Abstract
The neocortex of the prairie vole is composed of three well-defined sensory areas and one motor area: primary somatosensory, visual, auditory areas, and the primary motor area, respectively. The boundaries of these cortical areas are identifiable very early in development, and have been thought to resist alteration by all but the most extreme physical or genetic manipulations. Here we assessed the extent to which the boundaries of sensory/motor cortical areas can be altered by exposing young prairie voles ( Microtus ochrogaster ) to a chronic stimulus, high or low levels of parental contact, or an acute stimulus, a single dose of saline, oxytocin (OT), or oxytocin antagonist on the day of birth. When animals reached adulthood, their brains were removed, the cortex was flattened, cut parallel to the pial surface, and stained for myelin to identify the architectonic boundaries of sensory and motor areas. We measured the overall proportion of cortex that was myelinated, as well as the proportion of cortex devoted to the sensory and motor areas. Both the chronic and acute manipulations were linked to significant alterations in areal boundaries of cortical fields, but the areas affected differed with different conditions. Thus, differences in parental care and early exposure to OT can both change cortical organization, but their effects are not identical. Furthermore, the effects of both manipulations were sexually dimorphic, with a greater number of statistically significant differences in females than in males. These results indicate that early environmental experience, both through exposure to exogenous neuropeptides and parental contact can alter the size of cortical fields.
Keywords: prairie vole, neocortex, development, parental behavior, oxytocin
Introduction
The mammalian brain is characterized by the presence of a six-layered neocortex, which is involved in processing sensory inputs and generating motor output. Sensory cortex is segregated by modality and within each modality distinct cortical fields that form a topographic representation of the sensory receptor array are observed, as well as a motor cortical area in which roughly topographic maps of body part movements are found. These functional representations correspond with a unique architectonic appearance as well a specific pattern of connections. In adults, the boundaries of the primary cortical areas can be visualized using a myelin stain; primary sensory and motor areas stain more darkly for myelin than other areas of the neocortex (e.g. [ 1 ]).
The boundaries of primary sensory and motor cortex are identifiable as early as 5 days after birth in rats [ 2 ]. Under normal developmental circumstances these borders are stable across the lifespan, although the internal representations of the sensory arrays may change with alterations in the use or morphology of the sensory effector organ [ 2–5 ]. There have been a few instances where the size of primary sensory areas has been altered, but these have employed extreme experimental interventions at early developmental stages such as bilateral enucleation or limb amputation [ 3 , 6 , 7 ]. Likewise, alterations in gene expression patterns in the developing cortex can change the size of primary sensory and motor areas [ 8–10 ].
Although variations in gene expression have been demonstrated to exert macroscopic influences on cortical organization, the environmental and epigenetic factors driving cortical arealization have been less thoroughly investigated. It is well documented that within any given population there exists variation in the size, shape, and location of primary sensory and motor regions [ 11 , 12 ]. Furthermore, the existence of variation between individuals is one of the cornerstones upon which the theory of evolution via natural selection depends [ 11 , 13 ]. However, how early experience might contribute to individual differences in aspects of cortical organization, such as relative size of cortical fields, is currently unknown.
To address this question we used the prairie vole ( Microtus ochrogaster ), a monogamous and biparental rodent that is native to the grasslands of Illinois and Indiana in the central USA [ 14–17 ]. They exhibit a wide range of well-defined social behaviors in which the underlying neuroendocrine mechanisms are well understood [ 18–20 ]. Critically, they also exhibit natural variation in the style and amount of parental care expressed towards their pups [ 17 ], which directly translates into a variation in the amount and type of early sensory experience. Differences in parenting style in voles have been linked to differences in offspring behavior [ 17 ], neuroanatomical connections between cortical regions [ 21 ], stress reactivity [ 22 , 23 ], and oxytocin (OT) receptor binding [ 24 ].
The neuropeptide OT (see Table 1 for abbreviations) appears to be a common factor underlying differences in social behavior, parental behavior, and stress reactivity. Vole parents with high OT receptor density exhibit high levels of parental contact, which in turn yields offspring that exhibit high OT receptor density and high levels of alloparenting [ 24 ]. The converse is true for vole parents that exhibit low levels of OT receptor binding [ 24 ]. Humans also show alterations in OT levels in response to parental contact and social experience [ 25 ]. Furthermore, OT has been implicated in the plasticity of the developing neocortex [ 26 ].
Table 1.
Table of abbreviations
| List of abbreviations | |
|---|---|
| AC | auditory cortex |
| FM | frontal myelinated area |
| HC | high contact |
| LC | low contact |
| M1 | primary motor cortex |
| OT | Oxytocin |
| OTA | Oxytocin antagonist |
| OTR | oxytocin receptor |
| PV | parietal ventral area |
| S1 | primary somatosensory cortex |
| S2 | second somatosenory cortex |
| V1 | primary visual cortex |
| V2 | second visual cortex |
In these experiments, we exposed vole pups to exogenous OT. OT in the form of Pitocin is commonly used in American hospitals to induce and enhance labor [ 27 ]. We have previously administered OT within 24 hours of birth to simulate neonatal OT exposure. A single dose of exogenous OT can affect pair-bond formation in both males and females [ 28 , 29 ], as well as aggression [ 20 ], sexual behavior [ 30 ], and the vasopressin receptor system [ 18 ].
We asked two questions. First, are variations in early sensory experience (mediated by early parenting) associated with differences in the size of cortical fields? Second, is it possible to experimentally alter the relative size of cortical fields through the early administration of OT?
Results
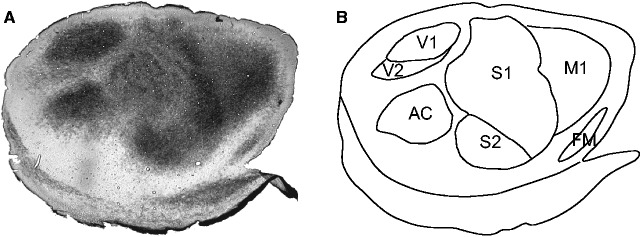
Staining the neocortex for myelin clearly revealed the borders of cortical fields, including the primary sensory areas ( Fig. 1A and B ). The myelination patterns of the prairie vole neocortex have been described previously in [ 21 , 31 ], and the cortical morphology observed is like that described in the previous studies. Briefly, V1 is located on the caudal pole of the neocortex and it stains darkly for myelin, whereas V2 is located immediately lateral to V1 and it stains less darkly for myelin. AC is a round structure that is found lateral to V1 and V2 and stains darkly for myelin. S1 is found rostral to V1, V2, and AC, and stains darkly, but non-uniformly, for myelin. As in other mammalian species, S1 of prairie voles includes a somatotopic representation of the body, with the hind limb represented most medially, followed by the forelimb, vibrissae, nose, and snout represented laterally [ 21 , 31 ]. The heterogenous staining within S1 is indicative of these different body part representations, with the most obvious being the barrel field. S2/PV is located adjacent and rostral to the lateral edge of S1, and stains uniformly darkly for myelin. M1 is found immediately rostral to S1 and stains moderately for myelin. Frontal myelinated (FM) is found rostral and lateral to M1, medial to the rhinal sulcus, and stains darkly for myelin.
