Abstract
The central mission for the development of the National Institute of Standards and Technology/National Institutes of Health/Environmental Protection Agency Mass Spectral Library is the acquisition of reference gas chromatography–mass spectrometry data for important compounds and their chemical modification products. The addition of reliable reference data of various derivatives of amino acids to The Library, and the study of their behavior under electron ionization conditions may be useful for their identification, structure elucidation, and a better understanding of the data obtained when the same derivatives are subjected to other ionization methods. N-Alkyl-N-perfluoroacyl derivatives of amino acids readily produce previously unreported alkylnitrilium cations of composition [HC≡N-alkyl]+. Homologous [HC≡N-aryl]+ cations are typical for corresponding N-aryl analogs. The formation of other ions characteristic for these derivatives involves oxygen rearrangement giving rise to ions [CnF2n+1–C≡N+–CnH2n+1] and [CnF2n+1–C≡N+-aryl]. The introduction of an N-benzyl substituent in a molecule favors a process producing benzylidene iminium cations. l-Threonine and l-cysteine derivatives exhibit more fragmentation pathways not typical for other α-amino acids; additionally, the Nω-amino group in l-lysine directs the dissociation process and provides structural information on the substitution at the amino functions in the molecule.
Keywords: amino acids, alkyl/perfluoroacyl derivatives, electron ionization, mass spectrometry, nitrilium ions
Introduction
Amino acids – the basic structural units of peptides and proteins – are important objects of analysis in metabolomics because of their critical functions in energy metabolism, neurotransmission, and lipid transport. Mass spectrometry (MS) and nuclear magnetic resonance spectroscopy are the core technologies used for the study of amino acids and peptides, both analytical techniques producing complementary structural information. Routine analysis of amino acids, peptides, and their chemical modification products by electron ionization (EI) began with the initial development of analytical organic MS,1–17 and were later enhanced by utilization of new ionization methods.18–28 Amino acid physical and chemical properties can be altered by derivatization to reduce their sensitivity to catalytic or thermal decomposition. Reactive functional groups can become more stable, more accessible to EI, and favor fragmentation pathways more useful for structure elucidation upon EI. Consequently, various derivatization methods have been employed for blocking amino, hydroxyl, mercapto, and carboxyl functions to produce alkyl, acyl, and trialkylsilyl derivatives, alkyl and aryl formates, and more. However, systematic studies of certain chemical derivatives in these series have not been performed. Consequently, reliable gas chromatography– mass spectrometry (GC-MS) reference data are not available. Further development of the chemical modification methods for amino acids and their residues and the study of their behavior under numerous ionization techniques remain an active area of investigation. Reference data are required to identify compounds in biological research and, thus, the acquisition of reference GC-MS data for compounds like amino acid derivatives remains the mission for the development of the National Institute of Standards and Technology/National Institutes of Health/Environmental Protection Agency Mass Spectral Library (The Library).29 The process of evaluating data for The Library requires assessing the consistency between a spectrum and a structure using ion thermochemistry, along with the known ion-fragmentation rules. Unexplained peaks are identified, followed by their thorough analysis to reconcile their formation. The proposal of unusual fragmentation processes specific for certain compounds requires additional compound and spectral analyses. Therefore, the National Institutes of Health Mass Spectrometry Data Center reports the results of an extensive EI study regarding the behavior of amino acid derivatives with partially and completely blocked functional groups by alkyl and perfluoroacyl groups. The identification and probable intermediacy of nitrilium cations is a key finding of this investigation.
Experimental
Materials
Glycine, l-alanine, l-phenylalanine, β-alanine, l-valine, l-leucine, l-isoleucine, l-serine, l-threonine, l-cysteine, l-methionine, l-aspartic acid, l-glutamic acid, l-lysine, methyl esters of N-benzylglycine and N-tritylglycine, 2-13C- and 3,3-dideuterol-alanines, 2-methyl-N-(4-methylphenyl)-l-alanine, methyl ester of N-benzyl-l-alanine, 2-methyl-N-benzyl-β-alanine,15N-l-phenylalanine, methyl ester of N-benzyl-l-phenylalanine, N-benzyl-l-valine methyl ether, ethyl ester of N-trityl-l-leucine, methyl esters of O-tert-butyl- and N-trityl-l-serines, methyl ester of O-tert-butyl-l-threonine, methyl ester of S-benzyl-l-cysteine, methyl ester of N-benzyl-l-methionine and dimethyl ester of l-glutamic acid were commercially available. Acylating reagents (trifluoroacetic, pentafluoropropionic and heptafluorobutyric anhydrides), as well as alkylating reagents (methyl, trideuteromethyl, ethyl, propyl, butyl and pentyl iodides), solvent (anhydrous acetonitrile and powdered sodium hydroxide) were also commercially available.
