Abstract
IMPORTANCE
Although blood donation is allowed every 8 weeks in the United States, recovery of hemoglobin to the currently accepted standard (12.5 g/dL) is frequently delayed, and some donors become anemic.
OBJECTIVE
To determine the effect of oral iron supplementation on hemoglobin recovery time (days to recovery of 80% of hemoglobin removed) and recovery of iron stores in iron-depleted (“low ferritin,” ≤26 ng/mL) and iron-replete (“higher ferritin,” >26 ng/mL) blood donors.
DESIGN, SETTING, AND PARTICIPANTS
Randomized, nonblinded clinical trial of blood donors stratified by ferritin level, sex, and age conducted in 4 regional blood centers in the United States in 2012. Included were 215 eligible participants aged 18 to 79 years who had not donated whole blood or red blood cells within 4 months.
INTERVENTIONS
One tablet of ferrous gluconate (37.5 mg of elemental iron) daily or no iron for 24 weeks (168 days) after donating a unit of whole blood (500 mL).
MAIN OUTCOMES AND MEASURES
Time to recovery of 80% of the postdonation decrease in hemoglobin and recovery of ferritin level to baseline as a measure of iron stores.
RESULTS
The mean baseline hemoglobin levels were comparable in the iron and no-iron groups and declined from a mean (SD) of 13.4 (1.1) g/dL to 12.0 (1.2) g/dL after donation in the low-ferritin group and from 14.2 (1.1) g/dL to 12.9 (1.2) g/dL in the higher-ferritin group. Compared with participants who did not receive iron supplementation, those who received iron supplementation had shortened time to 80% hemoglobin recovery in both the low-ferritin and higher-ferritin groups. Recovery of iron stores in all participants who received supplements took a median of 76 days (IQR, 20–126); for participants not taking iron, median recovery time was longer than 168 days (IQR, 147->168 days; P < .001). Without iron supplements, 67% of participants did not recover iron stores by 168 days.
|
Low-Ferritin Group (≤26 ng/mL)
|
Higher-Ferritin Group (>26 ng/mL)
|
|||
| Iron | No Iron | Iron | No Iron | |
|
| ||||
| Time to 80% hemoglobin recovery, mean (IQR), d | 32 (30–34) | 158 (126–>168) | 31 (29–33) | 78 (66–95) |
|
| ||||
| Time to recovery of baseline ferritin levels, median (IQR), d | 21 (12–84) | >168 (128–>168) | 107 (75–141) | >168 (>168–>168) |
|
| ||||
CONCLUSIONS AND RELEVANCE
Among blood donors with normal hemoglobin levels, low-dose iron supplementation, compared with no supplementation, reduced time to 80% recovery of the postdonation decrease in hemoglobin concentration in donors with low ferritin (≤26 ng/mL) or higher ferritin (>26 ng/mL).
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT01555060
It is estimated that 25% to 35% of blood donors become iron depleted from regular blood donation.1,2 In 2011, 9.2 million individuals in the United States donated 15.7 million units of red blood cells.3 Overall, 6.7% of these donors were deferred for not meeting the minimum capillary hemoglobin standard of 12.5 g/dL. Low hemoglobin, a late consequence of iron depletion, represents the single largest category of blood donor deferral and is more prevalent in women. Hemoglobin level and iron status are getting renewed attention as a donor safety issue based on increasing evidence that iron depletion is associated with fatigue,4 decreased exercise capacity,5 neurocognitive changes,6,7 pica, and restless legs syndrome.8,9
Blood donors in the United States are allowed to donate 500 mL of whole blood every 8 weeks (56 days). A study of “frequent” blood donors (women who donated ≥2 times/year and men ≥3 times/year) demonstrated that their risk for iron deficiency and hemoglobin deferral appears to be at least twice that of donors who donate 2 times per year or less frequently.2 Based on such findings, the US Food and Drug Administration has considered lengthening the minimum interdonation interval for all donors.10 Although available evidence indicates that iron supplementation improves iron status and reduces hemoglobin deferrals,11 the focus of many supplementation studies has been on a specific operational outcome (deferral) rather than physiologic end points, thus limiting their utility in evaluating variable donation intervals that are compatible with maintaining hemoglobin and iron equilibrium on a sustainable basis. To clarify the kinetics of hemoglobin recovery and associated replenishment of iron stores after blood donation, we assessed the effect of oral iron supplementation given for 24 weeks in a randomized nonblinded clinical trial.
Methods
Enrollment in the Hemoglobin and Iron Recovery Study (HEIRS) took place from April to December 2012 at 4 US blood centers participating in the National Heart, Lung, and Blood Institute (NHLBI) Recipient Epidemiology and Donor Evaluation Study–III (REDS-III) program: American Red Cross Blood Services, Farmington, Connecticut; BloodCenter of Wisconsin, Milwaukee; Blood Centers of the Pacific, San Francisco, California; and Institute for Transfusion Medicine, Pittsburgh, Pennsylvania (see the trial protocol in Supplement 1). Institutional review boards at each blood center, at the REDS-III Central Laboratory (Blood Systems Research Institute), and at the data coordinating center (RTI International, Research Triangle Park, North Carolina) approved the study. Volunteer whole blood donors 18 years and older were enrolled and assessed by ferritin testing for randomization and further participation in the trial. Each enrolled donor gave written informed consent to their participation.
An eligible participant was an individual who successfully donated a full (500-mL) whole blood unit on the day of enrollment and who had a history of 1 or more previous whole blood donations but no donations in the previous 4 months. Participants agreed not to use iron supplements other than those provided by study coordinators. Individuals with a baseline ferritin level exceeding 300 ng/mL were excluded so that individuals with iron loading conditions were not enrolled. Participants returned 3 to 8 days after blood donation and enrollment and, if meeting the stratified randomization requirements, were randomized to receive oral iron supplements or no treatment for 24 weeks of follow-up. During this time, they did not donate blood or take iron other than as assigned, and they provided blood samples. Pill adherence was assessed by asking questions of participants at each visit and examining blister packs returned at some visits. Participants were reimbursed $25 per visit.