Figure 1.
Anatomical organization of the vole cortex. (A) A tangential section of cortical tissue stained for myelin. Darkly stained fields correspond to primary sensory and motor regions. (B) By using an entire series of myelin sections we were able to identify the borders of sensory and motor areas. See Table 1 for abbreviations
Alterations in Cortical Field Size
In the following sections, we will discuss the effects of both the chronic (parental care) and acute (OT/ oxytocin antagonist (OTA)/saline) manipulations on the boundaries of specific cortical areas. See Table 2 for means.
Table 2.
Table of results
|
HC
|
LC
|
Saline
|
OT
|
OTA
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Area | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female |
| % Myelin | 59.29 ± 1.35 | 58.27 ± .66 | 58.80 ± 1.37 | 53.56 ± 1.70 | 56.60 ±2.05 | 59.13 ± 1.87 | 59.11 ± 1.29 | 56.90 ± 1.62 | 56.80 ± 1.38 | 59.53 ± 1.02 |
| %S1 | 22.60 ± .56 | 22.31 ± .35 | 21.69 ± .56 | 26.91 ± 1.67 | 23.43 ± 1.14 | 24.49 ± .61 | 23.27 ± .44 | 22.19 ± .15 | 24.87 ± .67 | 24.87 ± .67 |
| %S2/PV | 5.74 ± .31 | 5.00 ± .22 | 5.30 ± .30 | 4.71 ± .81 | 4.93 ± .36 | 5.16 ± .40 | 5.19 ± .32 | 6.23 ± .92 | 5.08 ± .40 | 5.08 ± .37 |
| %M1 | 10.88 ± .71 | 11.21 ± .70 | 11.77 ± .76 | 9.00 ± .30 | 10.15 ± .53 | 10.99 ± .54 | 11.58 ± .64 | 10.61 ± .58 | 11.30 ± .78 | 11.30 ± .65 |
| %V1 | 5.41 ± .42 | 4.32 ± .27 | 5.94 ± .44 | 4.74 ± .98 | 3.75 ± .38 | 3.60 ± .23 | 3.64 ± .25 | 4.78 ± .61 | 3.44 ± .36 | 3.44 ± .40 |
| %V2 | 2.80 ± .32 | 3.08 ± .56 | 2.75 ± .16 | 1.96 ± .52 | 2.12 ± .20 | 2.99 ± .69 | 2.51 ± .13 | 1.05 ± .61 | 2.11 ± .22 | 2.11 ± .33 |
| %AC | 9.16 ± .47 | 9.23 ± .38 | 8.50 ± .35 | 8.77 ± .57 | 9.82 ± .56 | 9.54 ± .54 | 10.75 ± .77 | 10.26 ± 1.02 | 9.76 ± .45 | 9.76 ± .68 |
The proportion of the cortical sheet that is occupied by different brain regions in males and females from each experimental group. Values are expressed as mean ± SE.
% Myelin
After determining the architectonic boundaries of different cortical regions, we calculated the proportion of the cortical sheet that was densely myelinated, (% Myelin = the sum of S1, S2/PV, M1, V1, V2, AC, and FM divided by the size of the entire cortical sheet; Figs 2 and3 ). The areas of the neocortex that stain most darkly for myelin are the primary sensory and motor areas (with the exception of the secondary visual area). In animals exposed to different amounts of parental care, there was a significant main effect of sex ( F = 9.504, P = 0.0067), a trend towards a significant effect of condition ( F = 4.023, P = 0.0611), and a significant sex by condition interaction ( F = 5.609, P = 0.0300). Post-hoc tests revealed that the % Myelin was significantly lower in LC females than in HC females, HC males, and LC males ( t = 2.110, P < 0.0137), which Cohen’s d indicated to be a large effect ( d > 1.785) ( Fig. 3 ). In animals treated with OT/OTA/saline, there were no significant main effects of sex ( F = 0.4763, P = 0.4944), condition (F = 0.0158, p = 0.9844), or a significant sex by condition interaction ( F = 1.0490, P = 0.3605).
Figure 2.
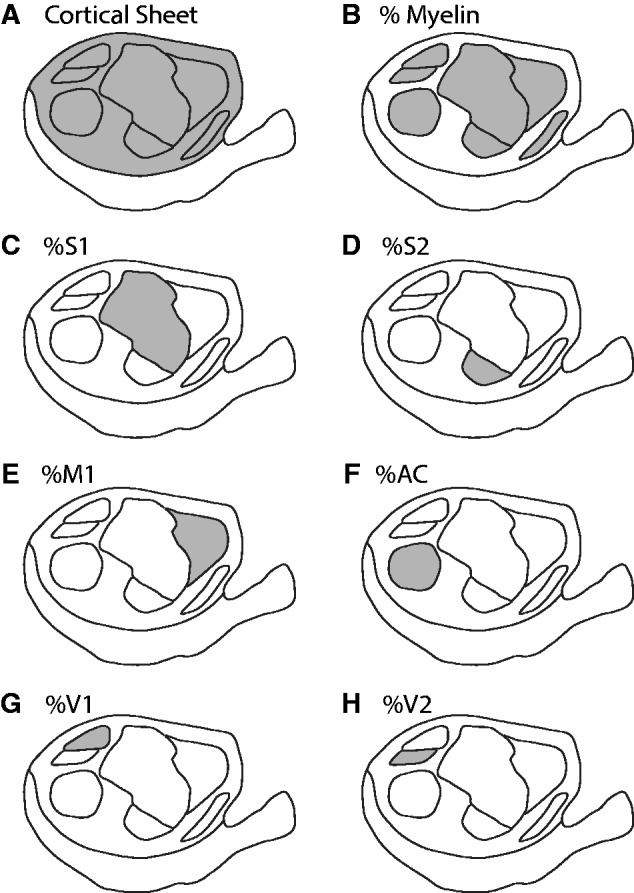
Schematic identifying the cortical regions included in each calculation. In each case, the shaded area represents the region of interest. To determine the proportion of cortex, the area of each region was divided by the area of the cortical sheet. (A) The cortical sheet. (B) The total area of myelination. (C) Primary somatosensory cortex (S1). D) Second somatosensory cortex (S2). (E) Primary motor cortex (M1). (F) Auditory cortex (AC). (G) Primary visual cortex (V1). H) Second visual cortex (S2)
Figure 3.