Microsynthesis
Several methods of derivatization30–33 were used. Initial perfluoroacylation followed by alkylation/esterification is best suited for amino acid polyhydrates, whereas alkylation followed by esterification may be preferable for “dry” amino acids. Procedures 1 and 2 (below) have been employed for the synthesis of alkyl esters/ethers of N-alkyl-N-perfluoroacyl amino acids. In addition, authentic samples of derivatives were prepared on the basis of partially alkylated or acylated amino acids. All N-aryl amino acids were the initial chemicals for the synthesis of N-aryl-N-acyl derivatives.
Procedure 1
Perfluoroacyl anhydride (ratio 1:5) was gradually added to a suspension of 1 µmol of amino acid in 30 µL of acetonitrile at 5°C. The mixture was stirred for 15 min, and analyzed by GC-MS. To accomplish alkylation, powdered NaOH and alkyl iodide (1 : 10 molar ratio) were added gradually to the previous mixture at 5°C, stirred for 15 min, and then the reaction mixture was analyzed by GC-MS after NaOH removal.
Procedure 2
The reaction conditions were the same as above, only the sequence was reversed: acylation was followed by alkylation.
Instrumentation
EI mass spectra were recorded on GC-MS systems with quadrupole analyzers (ionization energy 70 eV and ion source temperature 230°C), as previously described.34 Separation was achieved on a fused silica capillary column (30 m, 0.25 mm internal diameter, and nonpolar stationary liquid phase of polymethylsiloxane + 5% phenyl groups) with programmed oven temperature from 60°C to 270°C at a rate of 5 C min−1; the injection temperature was 230°C.GC tandem mass spectrometry (MS/MS) data were obtained at 2, 5, 10, and 17 eV collision energies on systems with quadrupole analyzers using nitrogen as the collision-induced dissociation gas.
Data analysis
The data evaluation is based on a comparison with the spectra of corresponding13C-,15N-, and2H-labeled analogs and N-alkyl and N-perfluoroacyl homologs.
Results and discussion