Participants randomized to receive iron pills were provided information on common adverse events (AEs) from iron and asked to report them by telephone to each center’s study coordinator. They were asked about AEs at each visit, including black stool, constipation, abdominal cramping, diarrhea, nausea, vomiting, bloating or indigestion, headache, metallic taste or sore mouth, hyperactive bowel sounds (loud grumbling sounds), itching or rash or hives, and leg cramps. Adverse events were managed according to a standard algorithm, typically by instructing affected participants to take pills every other day, except in the case of significant constipation, abdominal cramping, diarrhea, or vomiting. These AEs and others not listed were reported on a standard form to the principal investigator and evaluated by a physician expert in iron studies (safety medical monitor) who was not directly involved in the study. Participants in the no-iron groups were also asked to report AEs, which were evaluated in a similar manner. Serious AEs possibly related to iron or other aspects of the study resulted in withdrawal of the participant from the study. Serious AEs and reports of all AEs were reviewed by the safety medical monitor and reported to NHLBI staff and the chairman of their observational safety monitoring board, as well as to the appropriate institutional review boards.
Laboratory Testing
Ferritin, soluble transferrin receptor (sTfR), and complete blood cell count (CBC) were measured in a pre-donation sample. Laboratory testing was performed on pre-donation EDTA blood samples collected from a sampling pouch in line with tubing used to collect the whole blood unit and then on EDTA blood samples provided on 7 occasions: days 3 through 8 and at 2, 4, 8, 12, 16, and 24 weeks after donation. Samples were considered valid if collected within 5 days of the scheduled time. Samples were tested for CBC (all visits), plasma ferritin (all visits), reticulocytes (all visits at 2 of 4 centers), and sTfR at the pre-donation and final visits only. Plasma ferritin and sTfR were tested as previously reported, using a central laboratory.12 Complete blood cells counts (at each blood center) and reticulocyte counts (at 2 centers) were performed using similar automated hematology analyzers, which were standardized in accord with Clinical Laboratory Improvement Amendments requirements and validated by external proficiency testing.
Study Intervention
Participants were randomized to receive either oral ferrous gluconate, 325 mg, containing 37.5 mg of elemental iron (Major Pharmaceuticals), once a day for 24 weeks or no treatment. A placebo pill was not used, and the study intervention was not blinded. Pill counts and adherence assessment were performed at each visit.
Randomization
Enrolled participants were evaluated for randomization at a return visit between days 3 and 8 after blood donation and enrollment, based on their pre-donation ferritin values, sex, and age. A stratified enrollment and randomization scheme was followed to ensure inclusion of participants with varying iron stores and a slight overrepresentation of women (60% female/40% male) and individuals 60 years and older (20%). Participants were assessed for iron status per protocol as iron-depleted (“low ferritin,” ≤26 ng/mL) and iron-replete (“higher ferritin,” >26 ng/mL) based on the ferritin measurement at enrollment. The 2 ferritin strata were selected based on the REDS-II Iron Status Evaluation study, which suggested that a ferritin level of 26 ng/mL or less closely correlated with iron-deficient erythropoiesis.13 Participants with low- and higher-ferritin levels were randomized to receive supplements or no iron, comprising 4 nearly equal-sized groups, each with 4 age-sex subgroups, which were filled by a “first-entered-first-randomized” scheme. If the appropriate subgroup remained open, a centralized computer program randomized the participant to receive iron supplements or no iron. The delay in receiving ferritin results required initial overenrollment, resulting in 108 blood donors who were not randomized (because the appropriate subgroups were filled) and who were excused from further participation (Figure 1).
Figure 1. Hemoglobin and Iron Recovery Study (HEIRS) Flow Diagram.
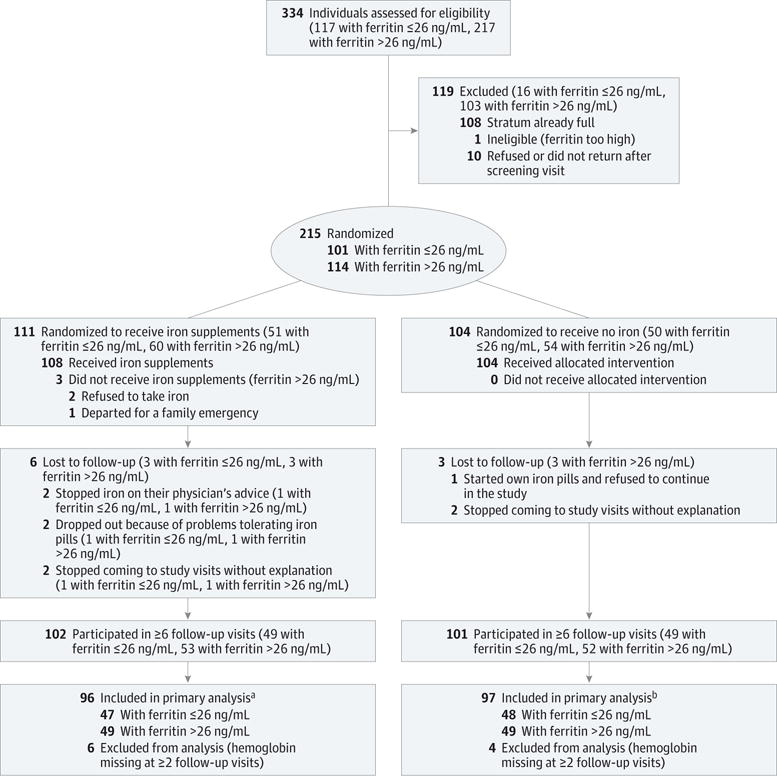
aAll participants had data from ≥6 follow-up visits; 3 individuals had 6 visits, and 93 had 7 visits.
bAll participants had data from ≥6 follow-up visits; 5 individuals had 6 visits, and 92 had 7 visits.