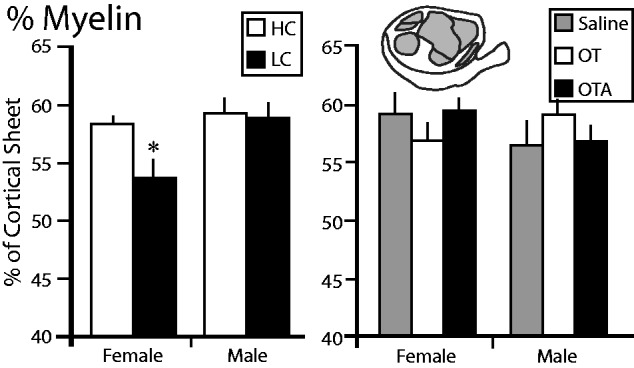
The proportion of the cortical sheet characterized by dense myelination (%Myelin). The left graph shows the %Myelin in female and male voles exposed to HC (white) or LC (black) amounts of parental care. The %Myelin in LC females was significantly lower than in HC females. The right graph shows the %Myelin in female and male voles treated with saline (gray), OT (white), or OTA (black). There were no differences between any of these groups. *, significantly different from other group within the same sex
%S1 and %S2/PV
We next compared the proportion of the cortical sheet occupied by S1 in each group ( Fig. 4 ). We found no significant main effect of sex ( F = 0.0044, P = 0.9477) or condition ( F = 0.1736, P = 0.6821), and no significant sex by condition interaction ( F = 0.0008, P = 0.9777) between the %S1 in HC females, LC females, HC males, and LC males. In animals treated with OT/OTA/saline, there were no significant main effects of sex (F = 1.4716, P = 0.2328), condition (F = 1.1014, p = 0.3431), or a significant sex by condition interaction ( F = 2.0745, P = 0.1400). However, a preplanned comparison revealed that %S1 in females treated with OT was significantly lower than in females treated with saline ( P = 0.0437) or OTA ( P = 0.0313), which Cohen’s d indicated to be a large effect ( d > 1.502).
Figure 4.
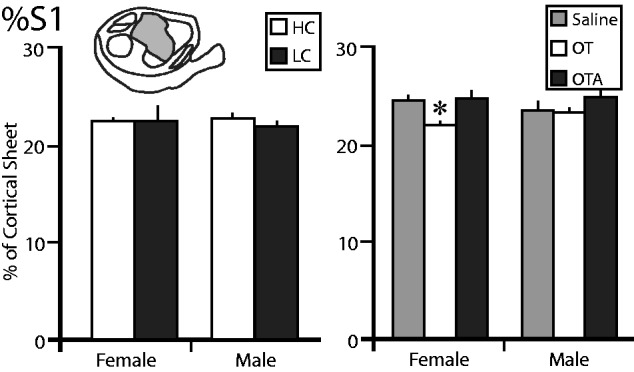
The proportion of the cortical sheet occupied by the primary somatosensory cortex (%S1). The left graph shows the %S1 in female and male voles exposed to HC (white) or LC (black) amounts of parental care. There are no differences between any of these groups. The right graph shows the %S1 in female and male voles treated with saline (gray), OT (white), or OTA (black). The %S1 of females treated with OT was significantly lower than females treated with saline or OTA, or males treated with OT. *, significantly different from other group within the same sex
We examined the proportion of the cortical sheet devoted to S2/PV (%S2/PV). In voles exposed to different amounts of parental contact we found no significant main effect of sex ( F = 3.1817, P = 0.0923) or condition ( F = 0.9983, P = 0.3317), and no significant sex by condition interaction ( F = 0.0107, P = 0.9188). In voles treated with OT/OTA/saline, there were no significant main effects of sex ( F = 0.3771, P = 0.5429), condition ( F = 1.1484, P = 0.3282), or a significant sex by condition interaction ( F = 1.5507, P = 0.2256).
%M1
We then examined the proportion of the cortical sheet occupied by M1 (%M1) in HC and LC males and females ( Fig. 5 ). There no significant main effect of sex ( F = 1.7187, P = 0.2073) or condition ( F = 1.3851, P = 0.2555), but there was a significant sex by condition interaction ( F = 5.4782, P = 0.0317). A post-hoc comparison revealed that the %M1 in LC females was significantly smaller than both LC males ( P = 0.0265) and HC females ( P = 0.0410), which Cohen’s d indicated to be a large effect ( d > 1.642). In animals treated with OT/OTA/saline, there were no significant main effects of sex ( F = 0.0349, P = 0.8529), condition ( F = 0.8150, P = 0.4504), or a significant sex by condition interaction ( F = 0.8379, P = 0.4407).
Figure 5.
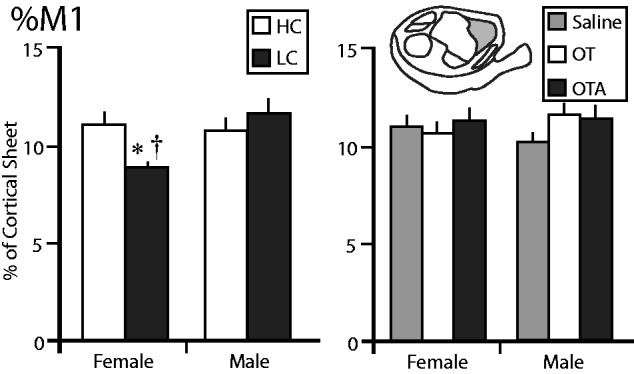
The proportion of the cortical sheet occupied by the primary motor cortex (%M1). The left graph shows the %M1 in female and male voles exposed to HC (white) or LC (black) amounts of parental care. The %M1 in LC females was significantly lower than in HC females or LC males. The right graph shows the %M1 in female and male voles treated with saline (gray), OT (white), or OTA (black). There were no differences between any of these groups. *, significantly different from other group within the same sex. †, significantly different from opposite sex within the same treatment group
%V1, %V2, and %AC
We also compared the proportion of the cortical sheet occupied by V1, V2, and AC ( Figs 6 and7 ). In voles exposed to different amounts of parental care the %V1 showed a significant effect of sex ( F = 5.3618, P = 0.0333), with females showing a significantly lower %V1 than males. There was no significant main effect of condition ( F = 1.0983, P = 0.3093), and no sex by condition interaction ( F = 0.0770, P = 0.7848) ( Fig. 6 ). In animals treated with OT/OTA/saline, there were no significant main effects of sex ( F = 1.3531, P = 0.2522), condition ( F = 2.1307, P = 0.1331), or a significant sex by condition interaction ( F = 1.6126, P = 0.2131). A preplanned comparison revealed that %V1 in females treated with OT was significantly higher than in females treated with saline ( P = 0.0494) or OTA ( P = 0.0404). Additionally, we found that females treated with OT had a significantly higher %V1 than males treated with OT ( t12 = 2.110, P = 0.0283). In all of these cases, Cohen’s d indicated that the effect size was large ( d > 1.374).