For N-alkyl-N-perfluoroacyl-α-amino acids and their alkyl esters, characteristic EI-induced cleavages of C(2)–C(1), C(2)–C(3), and C(2)–N bonds remain typical; sometimes these cleavages are accompanied by hydrogen rearrangement. Mass values of ions that result from the loss of carboxyl radicals or water molecules can be used for determination of the molecular weights of acids; ions resulting from the elimination of neutral alkanol and the alkyloxy and alkoxycarbonyl radicals from the ester functions are useful for the determination of M+• of esters when peaks of M+• are absent in the spectra. The fragmentation patterns observed in this work are similar to those reported earlier for homologous butyl esters of amino acids;35 the spectra of all the derivatives studied will be included in the next public release of The Library.
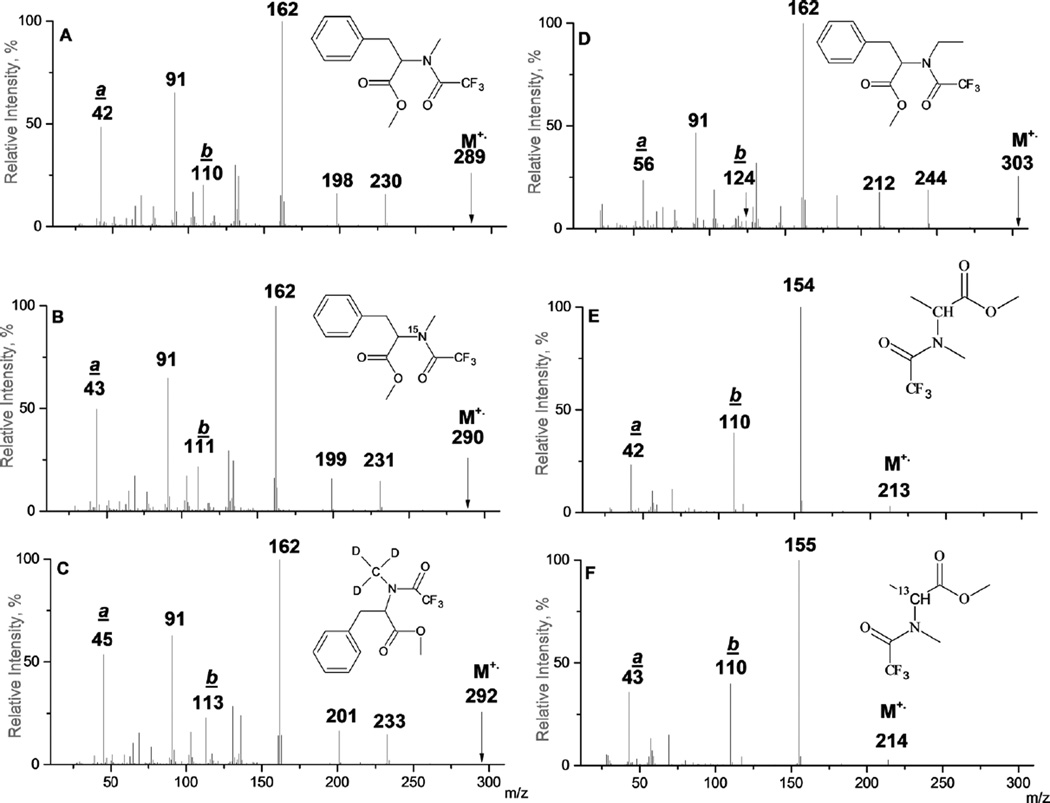
There are two types of ions, which most likely have structures of nitrilium cations [Figure 1 (a)–(f)] and apparently have been overlooked in earlier publications that are specific for all N-alkyl-N-perfluoroacyl-α-aminoalkanoic acids and their alkyl esters.
Figure 1.
EI mass spectra of methyl esters of N-trifluoroacetyl- derivatives of N-methyl-l-phenylalanine (a),15N-methyl-l-phenylalanine(b), N-trideuteromethyl-l-phenylalanine (c), N-ethyl-l-phenylalanine (d), N-methyl-l-alanine (e), and 2-13C-N-methyl-l-alanine (f).
The first type is a product of multiple cleavages and has a composition [HC≡N–CH3]+ and mass value 42 (ion a) in the spectra of N-trifluoroacetyl (TFA) derivatives of N-methyl-l-phenylalanines and N-methyl-l-alanines [Figure 1(a) and (e)]. This ion has not been discussed in prior reports of amino acid mass spectrometric fragmentation. Mass shifts of 1 Da are observed in the spectra of 2-13C- and15N-labeled compounds [Figure 1(f)] and (Figure 1(b), respectively], indicating the presence of a C(2)- and an amino N-atom in the ion a. This ion also includes the methyl group connected to nitrogen as demonstrated by a 3 Da increase (from 42 Da to 45 Da) in the case of the N-trideuteromethyl analog [Figure 1(c)], and by a 14 Da increase (to m/z 56) when N-methyl is replaced by an ethyl group [Figure 1(d)]. The remaining question is whether the hydrogen resident at C(2) remains. The introduction of a substituent at the C(2)-atom does not affect the formation of ion a. The presence of high-intensity peaks of ions of type a ([CH3–C≡N–C6H4CH3]+; 132 Da) in the spectrum of the methyl ester of 2-methyl-N-p-tolyl-N-TFA-l-alanine [Figure 2(a)] may suggest that the hydrogen atom at C(2) remains intact in the process of ion a formation.
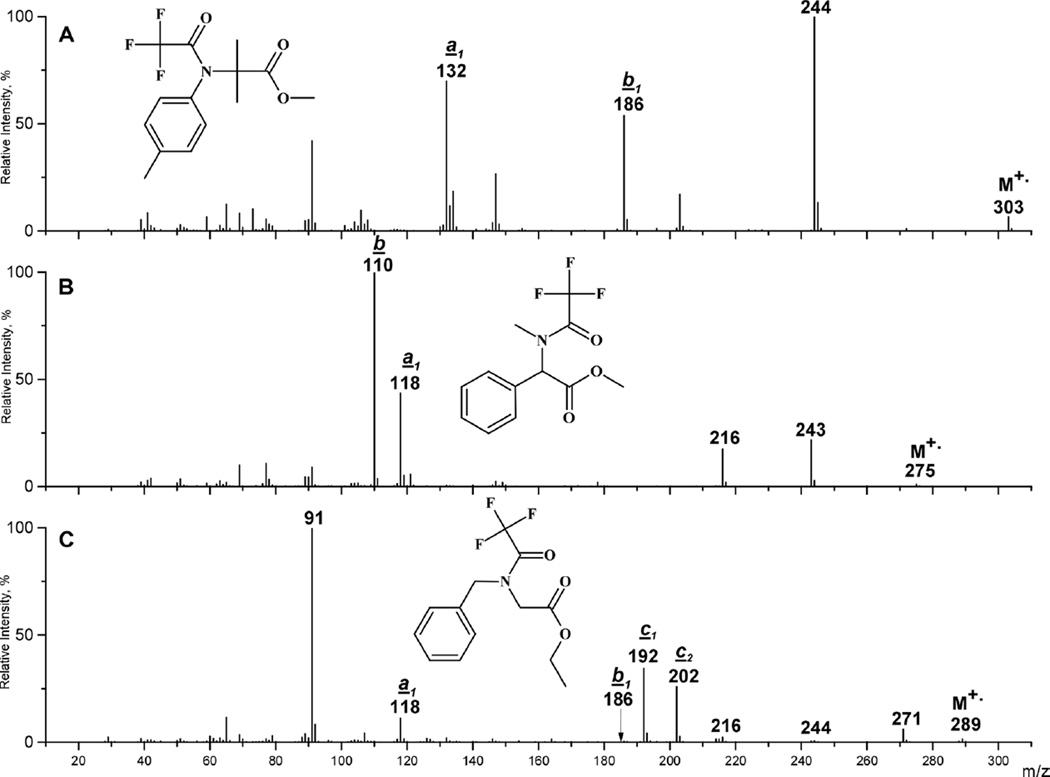
Figure 2.