Study Outcomes and Analytical Approach
The primary objective was to determine the effect of iron supplementation on hemoglobin recovery time among iron-depleted participants. The sampling and testing scheme allowed detection of the nadir in venous hemoglobin 3 to 8 days after whole blood donation, ie, sufficient time to account for postdonation plasma volume equilibration. Hemoglobin recovery was tracked at scheduled visits through 24 weeks (168 days). The primary outcome was hemoglobin recovery, defined as the time for restoration of 80% of the hemoglobin lost at donation, rather than 100% hemoglobin, because hemoglobin recovery occurs at a rate that decelerates as equilibrium is approached, resulting in a plot of hemoglobin vs time that rises and then flattens (eFigure 1 in Supplement 2). Estimates of time to a point of the flattened part of the curve are less reliable than estimates of time to a point at which the curve is rising more steeply. Additional rationale for selection of 80% recovery as an end point can be found in the eMethods in Supplement 2.
Mean time to 80% recovery was estimated by fitting a nonlinear regression of hemoglobin level vs time since donation to the visit-specific means in each group as described in “Statistical Methods.” Bootstrap confidence limits were calculated for each estimate of mean recovery time. The bootstrap method used an automated repeated sampling of the underlying population with replacement to generate multiple mean hemoglobin recovery curves and associated recovery times for each analysis group (by baseline ferritin and iron supplementation) or analysis subgroup (sex, ferritin quartiles). Both 95% confidence limits and interquartile ranges (IQRs) were derived from the distribution of 1000 replicates.
Secondary outcomes reflected 3 aspects of hemoglobin recovery time. First, how hemoglobin recovery time varied with pre-donation iron status was determined among donors who did not receive supplements. Hemoglobin recovery times and confidence intervals for iron-depleted participants not taking iron were developed as for the primary outcome and compared with hemoglobin recovery times for iron-replete participants not taking iron. Comparison of the confidence intervals allowed determination of the effect of pre-donation iron status on hemoglobin recovery times.
Second, the effect of iron supplementation on hemoglobin recovery time was determined among donors with higher ferritin levels. Hemoglobin recovery times and confidence limits were developed for the iron-replete participants who took iron and compared with the recovery times and confidence limits among iron-replete participants who did not take iron.
Third, how hemoglobin recovery time and the effects of iron supplementation varied with sex and age were determined, after correcting for iron status. In a similar manner, hemoglobin recovery times were derived for male and female donors and separately for older and younger participants, each subgroup being analyzed for hemoglobin recovery times. (Data on associations with age are not presented in this article.)
Simultaneous with hemoglobin decline and recovery, ferritin changes were tracked as a measure of recovery of iron stores. Ferritin recovery was determined in 2 ways: by determining the proportion of donors whose 168-day ferritin level was greater than or equal to baseline and by assessing the median time for a donor’s ferritin level to equal or exceed their baseline ferritin value. We also determined the time for ferritin to recover to 26 ng/mL or greater (ie, reach iron-replete status) in the iron-depleted donors who received iron.
The hemoglobin recovery time was also analyzed in the iron and no-iron groups by dividing the participants into subgroups by quartiles of enrollment ferritin. In addition, the time to recover a venous hemoglobin of 12.5 g/dL was determined for men and women separately, and the effect of iron supplementation was determined by comparing the proportion of male and female participants who achieved a venous hemoglobin of 12.5 g/dL at 56 days and whether they took iron supplements.
Sample Size
Sample size calculations were based on the comparison of hemoglobin recovery times among iron-depleted participants randomized to receive iron supplementation or no supplementation. Hemoglobin recovery time among iron-depleted participants taking iron supplementation was assumed to be similar to the 5-week recovery time previously shown in iron-replete donors.14 However, this time was extended to 7 weeks to allow for some lack of adherence with treatment. We considered iron supplementation in iron-depleted donors to be of clinical interest if the lack of iron supplementation doubled the mean hemoglobin recovery time, ie, from 7 to 14 weeks. Sample size calculations then indicated that a study with 100 participants, with 50 per treatment group, and a type I error rate of .05 would provide greater than 0.90 power to detect the difference between mean recovery times of 7 and 14 weeks. The prescribed sample size was then increased to 200 participants to provide for an equal number of iron-replete participants.
Statistical Methods
Baseline characteristics of participants receiving iron or not were compared using t tests for continuous variables, such as hemoglobin and log(ferritin), and Fisher exact tests for binary variables, such as sex and age subgroup. Separate comparisons were performed for low-ferritin and higher-ferritin participants. Changes in mean hemoglobin and mean ferritin over 168 days were compared among groups using repeated measures models. Time for restoration of 80% of the hemoglobin lost at donation was estimated by fitting a model of mean hemoglobin, ht, vs time since donation, t, to the 7 visit-specific means: ht = θ1 − θ2e−θ3t, where e is the base of natural logarithms and θ1, θ2, and θ3 are model parameters. Hemoglobin at recovery was hrec = h3 + 0.8(h0 − h3) where h0 is the average pre-donation hemoglobin and h3 is hemoglobin 3 days after donation calculated from the regression model. We used hemoglobin on day 3 to allow for postdonation volume equilibration. The estimates of mean recovery time presented here are the times at which the recovery curve reached hrec in various groups or subgroups of participants. Recovery times that exceeded maximum follow-up were censored at 168 days (24 weeks). Recovery time was also censored at 168 days if the curve of hemoglobin vs time since donation reached equilibrium at a level below 80% hemoglobin recovery; ie, if hrec > θ1. Approximate 95% confidence limits were estimated by bootstrapping with censoring at 168 days. Further methodological details are provided in Supplement 2.