Figure 6.
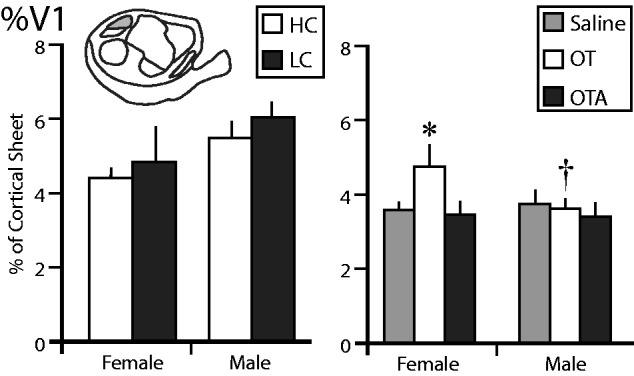
The proportion of the cortical sheet occupied by the primary visual cortex (%V1). The left graph shows the %V1 in female and male voles exposed to HC (white) or LC (black) amounts of parental care. There are no differences between any of these groups. The right graph shows the %V1 in female and male voles treated with saline (gray), OT (white), or OTA (black). The %V1 of females treated with OT was significantly higher than females treated with saline or OTA, as well as males treated with OT. *, significantly different from other group within the same sex. †, significantly different from opposite sex within the same treatment group
Figure 7.
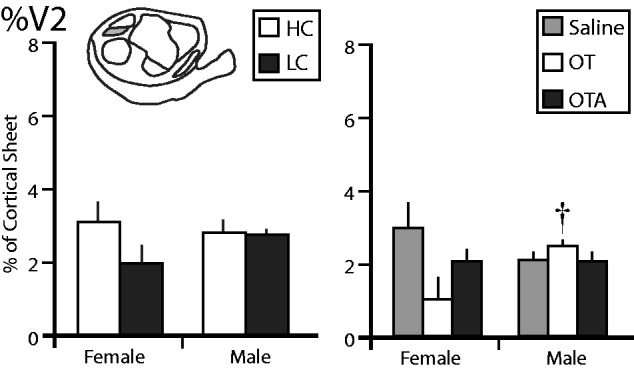
The proportion of the cortical sheet occupied by the second visual cortex (%V2). The left graph shows the %V1 in female and male voles exposed to HC (white) or LC (black) amounts of parental care. There are no differences between any of these groups. The right graph shows the %V2 in female and male voles treated with saline (gray), OT (white), or OTA (black). The %V2 of females treated with OT was significantly higher than males treated with OT. †, significantly different from opposite sex within the same treatment group
The %V2 in voles exposed to different levels of parental care did not show any significant main effects of sex ( F = 0.2645, P = 0.6136), condition ( F = 2.7988, P = 0.1126), or a significant sex by condition interaction ( F = 1.9758, P = 0.1779) ( Fig. 7 ). In voles treated with OT/OTA/saline, there was no significant main effect of sex ( F = 0.4577, P = 0.5029) or condition ( F = 1.7986, P = 0.1797). There was a significant sex by condition interaction ( F = 3.9497, P = 0.0279), with females treated with OT having significantly smaller %V2 than females treated with saline ( P = 0.0074) and males treated with OT ( P = 0.0325) which Cohen’s d indicated to be a large effect ( d > 1.080).
The %AC in voles exposed to different levels of parental care did not show any significant main effects of sex ( F = 0.0744, P = 0.7884), condition ( F = 1.3464, P = 0.2620), or a significant sex by condition interaction ( F = 0.0003, P = 0.9876). Similarly, in voles treated with OT/OTA/saline there were no significant main effects of sex ( F = 0.2653, P = 0.6096), condition ( F = 0.6573, P = 0.5242), or a significant sex by condition interaction ( F = 0.0240, P = 0.9763).
Discussion
Primary sensory and motor areas of the neocortex are defined by their functional organization, neuroanatomical connections, and architectonic boundaries [ 32 ]. Both during development and in adults, enhancing or reducing sensory input to these areas can alter both the functional and neuroanatomical organization of the primary sensory areas [ 3 , 5 , 7 , 33–35 ], but the architectonic boundaries which define the size of cortical fields, are set very early in development, around postnatal day (P)5 in rodents [ 3 ]. After this critical period these architectonic boundaries do not change, even following extreme peripheral manipulation, such as limb deafferentation or amputation, [ 3 ]. Even before the closure of the critical period; however, altering architectonic boundaries was only accomplished using the highly invasive peripheral manipulations, including nerve deafferentation or enucleation [ 3 , 7 ], or by altering patterns of gene expression during embryogenesis [ 10 , 36 ].
In this experiment, we tested two environmental manipulations that involved sensory mediated social experience, which should engage the OT system; as well as pharmacological manipulation of OT itself. We exposed young prairie voles to one of two experimental conditions: chronic exposure to differential levels of parental care, and acute exposure to a single dose of OT on the day of birth. Both of these treatments were linked to changes in the size of the sensory and motor areas of the neocortex. In both cases, exposure to the stimulus occurred before the closure of the critical period for the formation of architectonic boundaries around P5. Interestingly, these two interventions resulted in qualitatively different alterations to the boundaries. Voles exposed to differential levels of parental care exhibited changes in the total proportion of cortex that was myelinated, as well as the boundaries of M1. In contrast, voles that were given a single dose of OT on the day of birth exhibited changes in the boundaries of S1, V1, and V2. These differences could be explained by the intensity of the manipulation. The bolus dose of OT that was given exceeded normal physiological levels, but was only present in the body for a short period of time. In contrast, different parenting styles, which in rats are linked to alterations in both maternal and offspring OT levels [ 37 , 38 ], may have resulted in longer-term exposure to a lower dose of OT.
Furthermore, the age at which the exposure occurred may have influenced the cortical regions that were altered. Cortical arealization depends on a number of factors, including cell cycle regulation [ 39 , 40 ] and gene expression and epigenetic effects [ 8 , 41 , 42 ], as well as extrinsic factors such as sensory stimulation [ 7 , 43 , 44 ]. Sensory experience, in turn, regulates the synthesis and expression of OT within the cortex, which increases cross-modal plasticity [ 26 ]. Thus, increases in the amount of OT, whether through environmental stimulation or pharmacological administration, could induce cortical plasticity, thereby altering selected cortical borders.