EI mass spectra of N-TFA derivatives of 2,O-dimethyl-N-p-tolyl-l-alanine (a), (S)-(+)-2-phenyl-N,O-dimethyl-l-glycine (b) and N-benzyl-O-ethyl-l-glycine (c).
The second characteristic type of ion is formally a product of a rearrangement process involving migration of carbonyl oxygen at the amino group to C(2) followed by C(2)–N-cleavage, with the charge localization at a nitrilium N-atom (ion b, [CnF2n + 1–C≡N+–CnH2n + 1]). Peaks at m/z 110 with elemental composition C3H3NF3 were reported to be characteristic of n-butyl esters of N-methyl-N-TFA-derivatives of six α-amino acids along with some β- and γ-amino acids; however, the presence of this ion has not been observed in any non-N-methylated N-TFA amino acids.35
This ion and its homologs were investigated with the use of isotope-labeled amino acids. In the spectra of N-methylN-TFA alanines [Figure 1(e) and (f)], peaks of the ion [CF3C≡NCH3]+ at m/z 110 are prominent. For N-methyl-N-TFA-l-phenylalanine, 1 Da and 3 Da mass shifts are observed in the spectra of15N- and N-CD3 analogs [Figure 1(b), m/z 111 and Figure 1(c), m/z 113]. Homologs of b ions [C2F5C≡NCH3]+(m/z 160) and [C3F7C≡NCH3]+ (m/z 210) are characteristic for N-pentafluoropropionyl (PFP) and N-heptafluorobutyryl (HFB) derivatives.
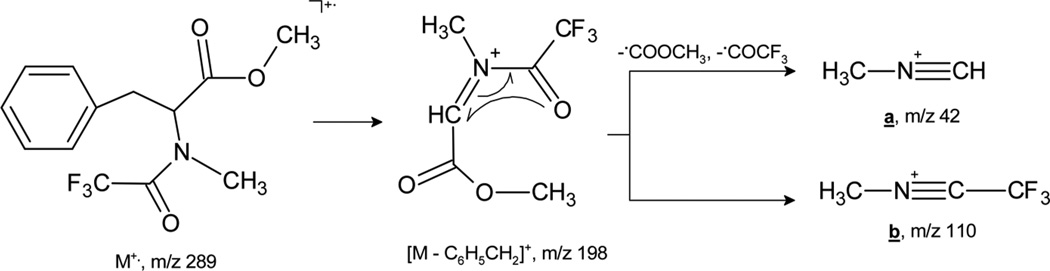
Type a and b ions are products of complex decomposition processes. Table 1 represents major routes for their formation in the cases of l-phenylalanine, l-methionine, l-aspartic acid, and l-lysine derivatives, and Table 2 summarizes the intensities of nitrilium cations in the spectra. General fragmentation patterns leading to the formation of nitrilium cations a and b in the case of the l-phenylalanine derivative are presented in Scheme 1.
Table 1.