Time to recovery of ferritin to baseline values among iron-replete and iron-depleted participants, or to 26 ng/mL or greater among iron-depleted participants, was estimated by linear interpolation between the last visit with ferritin below that level and the first visit with ferritin above that level. Medians and IQRs were calculated from the recovery times for individual participants. Nonparametric tests were used to compare recovery times among groups or subgroups. The analysis presented here was limited to participants with hemoglobin or ferritin data at 6 or more follow-up visits. An analysis of all participants using multiple imputation for missing values produced nearly identical results. Logistic regression was used to model the probability of recovery to venous hemoglobin of 12.5 g/dL or greater by 56 days as a function of treatment, adjusting for sex and baseline ferritin level. Ferritin was transformed to base 10 logarithms to eliminate a positive correlation between the variability of ferritin and mean ferritin. Two-sided statistical tests with a critical P value of .05 were used throughout the analysis. Data analysis was performed with SAS/STAT software (version 9.3 of the SAS System for Windows).
Results
There were 334 participants enrolled and screened for ferritin. Based on the stratified randomization scheme, 215 were randomized and followed up; 111 were randomized to receive ferrous gluconate (51 in the low-ferritin group and 60 in the higher-ferritin group), and 104 randomized to receive no supplemental iron (50 in the low-ferritin group, 54 in the higher-ferritin group) (Figure 1). The remaining 119 enrolled participants who were not randomized were not followed up. Among the 215 randomized participants, 136 (63%) were female and 52 (24%) were 60 years or older. Baseline characteristics according to iron assignment were comparable in both the low- and higher-ferritin groups, except for a slightly lower hemoglobin level in low-ferritin participants randomized to receive iron (Table 1). The hemoglobin levels of the low-ferritin groups were significantly lower, 0.6 to 0.9 g/dL, than the higher-ferritin groups. The sTfR, known as a measure of iron-deficient erythropoiesis,15 was higher in the low-ferritin groups than the higher-ferritin groups. Mean ferritin at entry for women (15.3 ng/mL) and men (14.7 ng/mL) in the low-ferritin groups was similar. In the higher-iron groups, mean ferritin was 52.2 ng/mL for women and 63.5 ng/mL for men.
Table 1.
Baseline Characteristics of Study Participants by Ferritin Level
| Low Ferritin (≤26 ng/mL) | Higher Ferritin (>26 ng/mL) | |||
|---|---|---|---|---|
| Iron (n = 51) | No Iron (n = 50) | Iron (n = 60) | No Iron (n = 54) | |
| Women, No. (%) | 33 (64.7) | 31 (62.0) | 38 (63.3) | 34 (63.0) |
| Age ≥60 y, No. (%) | 12 (23.5) | 11 (22.0) | 17 (28.3) | 12 (22.2) |
| Age, mean (SD), y | 47.5 (15.5) | 45.9 (15.7) | 49.3 (14.6) | 48.1 (14.6) |
| Weight, mean (SD), kg | 75.9 (16.4) | 76.8 (15.8) | 81.5 (16.4) | 77.9 (16.2) |
| Hemoglobin, mean (SD), g/dL | 13.2 (1.0) | 13.7 (1.3) | 14.1 (1.0) | 14.3 (1.2) |
| Ferritin, mean (SD), ng/mL | 14.9 (5.8) | 15.2 (6.0) | 54.0 (24.3) | 58.9 (32.9) |
| sTfR, mean (SD), mg/L | 4.0 (1.33) | 3.9 (1.19) | 3.1 (0.65) | 3.1 (0.62) |
| Estimated blood volume, mean (SD), L | 4.59 (0.8) | 4.66 (0.91) | 4.79 (0.84) | 4.64 (0.84) |
Abbreviation: sTfR, soluble transferrin receptor.
Adherence with oral iron supplements was 92.5%, and all retained participants verified they had no additional supplemental iron intake. Gastrointestinal AEs were reported by 3 participants receiving iron, who were advised, based on severity, either to discontinue iron (and leave the study) or to take the tablets every other day as tolerated. The dropout rate among the iron recipients, 10/111 (9%), was higher than among the recipients not receiving iron, 1/104 (1%). Symptoms directly attributable to iron (constipation and abdominal discomfort) that resulted in removal from the study were reported by only 2 participants.
Of the 215 randomized participants, hemoglobin was measured at 6 or more visits for 193 participants (96 iron, 97 no iron) and ferritin for 202 (Figure 1). Mean baseline hemoglobin in the 22 excluded participants differed from the mean in participants with 6 or more values by 0.08 g/dL. This difference is very small compared with the mean differences observed in this study between those who took and did not take iron.