Another interesting feature of the data is that administration of the OT antagonist did not produce a result opposite to that of OT administration. In every case, the OTA treatment did not differ from saline. This suggests that while OT receptors may be involved in the development of sensory and motor cortex, they are not strictly necessary. It is most probable that arginine vasopressin, a peptide that has cross-reactivity with OT, is also involved in this process; or even that OT itself is acting through vasopressin receptors [ 45–48 ]. One interesting line of research would be to study the sensory and motor cortex from animals in which the gene for OT receptors or vasopressin V1a receptors have been knocked out.
In this study, we found that both manipulations had sexually dimorphic effects, leading to significant alterations in cortical areas in females, but not in males. These results are not uncommon when dealing with neuropeptides like OT. In rats, high levels of maternal licking and grooming are linked to increase OTR binding in the amygdala and bed nucleus of the stria terminalis in females but not in males [ 37 ]. In prairie voles, exposure to OT results in sexually dimorphic effects, including increased aggression towards strangers [ 20 ], pair-bonding [ 49 , 50 ], alloparental care [ 29 ], as well as the distribution of vasopressin V1a receptors [ 18 ] and estrogen receptors [ 51 ]. Males and females often differed by the direction of effects or the dosage required.
The normal distribution of parental care in prairie voles may be analogous to a well-studied form of parental care in another species: maternal licking and grooming (LG) in rats [ 17 ]. There are several similarities between the two behaviors. First, rat dams exhibit variation in the amount of LG that they perform [ 52 ], much like prairie vole parents exhibit variation in the amount of time spent in contact with their offspring [ 17 ]. Furthermore, differences in the amount of LG received as pups is linked to variations in adult behavior [ 53 ], as is the amount of parental care received by young prairie voles [ 23 ]. The amount of time spent performing LG behaviors is mediated by maternal OT levels in rats [ 54 ], and maternal behaviors in prairie voles are mediated by OT levels [ 55 ]. The culmination of the LG literature in rats was the discovery that the mediation of the long-term consequences of early behavioral experiences occurred through epigenetic processes [ 56 , 57 ]. Although this has not yet been explicitly demonstrated in prairie voles, there are suggestions that the transmission of social behavior through parental care in voles may also be epigenetically regulated [ 24 ].
The administration of a single bolus dose of OT on the day of birth is designed to mirror the administration of OT as a labor-induction technique. Worldwide, the administration of OT, either alone or in combination with other techniques, is the most common method of labor induction [ 58 ]. In the USA in 2010, 23.8% of labors were induced [ 59 ]. Additionally, OT is routinely administered to postpartum mothers in order to aid in the contraction of the uterus and reduce the risk of maternal hemorrhage [ 60 ]. Despite the commonality of this practice, little is known about the long-term effects of perinatal OT administration. Recently, studies have indicated a relationship between OT-induced labor and attention deficit hyperactivity disorder diagnoses [ 61 ]. It has also been suggested that perinatal OT is linked to later diagnosis of autism spectrum disorders [ 62–64 ], although several studies have found no support for that link [ 59 , 63 ]. Further, many clinical trials investigating OT administration as a treatment for social and behavioral symptoms of ASD are underway or have been completed (i.e. [ 65 ]).
Little to nothing is known about the anatomical or physiological effects of early OT administration in humans. The recent findings in animals that OT may be involved in cortical plasticity [ 26 ], and that differences in parental care can alter behavior [ 17 , 24 ] as well as corticocortical connections [ 21 ] indicate that early alterations in OT levels may have major long-term consequences. In light of these recent findings, as well as the data presented here, we would urge caution when administering OT to patients whose brains are still developing.
Finally, perhaps the most intriguing aspect of these data is what they imply about the generation of phenotypic diversity within a population. In order to reduce the variability within studies, inbred strains of laboratory animals have been developed, which are phenotypically, if not genotypically, distinct from their wild counterparts [ 66 , 67 ]. Although we have long recognized phenotypic variability in wild organisms, the same cannot be said for laboratory animals. This is problematic, because phenotypic variability is one of the principles through which evolution by natural selection operates, and while we are beginning to understand how variation across species occurs, little is known about the origins of individual variation.
In humans, individual variation has been a focus of research, particularly in an attempt to understand the origins of specific behaviors. Characteristics such as the accuracy of introspection [ 68 ], musical ability [ 69 ], depression [ 70 ], working memory and attention [ 71 ], and language impairments [ 72 ] have all been linked to individual differences in brain anatomy. However, the origins of these differences remain unclear.
Although we appreciate that genetic variability obviously plays a large role in inducing individual differences within a population, here we demonstrate two methods by which individual variation can be achieved without alterations in gene sequence: differences in early parental care and administration of OT shortly after birth. Intermediary molecular mechanisms, such as gene expression and epigenetic markers such as methylation or histone acetylation, would be the logical next steps in exploring this variation.
Methods
Subjects
A total of 64 prairie voles were included in this study. A subset of subjects was used in other anatomical experiments, including functional mapping and neuroanatomical tracer experiments. Animals were born and housed in the UC Davis Psychology Department vivarium. These animals were descendants of a wild stock originally caught near Champaign, Illinois. The animals were pair housed in small laboratory cages (27 × 16 × 13 cm) in which food and water were available ad libitum. All animals were maintained on a 14:10-hour light/dark cycle, with lights on at 6 am. All experiments were performed under National Institutes of Health guidelines for the care of animals in research and were approved by the Institutional Animal Care and Use Committee of the University of California, Davis.
Behavioral Assessments: (HC vs LC)
Breeder pairs were observed to characterize the type and amount of parental behavior directed towards their offspring during the first several postnatal days. The observations performed are described in detail elsewhere [ 17 ]. Briefly, breeder pairs were observed four times during the P1–3, twice in the morning and twice in the afternoon. Observations lasted for 20 minutes and included maternal and paternal huddling, pseudohuddling, non-huddling contact, licking/grooming, anogenital licking/grooming, retrievals, hunching, nest building, autogrooming, and, in the mother, lateral, active, and neutral nursing. Behaviors were recorded using behavioral software ( www.behaviortracker.com ).
Rankings were calculated by summing the total amount of time the parents spent in contact with the pups to generate a parental behavior score. Scores were then ranked into quartiles, with the highest quartile becoming the high contact (HC) group, the lowest quartile becoming the low contact (LC) group, and the middle 50% of animals were excluded from analysis [ 17 ].