Major routes for the formation of types a and b ions according to MS-MS data.
| Amino acid derivative | Ion a | Ion b |
|---|---|---|
|
l-Phenylalanine, N-methyl-N- TFA-, methyl ester |
[M–C6H5CH2–COOCH3–COCF3]+ | [M–C6H5CH2–OCHCOOCH3]+ |
|
l-Methionine, N-methyl-N- TFA-, methyl ester |
[M–CH3SCHCH2–CH3OH- CO–COCF3]+ [M–COOCH3–HSCH3–COCF3–C3H3]+ [M–SCH3–HCOOCH3–HSCH3–COCF3-C3H3]+ |
[M–COOCH3–HSCH3–C3H4O]+ |
|
l-Aspartic acid, N-methyl-N- TFA-, dimethyl ester |
[M–COCF3–HCOOCH3–C3H3O3]+ | [M–COOCH3–CH3OH–C3H3O2]+ [M–COOCH3–CH2CO–C2H4O2]+ |
|
l-Lysine, N,N′-Dimethyl-N,N′- di(TFA), methyl ester |
[M–CH3OH–N(CH3)COCF3C4H7–CO–COCF3]+ | [M–CH3OH–N(CH3)COCF3C4H7–C2HO2]+ [M–COCF3–HCOOCH3–CH3N C2H4]+ |
Table 2.
Relative intensities of peaks of ions a ([HC≡N–CH3]+, m/z 42) and b ([CF3–C≡N+–CH3], m/z 110) in the spectra of methyl esters of N-methyl-N-TFA derivatives of l-glycine (I), l-alanine (II), l-phenylalanine (III), l-valine (IV), l-leucine (V), l-isoleucine (VI), l-serine (VII), l-threonine (VIII), l-cysteine (IX), l-methionine (X), l-lysine (XI), l-glutamic acid (XII) and l-aspartic acid (XIII).
| Compound | Im/z42 | Im/z110 | Compound | Im/z42 | Im/z110 | Compound | Im/z42 | Im/z110 |
|---|---|---|---|---|---|---|---|---|
| I | 22 | 33 | VI | 37 | 24 | XI | 29 | 31 |
| II | 24 | 39 | VII | 16 | 14 | XII | 14 | 29 |
| III | 48 | 20 | VIII | 50* | 7 | XIII | 90 | 69 |
| IV | 52 | 45 | IX | 33 | 16 | |||
| V | 44 | 31 | X | 34 | 14 |
May contain a contribution from an oxonium ion fragment.
Scheme 1.
Formation of ions a and b from M+• of N-methyl-N-trifluoroacetyl-l-phenylalanine.
Nitrilium cations (b1) are specific for N-aryl-N-perfluoroacyl α-amino acids when an aromatic carbon atom is directly connected to the nitrogen. Thus, a high-intensity peak of N-tolylacetonitrile cation at m/z 186 (55%) is observed in the spectrum of the methyl ester of N-p-tolyl-N-TFA-2-methyl-l-alanine [Figure 2(a)]. An aromatic ring may also stimulate the formation of ions of type a1 and b1 when it is introduced to C(2), as seen in the spectrum of 2-phenyl-N-methyl-N-TFA-glycine methyl ester [Figure (2(b)] where high-intensity peaks at m/z 118 (100%) and 110 (48%) are present.
Oxygen rearrangements leading to nitrilium cations have been observed previously for other compounds as well. Thus, N-alkyl-C-phenylnitrilium cations are the base peaks in the EI spectra of N-alkylsulfabenzamides;34 it was established that the mechanism involves an ipso attack of the amide oxygen and further fragmentation to the thermodynamically or kinetically favored products.36 Type a ions were observed in the EI spectra of N-methyl-N-TFA-amphetamines and a four-centered mechanism of oxygen migration has been suggested.37 The formation of nitrilium ions with a structure of a C-alkyl-N-phenylnitrilium cation have been observed in the electrospray ionization spectra of amides of N-acetylglycine and -alanine, and a mechanism for their formation has been proposed.38
Unlike N-phenyl compounds, the introduction of a benzyl radical at the amino function diminishes the formation of nitrilium cations and substitutes different fragmentation alternatives because of the presence of benzyl H-atoms and the ability to produce stable benzylidene iminium cations (c, [C6H5CH=NHR]+). Thus, iminium cations c (c1 and c2) along with benzyl cations (m/z 91) dominate in the spectrum of the methyl ester of N-benzyl-N-TFA-glycine [Figure 2(c)] whereas ions of type a and b are significantly reduced. Benzyl H-atoms also facilitate an additional fragmentation alternative, leading to the loss of a neutral water molecule from M+•.
Below are briefly described specific fragmentation patterns and diagnostically important ions typical for certain subclasses: (1) the “simple” amino acids, such as glycine and α- and phenyl-alanines, (2) amino acids with branched alkyl chains (valine, leucine, and isoleucine), and (3) amino acids with additional functional groups such as serine, threonine, cysteine, methionine, aspartic, and glutamic acids and lysine.