The mean baseline hemoglobin levels were comparable in the iron and no-iron groups and declined from a mean (SD) of 13.4 (1.1) g/dL to 12.0 (1.2) g/dL after donation in the low-ferritin group and from 14.2 (1.1) g/dL to 12.9 (1.2) g/dL in the higher-ferritin group. Figure 2A shows the mean hemoglobin levels by visit for each of the 4 groups stratified by treatment and baseline ferritin. Immediate postdonation hemoglobin and the absolute postdonation decrease in hemoglobin were comparable, except for a significantly greater decrease in postdonation hemoglobin in women compared with men, related to hemoglobin level and blood volume differences (eTable in Supplement 2). Compared with the groups who did not receive supplements, participants in the low-ferritin group who received iron experienced more rapid hemoglobin recovery (mean, 32 days; IQR, 30–34, vs 158 days; IQR, 126->168) as did the higher-ferritin group who took iron (31 days; IQR, 29–33, vs 78 days; IQR, 66–95). Model estimates of hemoglobin recovery for the groups not receiving iron were highly variable with no statistically significant difference found between the higher-ferritin group and the low-ferritin group. In contrast, participants randomized to receive iron recovered their hemoglobin more quickly, with considerably less variability in their recovery model estimates and with no discernible difference between higher-ferritin and low-ferritin groups. Whole blood donation removed an average of 10% of baseline hemoglobin (Figure 2B). Among participants receiving iron, hemoglobin levels increased to 106% of baseline (95% CI, 104.3%–107.7%) in the low-ferritin group, plateauing after about 120 days of iron intake. In contrast, the hemoglobin in the 2 groups not taking supplements did not fully recover to baseline at 168 days.
Figure 2. Time to Hemoglobin Recovery According to Treatment and Baseline Ferritin Level.
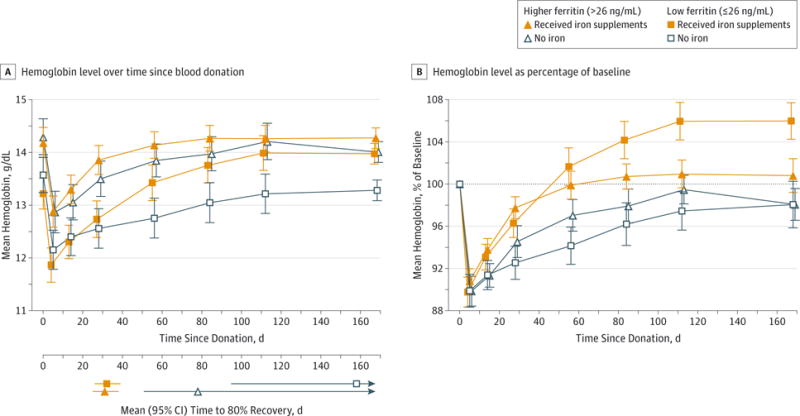
A, Mean hemoglobin for each group vs time since donation. Time “0” is the day of donation. B, Hemoglobin expressed as a percentage of initial hemoglobin value. Hemoglobin recovery was significantly higher than baseline in the low-ferritin group (≤26 ng/mL) who took iron (mean, 106.0%; 95% CI, 104.3%–107.7%; P < .001) and significantly lower than baseline in the higher-ferritin group (>26 ng/mL) who took no iron (mean, 98.1%; 95% CI, 96.6%–99.6%; P = .02). Dotted line indicates 100% of initial hemoglobin value; error bars, 95% confidence intervals.
No significant differences between men and women were found in hemoglobin recovery or in the effect of iron supplementation, independent of baseline ferritin (Table 2).
Table 2.
Hemoglobin Recovery to Pre-donation Level by Sex, Treatment, and Baseline Ferritin Level
| Ferritin Group (ng/mL) | No. of Participants | Time to 80% Hemoglobin Recovery, Mean (IQR), da | (95% CI)b | |
|---|---|---|---|---|
| Women | ||||
| Iron | Low (≤26) | 30 | 36 (33–39) | (29–46) |
| Iron | Higher (>26) | 29 | 32 (29–34) | (25–41) |
| No iron | Low (≤26) | 30 | 153 (126–>168) | (94–>168) |
| No iron | Higher (>26) | 29 | 92 (76–130) | (55–>168) |
| Men | ||||
| Iron | Low (≤26) | 17 | 25 (23–28) | (18–35) |
| Iron | Higher (>26) | 20 | 30 (27–35) | (22–49) |
| No iron | Low (≤26) | 18 | >168 (102–>168) | (50–>168) |
| No iron | Higher (>26) | 20 | 68 (50–95) | (34–>168) |
Abbreviation: IQR, interquartile range.
Interquartile range of bootstrap estimates.
95% CI of bootstrap estimates.
In an exploratory analysis, we found the proportion with venous hemoglobin greater than 12.5 g/dL by 56 days was higher among 95 participants taking iron than among 96 participants not taking iron (0.92 vs 0.73; P < .001 from logistic regression) (eFigure 2 in Supplement 2).
Figure 3 depicts the recovery of ferritin in the 4 groups. As for hemoglobin recovery, ferritin took less time to recover for participants receiving iron. Median time to recovery for all participants taking iron was 76 days (IQR, 20–126); for participants not taking iron, median recovery time was longer than 168 days (IQR, 147->168 days; P < .001), and 67% of the participants not taking iron did not reach their baseline ferritin levels by 168 days. Median time to recovery to baseline ferritin levels in the low-ferritin group taking iron was 21 days (IQR, 12–84), and time to recovery to levels of 26 ng/mL or greater was 120 days (IQR, 64->168). For participants not taking iron, recovery to baseline was longer than 168 days (IQR, 128->168) and recovery to ferritin levels of 26 ng/mL or greater was longer than 168 days (IQR, >168->168). Median time to recovery to baseline in the higher-ferritin group taking iron was 107 days (IQR, 75–141), and for participants not taking iron, recovery to baseline was longer than 168 days (IQR, 168->168). The rate of recovery of ferritin increased after hemoglobin recovery occurred (Figure 3). This effect was noted in all groups except the low-ferritin, no-supplement group and was most pronounced in the iron-replete, no-supplement group.
Figure 3. Mean Ferritin Level for Each Group vs Time Since Donation.