Twenty-one animals (5 HC females, 6 HC males, 3 LC females, and 7 LC males) were included in this experimental group. Because these subjects were exposed to different amounts of parental care over a long period of time (the first (P)20), and because differences in parental care are linked to differences in OT receptor levels [ 24 ], subjects in these groups were exposed to chronic differences in OT levels.
Pharmacological Treatments: (Saline/OT/OTA)
Forty-three animals were included in this experimental group. Within 24 hours of birth, experimental subjects were briefly removed from the cage, sexed, weighed, and toe-clipped for identification. All pups, both male and female, were randomly assigned to treatment groups, receiving an intraperitoneal injection of isotonic saline (eight males, eight females), OT (11 males received 3 µg and 3 females received 6 µg), or OTA ([d(CH 2 ) 5 , Tyr(Me) 2 , Orn 8 ]-vasotocin; five females and eight males each received 0.3 µg). OT doses were chosen based on previous studies, as the ones that would maximize facilitation of pair bonding in adult animals [ 28 , 29 ]. All injections were 25.0 µl in volume and administered via a 250 μl gas-tight Hamilton syringe. In contrast to the subjects exposed to HC or LC parenting styles, these subjects were all the offspring of medium contact parents and experienced a single acute dose of OT, OTA, or saline.
Histology
Animals were euthanized with an overdose of sodium pentobarbital (250 mg/kg, IP) and transcardially perfused with 15 ml of 0.9% saline, followed by 15 ml of 4% paraformaldehyde in phosphate buffer and then 15 ml of 4% paraformaldehyde with 10% sucrose. After perfusion, the brain was extracted and the cortex was removed from the subcortical structures. The neocortex was flattened and postfixed in 30% sucrose overnight. The flattened tissue was sectioned at 20 µm using a freezing microtome, and the resulting sections were stained for myelin [ 73 ].
Analysis
Borders were drawn identifying the boundaries of sensory regions within the neocortex. As described previously in [ 2 ], while individual sections of tissue can contain many partial anatomical boundaries, complete boundaries were obtained by combining the entire series of sections into a single comprehensive reconstruction. This was accomplished by taking photomicrographs of individual sections that were stained for myelin. All the photomicrographs for an individual case were imported into Adobe Illustrator (Adobe Systems, San Jose, CA), and sections were aligned using landmarks, including blood vessels, tissue artifacts, and the outline of the section ( Fig. 1 ). In all cases, the largest section in the series was used to define the outline of the cortical hemisphere, and the person drawing the boundaries was blind to the condition of the subject.
Once the consolidated architectonic boundaries were determined we found the area in mm 2 of the following regions: the cortical sheet (excluding the olfactory bulb, pyriform cortex, and entorhinal cortex), primary somatosensory cortex (S1), second somatosensory cortex/parietal ventral area (S2/PV), primary motor cortex (M1), primary visual cortex (V1), second visual cortex (V2), auditory cortex (AC), and FM area. We then calculated the proportion of the cortical sheet that was comprised of each of those regions, as well as the total proportion of myelinated cortical regions ( Fig. 2 ). Due to differences in the method of flattening, in some cases the cingulate cortex was visible and increased the area of the cortical sheet. In those cases, we subtracted the area of the cingulate cortex from the area of the cortical sheet and used that value as the denominator when calculating the proportion of the cortical sheet that was comprised of each region. In these cases, the resulting area of the cortical sheet was comparable to that of cases where the cingulate cortex was not visible.
In cases where both hemispheres were available, the values were averaged between hemispheres to generate a single set of data for the animal. Values were then averaged within groups. Animals that were exposed to HC and LC parenting styles were analysed separately from animals treated with OT/OTA/Saline. Differences within each group were compared using two-way analysis of variance (ANOVA; JMP, SAS, Cary, NC). Individual differences between specific groups were determined using Student’s t-tests. Effect size was calculated using Cohen’s d. For all tests, α = 0.05.
Funding
This work was supported by grants to Karen L Bales (NIH HD060117 and NIH HD071998).
Conflict of interest statement . None declared.
References
- 1. Campi KL, Bales KL, Grunewald R, Krubitzer L. Connections of auditory and visual cortex in the prairie vole (Microtus ochrogaster): evidence for multisensory processing in primary sensory areas . Cereb Cortex 2010. ; 20 : 89 – 108 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seelke AMH, Dooley JC, Krubitzer LA. The emergence of somatotopic maps of the body in s1 in rats: the correspondence between functional and anatomical organization . PLoS One 2012. ; 7 : e32322.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dawson DR, Killackey HP. The organization and mutability of the forepaw and hindpaw representations in the somatosensory cortex of the neonatal rat . J. Comp Neurol . 1987. ; 256 : 246 – 56 . [DOI] [PubMed] [Google Scholar]
- 4. Recanzone GH, Merzenich MM, Dinse HR. Expansion of the cortical representation of a specific skin field in primary somatosensory cortex by intracortical microstimulation . Cereb Cortex 1992. ; 2 : 181 – 96 . [DOI] [PubMed] [Google Scholar]
- 5. Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR. Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task . J Neurophysiol 1992. ; 67 : 1031 – 56 . [DOI] [PubMed] [Google Scholar]
- 6. Karlen SJ, Krubitzer L. Effects of bilateral enucleation on the size of visual and nonvisual areas of the brain . Cereb Cortex 2009. ; 19 : 1360 – 71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kahn DM, Krubitzer L. Massive cross-modal cortical plasticity and the emergence of a new cortical area in developmentally blind mammals . Proc Natl Acad Sci U S A 2002. ; 99 : 11429 – 434 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Leary DD, Sahara S. Genetic regulation of arealization of the neocortex . Curr Opin Neurobiol 2008. ; 18 : 90 – 100 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Armentano M, Chou SJ, Tomassy GS, Leingärtner A, O’Leary DD, Studer M. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas . Nat Neurosci 2007. ; 10 : 1277 – 86 . [DOI] [PubMed] [Google Scholar]