Only the glycine derivative with no substituents at C(2), the simplest representative of the class of amino acids, can produce the radical cation [M–CO2]+.under EI as a result of the interaction of alkoxycarbonyl and perfluoroacyl moieties. These [M–CO2]+• ions are prominent in the spectra of N-methyl-N-TFA-l-glycine methyl esters and their ethyl and PFP and HFP analogs, and readily eliminate a molecule of water. A similar unique behavior was reported earlier39 for N-TFA-glycine methyl ester when compared to its alanine, phenylalanine, and aspartic acid analogs.
The distinguishable decomposition under the EI of derivatives of amino acids with branched alkyl chains, such as l-valine, l-leucine, and l-isoleucine, result from the formation of iminium cations [HN(CH3)=C(CF3)OH]+ at 128 Da for their corresponding N-methyl-N-TFA acids and methyl esters. In the spectra of N-PFP- and N-HFB-homologs the peaks at m/z 178 and m/z 228, respectively, confirm the presence of perfluoroacyl moieties in the ions.
Incorporation of a hydroxyl function in the molecules of l-serine and l-threonine complicates the mass spectra for their derivatives by generating major competing processes caused by the fragmentation-directing effects of the alkanol moiety.
The molecular ion of N,O-dimethyl-N-TFA-threonine methyl ester eliminates a carbomethoxy radical and a molecule of methanol to produce the base peak in the spectrum. Replacement of O-methyl with O-tert-butyl- or O-TFA changes the primary fragmentation processes. Decomposition of the M+• of N-methyl-O-tert-butyl-N-TFA-threonine mainly starts with the alcohol function, producing the [M–C4H9]+, [M–OC4H9]+, and [M–C4H9OCH(CH3)]+ ions; the loss of the carbomethoxy radical is observed only from the [M–OC4H9]+ fragment. In the molecular ion of the O-TFA-derivative, both the acidic and alcoholic moieties participate in the primary fragmentation, and the [M–C(CH3)OC(O)CF3]+ ion is the base peak in the spectrum.
The EI-induced fragmentation of l-cysteine and l-methionine N-methyl-N-TFA derivatives is affected by the sulfur atom generating competing processes of decomposition. In both cases, peaks of thionium ions [CH3S=CH2]+ (m/z 61) of significant intensity are observed along with type a and b ions. In addition, N-methyl-N-TFA-l-cysteine can readily produce the methylthioacrylic acid radical cation as a result of elimination of neutral trifluoroacetamide; the corresponding methyl ester eliminates the neutral N-methyltrifluoroacetamide molecule with the formation of methyl methylthioacrylate radical cations. Cleavage of C(2)–N does not appear typical for the same kind of derivatives of a homologous l-methionine.
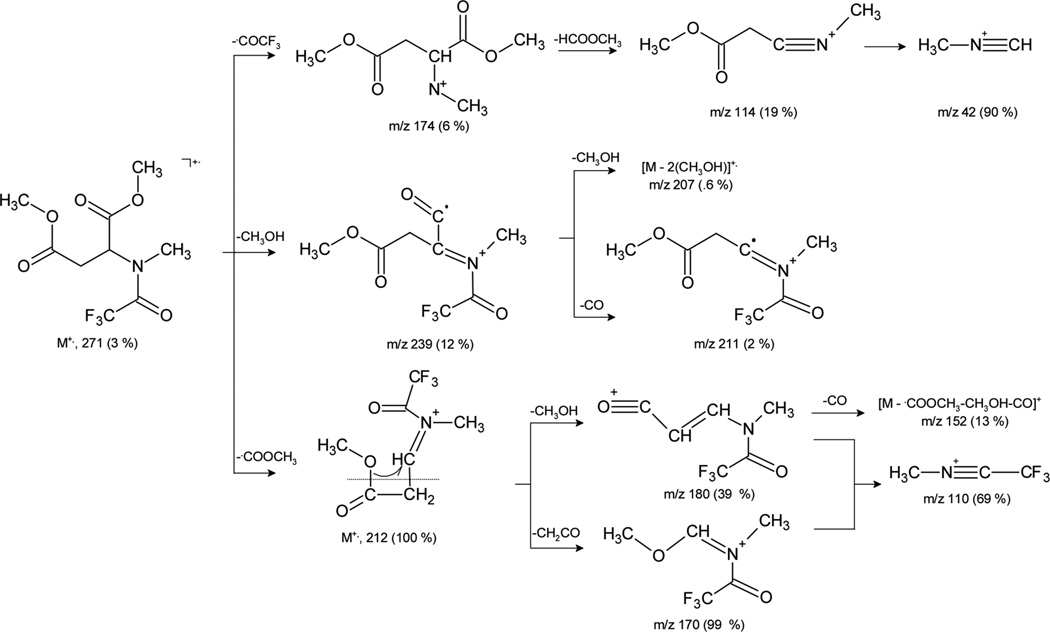
The decomposition of molecular ions of dimethyl esters of l-aspartic and l-glutamic acid derivatives proceeds via the loss of carbomethoxy or TFA radicals and methanol elimination. The resulting cations undergo hydrogen and skeletal rearrangement processes giving rise to the ions presented in Scheme 2.