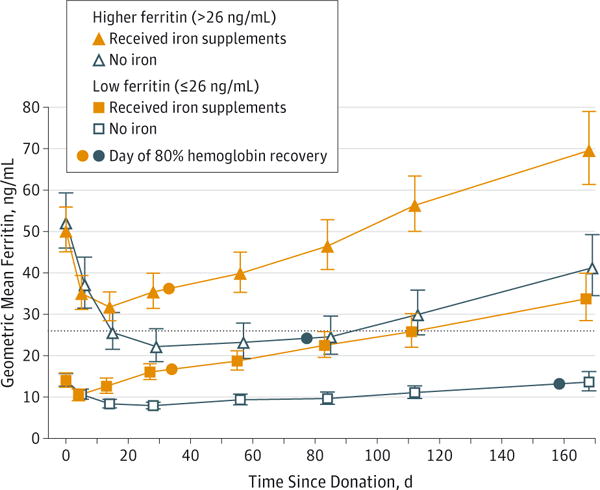
After a donor gives 500 mL of whole blood, ferritin decreases by approximately 30 ng/mL over a 30-day period, as depicted in the no-iron, iron-replete group (open triangles). Each ng/mL of ferritin equates to 8 to 10 mg of storage iron; thus, 30 × 8 mg = 240 mg, approximately the amount of iron contained in each unit of blood. Reconstitution of storage iron was not observed until after recovery of hemoglobin, as indicated in the upward change in slope of the ferritin recovery curve in the no-iron, higher-ferritin group, at approximately day 84. Dotted line indicates 26 ng/mL, the value used to stratify iron-deficient and iron-replete donors at enrollment; error bars, 95% confidence intervals.
We also examined hemoglobin recovery by pre-donation ferritin quartiles (Figure 4). In participants who did not take supplements, hemoglobin recovery was significantly faster in the highest-quartile ferritin subgroup (51–192 ng/mL) compared with the lowest ferritin subgroup (4–15 ng/mL). (All other subgroup comparisons in no-supplement participants were not significant.) Hemoglobin recovery was uniformly rapid in all participants taking supplements, with recovery for the lower 3 quartiles of ferritin (4–50 ng/mL) significantly faster than among comparable participants who did not take supplements. Only in the highest ferritin quartile (51–192 ng/mL) was there no significant difference in hemoglobin recovery time with iron supplementation.
Figure 4. Mean Time to 80% Hemoglobin Recovery by Quartile of Ferritin and Treatment Assignment.
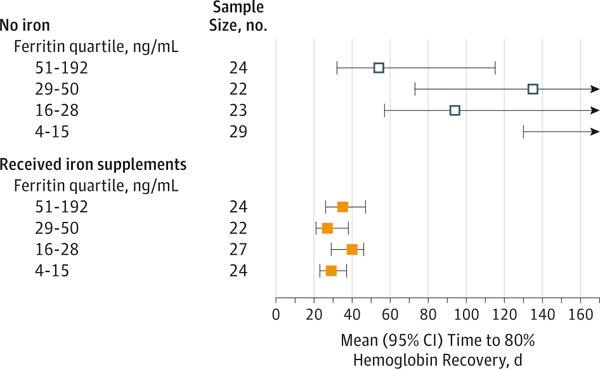
The confidence intervals are censored at the end of follow-up at 168 days. No mean is shown for the lowest ferritin quartile for participants not taking iron because the mean was longer than 168 days.
Discussion
This trial of oral iron supplementation after blood donation found wide variation in the rate of hemoglobin recovery among participants who did not take supplements and recovery times well beyond the 56-day minimum donation interval among iron-depleted individuals. Uniformly increased hemoglobin recovery was found in participants with pre-donation ferritin levels less than 50 ng/mL who took 37.5 mg of elemental iron per day. The increase in hemoglobin to levels above baseline among iron-depleted participants receiving iron supplementation reflected a preexisting relative anemia, even though at least 120 days had elapsed since their previous donation. Participants who did not take iron supplements did not fully recover ferritin to pre-donation levels by 168 days. We also found that iron supplementation equally improved hemoglobin and iron recovery times among male and female participants, and in an exploratory analysis, we showed that participants were more likely to recover venous hemoglobin to 12.5 g/dL or higher at 56 days with supplemental iron.
Although the absolute amount of hemoglobin decrease was relatively small and of marginal clinical consequence after a single blood donation, donating blood is an iterative process that leads to progressive iron loss and anemia in some frequent blood donors, so it is important that the hemoglobin decrease after blood donation be recovered before the next blood donation. Our findings support studies showing that iron supplementation reduces the high rate of low-hemoglobin donor deferrals,11 currently 17.7% among all presenting women and 1.6% among men based on capillary hemoglobin of less than 12.5 g/dL.16
This randomized, nonblinded multicenter study directly assessed the effect of iron supplementation on the recovery of hemoglobin and iron stores in repeat blood donors after a single whole blood donation. Male and female donors with a wide range of iron status were successfully enrolled and followed up over the course of the 24-week study. Although our enrollment process did not reflect a random sampling of the donor population, it included donors representing the range of donation intensity that is most common in routine blood bank operations. Our study reinforces earlier investigations that documented declining ferritin levels in frequent blood donors in the absence of an iron supplementation regimen.17 One iron supplementation trial conducted in frequent (4–6 times/year) blood donors showed that ferritin levels were maintained among donors taking 20 mg of elemental iron daily but declined among the placebo controls. However, it was only among donors taking 40 mg daily that ferritin levels increased, resulting in positive iron balance.18 Our choice of ferrous gluconate containing 37.5 mg of elemental iron appears to have been suitable in terms of both efficacy and a low frequency of gastrointestinal AEs.
