelial dysfunction. Ophthalmology. 2001;108:705–10. doi: 10.1016/s0161-6420(00)00663-1. [DOI] [PubMed] [Google Scholar]
- 9.Castro R, Rivera I, Blom HJ, Jakobs C, Tavares I, de Almeida I. Homocysteine metabolism, hyperhomocysteinaemia and vascular disease: an overview. J Inherit Metab Dis. 2006;29:3–20. doi: 10.1007/s10545-006-0106-5. [DOI] [PubMed] [Google Scholar]
- 10.Jakubowski H. Pathophysiological consequences of homocysteine excess. J Nutr. 2006;136:1741S–9S. doi: 10.1093/jn/136.6.1741S. [DOI] [PubMed] [Google Scholar]
- 11.Hughes WM, Rodriguez WE, Rosenberger D, Chen J, Sen U, Tyagi N, et al. Role of copper and homocysteine in pressure overload heart failure. Cardiovasc Toxicol. 2008;8:137–44. doi: 10.1007/s12012-008-9021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dionisio N, Jardin I, Salido GM, Rosado JA. Homocysteine, intracellular signaling and thrombotic disorders. Curr Med Chem. 2010;17:3109–19. doi: 10.2174/092986710791959783. [DOI] [PubMed] [Google Scholar]
- 13.The Age-Related Eye Disease Study (AREDS): design implications. AREDS report no. The Age-Related Eye Disease Study Research Group. Control Clin Trials. 1999;20:573–600. doi: 10.1016/s0197-2456(99)00031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devasagayam TP, Tarachand U. Decreased lipid peroxidation in the rat kidney during gestation. Biochem Biophys Res Commun. 1987;145:134–8. doi: 10.1016/0006-291x(87)91297-6. [DOI] [PubMed] [Google Scholar]
- 15.Hu ML. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994;233:380–5. doi: 10.1016/s0076-6879(94)33044-1. [DOI] [PubMed] [Google Scholar]
- 16.Bharathselvi M, Biswas J, Selvi R, Coral K, Narayanasamy A, Ramakrishnan S, et al. Increased homocysteine, homocysteine-thiolactone, protein homocysteinylation and oxidative stress in the circulation of patients with Eales’ disease. Ann Clin Biochem. 2013;50:330–8. doi: 10.1177/0004563213492146. [DOI] [PubMed] [Google Scholar]
- 17.Jakubowski H, Zhang L, Bardeguez A, Aviv A. Homocysteine thiolactone and protein homocysteinylation in human endothelial cells: implications for atherosclerosis. Circ Res. 2000;87:45–51. doi: 10.1161/01.res.87.1.45. [DOI] [PubMed] [Google Scholar]
- 18.Chwatko G, Jakubowski H. The determination of homocysteine-thiolactone in human plasma. Anal Biochem. 2005;337:271–7. doi: 10.1016/j.ab.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 19.Ramakrishnan S, Sulochana KN, Selvaraj T, Abdul Rahim A, Lakshmi M, Arunagiri K. Smoking of beedies and cataract: cadmium and vitamin C in the lens and blood. Br J Ophthalmol. 1995;79:202–6. doi: 10.1136/bjo.79.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorota S, Artur M, Krystyna R. Copper levels in patients with rheumatoid Arthritis. Ann Agric Environ Med. 2013;20:312–6. [PubMed] [Google Scholar]
- 21.Huesgen AG. Sensitive and reliable amino acid analysis in protein hydrolysates using Hp 1100 series HPLC. Palo Alto, CA: Hewlett Packard; 1999. pp. 1–12. [Google Scholar]
- 22.Kamburoglu G, Gumus K, Kadayifcilar S, Eldem B. Plasma homocysteine, vitamin B12 and folate levels in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2006;244:565–9. doi: 10.1007/s00417-005-0108-2. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh S, Saha M, Das D. A study on plasma homocysteine level in age-related macular degeneration. Nepal J Ophthalmol. 2013;5:195–200. doi: 10.3126/nepjoph.v5i2.8728. [DOI] [PubMed] [Google Scholar]
- 24.Jakubowski H. Homocysteine is a protein amino acid in humans. Implications for homocysteine-linked disease. J Biol Chem. 2002;277:30425–8. doi: 10.1074/jbc.C200267200. [DOI] [PubMed] [Google Scholar]
- 25.Liu G, Nellaiappan K, Kagan HM. Irreversible inhibition of lysyl oxidase by homocysteine thiolactone and its selenium and oxygen analogues. Implications for homocystinuria. J Biol Chem. 1997;272:32370–7. doi: 10.1074/jbc.272.51.32370. [DOI] [PubMed] [Google Scholar]
- 26.Chang HH, Lin DP, Chen YS, Liu HJ, Lin W, Tsao ZJ, et al. Intravitreal homocysteine-thiolactone injection leads to the degeneration of multiple retinal cells, including photoreceptors. Mol Vis. 2011;17:1946–56. [PMC free article] [PubMed] [Google Scholar]
- 27.Burtis CA, Ashwood ER. Tietz textbook of clinical chemistry. 2nd ed. Philadelphia: WB. Saunders; 1986. [Google Scholar]
- 28.Moriarty SE, Shah JH, Lynn M, Jiang S, Openo K, Jones DP, et al. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic Biol Med. 2003;35:1582–8. doi: 10.1016/j.freeradbiomed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Coral K, Raman R, Rathi S, Rajesh M, Sulochana KN, Angayarkanni N, et al. Plasma homocysteine and total thiol content in patients with exudative age-related macular degeneration. Eye. 2006;20:203–7. doi: 10.1038/sj.eye.6701853. [DOI] [PubMed] [Google Scholar]
- 30.Valentine JS, Doucette PA, Zittin Potter S. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu Rev Biochem. 2005;74:563–93. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- 31.Behndig A, Svensson B, Marklund SL, Karlsson K. Superoxide dismutase isoenzymes in the human eye. Invest Ophthalmol Vis Sc. 1998;39:471–5. [PubMed] [Google Scholar]
- 32.Brown JC, Strain JJ. Effect of dietary homocysteine on copper status in rats. J Nutr. 1990;120:1068–74. doi: 10.1093/jn/120.9.1068. [DOI] [PubMed] [Google Scholar]