- 10. Bishop KM, Rubenstein JL, O’Leary DD. Distinct actions of Emx1, Emx2, and Pax6 in regulating the specification of areas in the developing neocortex . J Neurosci 2002. ; 22 : 7627 – 38 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krubitzer LA, Seelke AMH. Cortical evolution in mammals: the bane and beauty of phenotypic variability . Proc Natl Acad Sci U S A 2012. ; 109 : 10647 – 54 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karlen SJ, Krubitzer L. Phenotypic diversity is the cornerstone of evolution: variation in cortical field size within short-tailed opossums . J Comp Neurol 2006. ; 499 : 990 – 9 . [DOI] [PubMed] [Google Scholar]
- 13. Darwin C. The Origin of Species . Harmondsworth: : Penguin Books Ltd; ., 1859/1983, 477 . [Google Scholar]
- 14. Getz LL, Carter CS, Gavish L. The mating system of the prairie vole, Microtus-ochrogaster - field and laboratory evidence for pair-bonding . Behav Ecol Sociobiol 1981. ; 8 : 189 – 94 . [Google Scholar]
- 15. Thomas JA, Birney EC. Parental care and mating system of the prairie vole, Microtus-ochrogaster . Behav Ecol Sociobiol 1979. ; 5 : 171 – 86 . [Google Scholar]
- 16. Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus-ochrogaster) - the role of social and sexual experience . Hormones Behav 1992. ; 26 : 339 – 49 . [DOI] [PubMed] [Google Scholar]
- 17. Perkeybile AM, Griffin LL, Bales KL. Natural variation in early parental care correlates with social behaviors in adolescent prairie voles ( Microtus ochrogaster ) . Front Behav Neurosci 2013. ; 7 : 21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, Carter CS . Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors . Neuroscience 2007. ; 144 : 38 – 45 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kenkel WM, Paredes J, Yee JR, Pournajafi-Nazarloo H, Bales KL, Carter CS. Neuroendocrine and behavioural responses to exposure to an infant in male prairie voles . J Neuroendocrinol 2012. ; 24 : 874 – 86 . [DOI] [PubMed] [Google Scholar]
- 20. Bales KL, Carter CS. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster) . Horm Behav 2003. ; 44 : 178 – 84 . [DOI] [PubMed] [Google Scholar]
- 21. Seelke AMH, Perkeybile AM, Grunewald R, Bales KL, Krubitzer LA . Individual differences in cortical connections of somatosensory cortex are associate with parental rearing style in prairie voles (Microtus ochrogaster) . J Comp Neurol 2016. ; 524 : 564 – 77 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perkeybile AM, Bales KL. Early rearing experience is associated with vasopressin immunoreactivity but not reactivity to an acute non-social stressor in the prairie vole . Physiol Behav 2015. ; 147 : 149 – 56 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Perkeybile AM, Bales KL. Early rearing experience is related to altered aggression and vasopressin production following chronic social isolation in the prairie vole . Behav Brain Res 2015. ; 283 : 37 – 46 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perkeybile AM, Delaney-Busch N, Hartman S, Grimm KJ, Bales KL . Intergenerational transmission of alloparental behavior and oxytocin and vasopressin receptor distribution in the prairie vole . Front Behav Neurosci 2015. ; 9 : 191 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wismer Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollack SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior . Proc Natl Acad Sci U S A 2005. ; 102 : 17237 – 40 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zheng JJ, Li SJ, Zhang SD, Miao WY, Zhang D, Yao H, Yu X. Oxytocin mediates early experience-dependent cross-modal plasticity in the sensory cortices . Nat Neurosci 2014. ; 17 : 391 – 9 . [DOI] [PubMed] [Google Scholar]
- 27. Kenkel WM, Yee JR, Carter CS. Is oxytocin a maternal-foetal signalling molecule at birth? Implications for development . J Neuroendocrinol 2014. ; 26 : 739 – 49 . [DOI] [PubMed] [Google Scholar]
- 28. Bales KL, Carter CS. Developmental exposure to oxytocin facilitates partner preferences in male prairie voles (Microtus ochrogaster) . Behav Neurosci 2003. ; 117 : 854 – 9 . [DOI] [PubMed] [Google Scholar]
- 29. Bales KL, van Westerhuyzen JA, Lewis-Reese AD, Grotte ND, Lanter JA, Carter CS. Oxytocin has dose-dependent developmental effects on pair-bonding and alloparental care in female prairie voles . Horm Behav 2007. ; 52 : 274 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bales KL, Abdelnabi M, Cushing BS, Ottinger MA, Carter CS. Effects of neonatal oxytocin manipulations on male reproductive potential in prairie voles . Physiol Behav 2004. ; 81 : 519 – 26 . [DOI] [PubMed] [Google Scholar]
- 31. Campi KL, Karlen SJ, Bales KL, Krubitzer L. Organization of sensory neocortex in prairie voles (Microtus ochrogaster) . J Comp Neurol 2007. ; 502 : 414 – 26 . [DOI] [PubMed] [Google Scholar]
- 32. Kaas JH. What, if anything, is S1? Organization of first somatosensory area of the cortex . Physiol Rev 1983. ; 63 : 206 – 31 . [DOI] [PubMed] [Google Scholar]
- 33. Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys . J Neurosci 1993. ; 13 : 87 – 103 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lebedev MA, Mirabella G, Erchova I, Diamond ME. Experience-dependent plasticity of rat barrel cortex: redistribution of activity across barrel-columns . Cereb Cortex 2000. ; 10 : 23 – 31 . [DOI] [PubMed] [Google Scholar]
- 35. Erzurumlu RS, Gaspar P. Development and critical period plasticity of the barrel cortex . Eur J Neurosci 2012. ; 35 : 1540 – 53 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O’Leary DD AM., Stocker A, Zembrzycki Area patterning of the mammalian cortex . In: Rubenstein JL, Rakic P. (eds.), Patterning and Cell Type Specification in the Developing CNS and PNS: Comprehensive Developmental Neuroscience , New York: : Elsevier; , 2013. , 61 – 85 . [Google Scholar]
- 37. Francis DD, LJ Young, Meaney MJ, Insel TR . Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences . J Neuroendocrinol 2002. ; 14 : 349 – 53 . [DOI] [PubMed] [Google Scholar]
- 38. Kojima S, Stewart RA, Demas GE, Alberts JR. Maternal contact differentially modulates central and peripheral oxytocin in rat pups during a brief regime of mother-pup interaction that induces a filial huddling preference . J Neuroendocrinol 2012. ; 24 : 831 – 40 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Noctor SC, Martínez-Cerdeño V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases . Nat Neurosci 2004. ; 7 : 136 – 44 . [DOI] [PubMed] [Google Scholar]