The HEIRS suggests that recovery of hemoglobin takes considerably longer for many donors than reported in early studies that informed decisions to establish interdonation intervals at 8 weeks. Evidence supporting an 8-week standard was based on studies conducted in small numbers of young, iron-replete participants19 or on limited data on regular blood donors before reliable iron measurements were available.20 More recently, a novel study that quantified total hemoglobin mass using a carboxyhemoglobin method found that hemoglobin was replenished after donation in a mean (SD) of 36 (11) days, but it too was of limited generalizability because it was restricted to young men.14 The HEIRS enrolled participants with diverse demographic characteristics to report the effect of supplemental iron on the kinetics of hemoglobin and ferritin recovery to important physiologic and operational thresholds.
A limitation in this study is that our curve-fitting analytical method could not compute recovery time of hemoglobin in some participants, and thus we could not determine the individual variation in hemoglobin recovery times. However, we were able to use the mean of each group (n = 50–60) to obtain reliable hemoglobin recovery measurements and then use bootstrap methods to obtain confidence limits for the mean (Table 2). This method took advantage of all hemoglobin data points available, which tended to reduce variability due to a single measurement (eMethods in Supplement 2).21 Although we found that more participants who were iron-depleted recovered to hemoglobin levels of 12.5 g/dL by 56 days of taking iron, implementing a supplement plan may not result in reduced hemoglobin deferrals because capillary hemoglobin measurements, which are used in the blood center setting, tend to be higher than venous hemoglobin measurements.22 Finally, it is also possible that the motivation to participate in a research setting or participant reimbursement may have affected adherence with the pill regimen. The willingness of blood donors to take a daily iron tablet on a regular basis might be reduced in routine practice, as suggested by the 9% dropout rate among the participants receiving iron compared with 1% among the donors not receiving iron.
An important policy consideration raised by our findings concerns the 8-week minimum required interdonation interval in the United States and Canada, which is shorter than that allowed in many countries.23 As noted earlier, when this standard was instituted, the expectation was that most donors would recover the hemoglobin lost from blood donation within this time period. The data presented here demonstrate that hemoglobin recovery differs by pre-donation ferritin level, and even in iron-replete donors, the mean recovery was only 70% at 8 weeks. Iron loss from blood donation is substantial (≈230 mg iron in a unit of packed red blood cells), and absorption of dietary iron is a limiting factor for new hemoglobin synthesis and subsequently replenishment of iron stores. Although longer minimum intervals would by definition allow for more thorough recovery between donations, simulations of the effect of prolonging the minimum donation interval for blood donation to 12 or 16 weeks suggest that the blood supply in the United States might be significantly compromised.24 Further, based on the results presented here, an increase in the interdonation interval would still not provide adequate time for hemoglobin and iron recovery in many donors who do not take supplements.
Conclusions
Among blood donors with normal hemoglobin levels, low-dose iron supplementation, compared with no supplementation, reduced time to 80% recovery of the postdonation decrease in hemoglobin concentration in donors with low ferritin (≤26 ng/mL) or higher ferritin (>26 ng/mL).
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by National Heart, Lung, and Blood Institute (NHLBI) contracts HHSN268201100001I, HHSN268201100002I, HHSN268201100003I, HHSN268201100004I, HHSN268201100005I, and HHSN268201100006I.
Role of the Funder/Sponsor: The study sponsor, NHLBI, participated in the design, approval, and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Video Interview and JAMA Report Video at jama.com
Supplemental content at jama.com
Author Contributions: Drs Kiss and Brambilla had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kiss, Brambilla, Glynn, Mast, Spencer, Kleinman, Cable.
Acquisition, analysis, or interpretation of data: Kiss, Brambilla, Glynn, Mast, Spencer, Stone, Cable.
Drafting of the manuscript: Kiss, Brambilla, Glynn, Stone, Cable.
Critical revision of the manuscript for important intellectual content: Kiss, Brambilla, Glynn, Mast, Spencer, Stone, Kleinman, Cable.
Statistical analysis: Kiss, Brambilla, Glynn, Spencer.
Obtained funding: Kiss, Brambilla, Mast, Cable.
Administrative, technical, or material support: Spencer, Stone, Cable.
Study supervision: Kiss, Mast, Stone, Cable.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Mast reported having received a grant from Novo Nordisk and honoraria from Siemens. No other disclosures were reported.
Additional Contributions: We wish to recognize the special expertise of our friend and colleague, Leslie H. Tobler, DrPH (deceased), of Blood Systems Research Institute, San Francisco, California, who coordinated the Central Laboratory and assisted with the design and analysis of this study. We thank the safety medical monitor for the study, Ann Zimrin, MD, of the University of Maryland, and the research staff responsible for donor enrollment, follow-up, and data collection at the 4 REDS-III hubs (Walt Bialkowski, MS; Shanita Thomas, BS; Zoya Udee, BA; Pam D’Andrea, RN; Barb Kletter, CMA; Chris Sayre, BS, MT, ASCP, MBA; Anne Guiltinan, MA, LMFT; Daniel A. Hindes, BA; Monalissa Doane, MS), the staff responsible for protocol and data management at the coordinating center (Christopher Siege, BS, MS; Anne-Lyne McCalla, BS, MPH), and the staff responsible for laboratory testing and data management at the central laboratory (BSRI Viral Reference Laboratory and Repository Core) for their commitment to the study and skillful support. All staff received contractual financial support.