Scheme 2.
Fragmentation of dimethyl ester of N-methyl-N-trifluoroacetyl-l-aspartic acid.
The ω-amino group in l-lysine is distant from the amino acid moiety and therefore does not participate in analogous fragmentation processes, as described above for N-methylN-TFA-amino acid derivatives. However, the ω-amino group directs fragmentation, clarifying the interpretation of fragmentation and the determination of substitutions at N-atoms: the base peak at 140 Da that is typical for Nω-methyl-Nω-TFA derivatives and 180 Da for Nω-TFA derivatives (Table 3).
Table 3.
Characteristic ions [m/z (relative intensity)] in the spectra of methyl esters of N,N′-di(trifluoroacetyl)- (A), N-α -methyl-N,N′di(trifluoroacetyl)- (B), N-methyl-N,N′-di(trifluoroacetyl)- (C) and N,N′-dimethyl-N,N′-di(trifluoroacetyl)- (D)-l-lysine.
| Ions | A | B | C | D |
|---|---|---|---|---|
| M+• | 352 (0) | 366 (0.3) | 366 (0.4) | 380 (0.5) |
| [M–CH3OH]+ • | 320 (7) | 334 (1) | 334 (11) | 348 (8) |
| [M–COOCH3]+ | 293 (4) | 307 (3) | 307 (3) | 321 (26) |
| [M–COCF3]+ | 255 (0.1) | 269 (0.4) | 269 (3) | 283 (3) |
| [M–COOCH3–HN(CH3)COCF3]+ | 180 (100) | 180 (100) | 180 (20) | 194 (17) |
| [HN(COCF3)=CH2]+ | 126 (27) | 126 (15) | 126 (4) | 126 (3) |
| [CH3N(COCF3)=CH2]+ | 140 (3) | 140 (12) | 140 (100) | 140 (100) |
| [HC≡N–CH3]+ (a) | 42 (2) | 42 (17) | 42 (10) | 42 (29) |
| [CF3–N≡C–CH3]+ (b) | 110 (2) | 110 (15) | 110 (6) | 110 (31) |
| [C5H7]+ | 67 (19) | 67 (20) | 67 (16) | 67 (23) |
| [CF3]+ | 69 (18) | 69 (14) | 69 (19) | 69 (19) |
Conclusion
In summary, nitrilium ions of type a [R1–C≡N–R]+ and b [CnF2n + 1–C≡N+–CnH2n + 1] are characteristic for N-alkyl-N-perfluoroacyl-amino acids and their alkyl esters. These ions are also typical for N-aryl analogs when the amino N-atom is directly connected to an aromatic carbon. In addition, the presence of an aromatic ring at N or C(2) enhances the formation of ions of type a and b. Iminium cations become specific for N-benzyl-N-alkyl-α-amino acids because of the production of stable benzylidene iminium cations c (C6H5CH=NHR]+) with a diminishing formation of ions a and b.
The decomposition of alkyl-perfluoroacyl-amino acids and their derivatives with the formation of nitrilium cations may be particularly useful to a better understanding of the origin of toxic cyanides in the environment40,41 and in prebiotic chemistry.42,43
Acknowledgments
We thank Dr Sanford Markey and Dr Jacolin A. Murray for useful reviews of the manuscript.