- 40. Noctor SC, Martinez-Cerdeno V, Kriegstein AR. Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis . J Comp Neurol 2008. ; 508 : 28 – 44 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lister R, Mukamel EA, Nery JR, Urich M, Puddifoot CA, Johnson ND, Lucero J, Huang Y, Dwork AJ, Schultz MD. et al. . Global epigenomic reconfiguration during mammalian brain development . Science 2013. ; 341 : 1237905 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ypsilanti AR, Rubenstein JL. Transcriptional and epigenetic mechanisms of early cortical development: an examination of how Pax6 coordinates cortical development . J Comp Neurol 2016. ; 524 : 609 – 29 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hunt DL, Yamoah EN, Krubitzer L. Multisensory plasticity in congenitally deaf mice: how are cortical areas functionally specified? Neuroscience 2006. ; 139 : 1507 – 24 . [DOI] [PubMed] [Google Scholar]
- 44. Lomber SG, Meredith MA, Kral A. Cross-modal plasticity in specific auditory cortices underlies visual compensations in the deaf . Nat Neurosci 2010. ; 13 : 1421 – 7 . [DOI] [PubMed] [Google Scholar]
- 45. Barberis C, Mouillac B, Durroux T. Structural bases of vasopressin/oxytocin receptor function . J. Endocrinol 1998. ; 156 : 223 – 9 . [DOI] [PubMed] [Google Scholar]
- 46. Barberis C, Tribollet E. Vasopressin and oxytocin receptors in the central nervous system . Crit Rev Neurobiol 1996. ; 10 : 119 – 54 . [DOI] [PubMed] [Google Scholar]
- 47. Mouillac B, Chini B, Balestre MN, Jard S, Barberis C, Manning M, Tribollet E, Trumpp-Kallmeyer S, Hoflack J, Elands J. et al. . Identification of agonist binding sites of vasopressin and oxytocin receptors . Oxytocin 1995. ; 395 : 301 – 10 . [PubMed] [Google Scholar]
- 48. Tribollet E, Dubois-Dauphin M, Dreifuss JJ, Barberis C, Jard S. Oxytocin receptors in the central-nervous-system - distribution, development, and species-differences . Ann N Y Acad Sci 1992. ; 652 : 29 – 38 . [DOI] [PubMed] [Google Scholar]
- 49. Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles . Behav Neurosci 1995. ; 109 : 782 – 9 . [DOI] [PubMed] [Google Scholar]
- 50. Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) . J Neuroendocrinol 1994. ; 6 : 247 – 50 . [DOI] [PubMed] [Google Scholar]
- 51. Yamamoto Y, Carter CS, Cushing BS. Neonatal manipulation of oxytocin affects expression of estrogen receptor alpha . Neuroscience 2006. ; 137 : 157 – 64 . [DOI] [PubMed] [Google Scholar]
- 52. Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ . Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress . Science 1997. ; 277 : 1659 – 62 . [DOI] [PubMed] [Google Scholar]
- 53. Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat . Science 1999. ; 286 : 1155 – 8 . [DOI] [PubMed] [Google Scholar]
- 54. Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat . J Neuroendocrinol 2000. ; 12 : 1145 – 8 . [DOI] [PubMed] [Google Scholar]
- 55. Olazabal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles . Neuroscience 2006. ; 141 : 559 – 68 . [DOI] [PubMed] [Google Scholar]
- 56. Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ . Epigenetic programming by maternal behavior . Nat Neurosci 2004. ; 7 : 847 – 54 . [DOI] [PubMed] [Google Scholar]
- 57. Beery AK, McEwen LM, MacIsaac JL, Francis DD, Kobor MS. Natural variation in maternal care and cross-tissue patterns of oxytocin receptor gene methylation in rats . Horm Behav 2016. ; 77 : 42 – 52 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Alfirevic Z, Kelly AJ, Dowswell T. Intravenous oxytocin alone for cervical ripening and induction of labour . Cochrane Database Syst Rev 2009. ; CD003246 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Osterman MJ, Martin JA. Recent declines in induction of labor by gestational age . NCHS Data Brief 2014. ; 155 : 1 – 8 . [PubMed] [Google Scholar]
- 60. A.P.H.P.Q.I. Panel . Practice Brief: Clinical management guidelines for women’s health and perinatal nurses, In: O.a.N.N. Association of Women’s Health (ed.), 2014. .
- 61. Kurth L, Haussmann R. Perinatal Pitocin as an early ADHD biomarker: neurodevelopmental risk? J Atten Disord 2011. ; 15 : 423 – 31 . [DOI] [PubMed] [Google Scholar]
- 62. Gialloreti LE, Benvenuto A, Benassi F, Curatolo P. Are caesarean sections, induced labor and oxytocin regulation linked to Autism Spectrum Disorders? Med Hypotheses 2014. ; 82 : 713 – 8 . [DOI] [PubMed] [Google Scholar]
- 63. Weisman O, Agerbo E, Carter CS, Harris JC, Uldbjerg N, Henriksen TB, M Thygesen, Mortensen PB, Leckman JF, Dalsgaard S. Oxytocin-augmented labor and risk for autism in males . Behav Brain Res 2015. ; 284 : 207 – 12 . [DOI] [PubMed] [Google Scholar]
- 64. Wahl RU. Could oxytocin administration during labor contribute to autism and related behavioral disorders? A look at the literature . Med Hypotheses 2004. ; 63 : 456 – 60 . [DOI] [PubMed] [Google Scholar]
- 65. Guastella AJ, Gray KM, Rinehart NJ, Alvares GA, Tonge BJ, Hickie IB, Keating CM, Cacciotti-Saija C, Einfeld SL . The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: a randomized controlled trial . J Child Psychol Psychiatry 2015. ; 56 : 444 – 52 . [DOI] [PubMed] [Google Scholar]
- 66. Campi KL, Collins CE, Todd WD, Kaas J, Krubitzer L. Comparison of area 17 cellular composition in laboratory and wild-caught rats including diurnal and nocturnal species . Brain Behav Evol 2011. ; 77 : 116 – 30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Calisi RM, Bentley GE. Lab and field experiments: are they the same animal? Horm Behav 2009. ; 56 : 1 – 10 . [DOI] [PubMed] [Google Scholar]
- 68. Fleming SM, Weil RS, Nagy Z, Dolan RJ, Rees G. Relating introspective accuracy to individual differences in brain structure . Science 2010. ; 329 : 1541 – 3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians . J Neurosci 2003. ; 23 : 9240 – 5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Holmes AJ, Lee PH, Hollinshead MO, Bakst L, Roffman JL, Smoller JW, Buckner RL . Individual differences in amygdala-medial prefrontal anatomy link negative affect, impaired social functioning, and polygenic depression risk . J Neurosci 2012. ; 32 : 18087 – 100 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective . Psychon Bull Rev 2002. ; 9 : 637 – 71 . [DOI] [PubMed] [Google Scholar]
- 72. Leonard C, Eckert M, Given B, Virginia B, Eden G. Individual differences in anatomy predict reading and oral language impairments in children . Brain 2006. ; 129 : 3329 – 42 . [DOI] [PubMed] [Google Scholar]
- 73. Gallyas F. Silver staining of myelin by means of physical development . Neurol Res 1979. ; 1 : 203 – 9 . [DOI] [PubMed] [Google Scholar]