References
- 1.Baart AM, van Noord PAH, Vergouwe Y, et al. High prevalence of subclinical iron deficiency in whole blood donors not deferred for low hemoglobin. Transfusion. 2013;53(8):1670–1677. doi: 10.1111/j.1537-2995.2012.03956.x. [DOI] [PubMed] [Google Scholar]
- 2.Cable RG, Glynn SA, Kiss JE, et al. NHLBI Retrovirus Epidemiology Donor Study-II (REDS-II) Iron deficiency in blood donors: the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2012;52(4):702–711. doi: 10.1111/j.1537-2995.2011.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitaker BI, Hinkins S. 2011 National blood collection and utilization survey report. US Dept of Health and Human Services; http://www.hhs.gov/ash/bloodsafety/2011-nbcus.pdf. Accessed November 25, 2014. [Google Scholar]
- 4.Krayenbuehl PA, Battegay E, Breymann C, Furrer J, Schulthess G. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood. 2011;118(12):3222–3227. doi: 10.1182/blood-2011-04-346304. [DOI] [PubMed] [Google Scholar]
- 5.Brownlie T, IV, Utermohlen V, Hinton PS, Haas JD. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. Am J Clin Nutr. 2004;79(3):437–443. doi: 10.1093/ajcn/79.3.437. [DOI] [PubMed] [Google Scholar]
- 6.Murray-Kolb LE, Beard JL. Iron treatment normalizes cognitive functioning in young women. Am J Clin Nutr. 2007;85(3):778–787. doi: 10.1093/ajcn/85.3.778. [DOI] [PubMed] [Google Scholar]
- 7.Falkingham M, Abdelhamid A, Curtis P, Fairweather-Tait S, Dye L, Hooper L. The effects of oral iron supplementation on cognition in older children and adults: a systematic review and meta-analysis. Nutr J. 2010;9:4. doi: 10.1186/1475-2891-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryant BJ, Yau YY, Arceo SM, Hopkins JA, Leitman SF. Ascertainment of iron deficiency and depletion in blood donors through screening questions for pica and restless legs syndrome. Transfusion. 2013;53(8):1637–1644. doi: 10.1111/trf.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spencer BR, Kleinman S, Wright DJ, et al. REDS-II RISE Analysis Group Restless legs syndrome, pica, and iron status in blood donors. Transfusion. 2013;53(8):1645–1652. doi: 10.1111/trf.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blood Products Advisory Committee meeting: July 27, 2010. US Food Drug Administration; http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/BloodProductsAdvisoryCommittee/ucm221857.htm. Accessed December 22, 2014. [Google Scholar]
- 11.Smith GA, Fisher SA, Doree C, Di Angelantonio E, Roberts DJ. Oral or parenteral iron supplementation to reduce deferral, iron deficiency and/or anaemia in blood donors. Cochrane Database Syst Rev. 2014;7:CD009532. doi: 10.1002/14651858.CD009532.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cable RG, Glynn SA, Kiss JE, et al. NHLBI Retrovirus Epidemiology Donor Study-II Iron deficiency in blood donors: analysis of enrollment data from the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2011;51(3):511–522. doi: 10.1111/j.1537-2995.2010.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiss JE, Steele WR, Wright DJ, et al. NHLBI Retrovirus Epidemiology Donor Study-II Laboratory variables for assessing iron deficiency in REDS-II Iron Status Evaluation (RISE) blood donors. Transfusion. 2013;53(11):2766–2775. doi: 10.1111/trf.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pottgiesser T, Specker W, Umhau M, Dickhuth HH, Roecker K, Schumacher YO. Recovery of hemoglobin mass after blood donation. Transfusion. 2008;48(7):1390–1397. doi: 10.1111/j.1537-2995.2008.01719.x. [DOI] [PubMed] [Google Scholar]
- 15.Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89(3):1052–1057. [PubMed] [Google Scholar]
- 16.Mast AE, Schlumpf KS, Wright DJ, et al. NHLBI Retrovirus Epidemiology Donor Study-II Demographic correlates of low hemoglobin deferral among prospective whole blood donors. Transfusion. 2010;50(8):1794–1802. doi: 10.1111/j.1537-2995.2010.02649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon TL, Garry PJ, Hooper EM. Iron stores in blood donors. JAMA. 1981;245(20):2038–2043. [PubMed] [Google Scholar]
- 18.Radtke H, Tegtmeier J, Röcker L, Salama A, Kiesewetter H. Daily doses of 20 mg of elemental iron compensate for iron loss in regular blood donors: a randomized, double-blind, placebo-controlled study. Transfusion. 2004;44(10):1427–1432. doi: 10.1111/j.1537-2995.2004.04074.x. [DOI] [PubMed] [Google Scholar]
- 19.Wadsworth GR. Recovery from acute haemorrhage in normal men and women. J Physiol. 1955;129(3):583–593. doi: 10.1113/jphysiol.1955.sp005380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fowler WM, Barer AP. Rate of hemoglobin regeneration in blood donors. JAMA. 1942;118(6):421–427. [Google Scholar]
- 21.Dot D, Miró J, Fuentes-Arderiu X. Within-subject biological variation of hematological quantities and analytical goals. Arch Pathol Lab Med. 1992;116(8):825–826. [PubMed] [Google Scholar]
- 22.Cable RG, Steele WR, Melmed RS, et al. NHLBI Retrovirus Epidemiology Donor Study-II (REDS-II) The difference between fingerstick and venous hemoglobin and hematocrit varies by sex and iron stores. Transfusion. 2012;52(5):1031–1040. doi: 10.1111/j.1537-2995.2011.03389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karp JK, King KE. International variation in volunteer whole blood donor eligibility criteria. Transfusion. 2010;50(2):507–513. doi: 10.1111/j.1537-2995.2009.02392.x. [DOI] [PubMed] [Google Scholar]
- 24.Eder A. Proposed changes to hemoglobin and donation interval criteria for whole blood donors: projected impact on current donor base. Paper presented at: 98th meeting of Blood Products Advisory Committee, US Food and Drug Administration; July 28, 2008; Gaithersburg, MD. http://www.fda.gov/ohrms/dockets/ac/cber08.html#BloodProducts. Accessed November 14, 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