References
- 1.Jones JH. The mass spectra of amino acid and peptide derivatives. Q. Rev. Chem. Soc. 1968;22:302. doi: 10.1039/j39680001011. doi: http://dx.doi.org/10.1039/qr9682200302. [DOI] [PubMed] [Google Scholar]
- 2.Shemyakin MM. Primary structure determination of peptides and proteins by mass spectrometry. Pure Appl. Chem. 1968;17:313. doi: http://dx.doi.org/10.1351/pac196817030313. [Google Scholar]
- 3.Lederar E. Mass spectrometry of natural and synthetic peptide derivatives. Pure Appl. Chem. 1968;17:489. doi: http://dx.doi.org/10.1351/pac196817030489. [Google Scholar]
- 4.Jonstone RAW, Rose ME. In: Chemistry and Biochemistry of the Amino Acids. Barrett GC, editor. London: Chapman and Hall; 1985. p. 480. doi: http://dx.doi.org/10.1007/978-94-009-4832-7_17. [Google Scholar]
- 5.Zaikin VG, Sysoev AA. Mass spectrometry in Russia. Eur. J. Mass Spectrom. 2013;19:399. doi: 10.1255/ejms.1248. doi: http://dx.doi.org/10.1255/ejms.1248. [DOI] [PubMed] [Google Scholar]
- 6.Bouchoux G. Gas phase basicities of polyfunctional molecules. Part 3. Amino acids. Mass Spectrom. Rev. 2012;31:391. doi: 10.1002/mas.20349. doi: http://dx.doi.org/10.1002/mas.20349. [DOI] [PubMed] [Google Scholar]
- 7.Zaikin VG, Mikaia AI. Reaction gas chromatography–mass spectrometry. Mass Spectrom. Rev. 1990;9:115. doi: http://dx.doi.org/10.1002/mas.1280090105. [Google Scholar]
- 8.Paizs B, Suhai S. Fragmentation pathways of protonated peptides. Mass Spectrom. Rev. 2005;24:508. doi: 10.1002/mas.20024. doi: http://dx.doi.org/10.1002/chin.200621280. [DOI] [PubMed] [Google Scholar]
- 9.Andersson C-O. Mass spectrometric studies on amino acid and peptide derivatives. Acta Chem. Scand. 1958;12:1353. doi: http://dx.doi.org/10.3891/acta.chem.scand.12-1353a. [Google Scholar]
- 10.Weygard F, Geiger H, Swodenk W. Trennung von Aminosauren, Dipeptiden und Tripeptiden durch Vacuumsublimation der N-trifluoracetylierten Ester. N-trifluoroacetylierte-aminosauren, VII. Mitt. Angew. Chem. 1956;68:307. doi: http://dx.doi.org/10.1002/ange.19560680816. [Google Scholar]
- 11.Simpson JT, Torok DS, Markey SP. Pentafluorobenzyl chloroformate derivatization for enhancement of detection of amino acids or alcohols by electron capture negative ion chemical ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 1995;6:525. doi: 10.1016/1044-0305(95)00231-2. doi: http://dx.doi.org/10.1016/1044-0305(95)00231-2. [DOI] [PubMed] [Google Scholar]
- 12.Leimer KR, Rice RH, Gehrke CW. Complete mass spectra of the per-trimethylsilylated amino acids. J. Chromatogr. A. 1977;141:355. doi: 10.1016/s0021-9673(00)99131-3. doi: http://dx.doi.org/10.1016/s0021-9673(00)93539-8. [DOI] [PubMed] [Google Scholar]
- 13.Biemann K, Seibl J, Gapp F. Mass spectra of organic molecules. I. Ethyl esters of amino acids. J. Am. Chem. Soc. 1961;83:3795. doi: http://dx.doi.org/10.1021/ja01479a016. [Google Scholar]
- 14.Junk G, Svec H. The mass spectra of alpha-amino acids. J. Am. Chem. Soc. 1963;85:839. doi: http://dx.doi.org/10.2172/12645908. [Google Scholar]
- 15.Low GK-C, Duffield AM. Positive and negative chemical ionization mass spectra of amino acid carboxy-n-butyl ester N-pentafluoropropionate derivative. Biomed. Mass Spectrom. 1984;11:223. doi: http://dx.doi.org/10.1002/bms.1200110506. [Google Scholar]
- 16.Gelpi E, Koenig WA, Gibert J, Oro J. Combined gas chromatography–mass spectrometry of amino acid derivatives. J. Chromatogr. Sci. 1969;7:604. doi: http://dx.doi.org/10.1093/chromsci/7.10.604. [Google Scholar]
- 17.Stalling DL, Gehrke CW, Zuwalt R. A new silylation reagent for amino acids bis(trimethylsilyl)trifluoroacetamide (BSTFA. Biochem. Biophys. Res. Commun. 1968;31:616. doi: 10.1016/0006-291x(68)90523-8. doi: http://dx.doi.org/10.1016/0006-291x(68)90523-8. [DOI] [PubMed] [Google Scholar]
- 18.Zaikin V, Halket J. A Handbook of Derivatization for Mass Spectrometry. Chichester: IM Publications; 2009. [Google Scholar]
- 19.Revelsky IA, Yashin YS, Sobolevsky TG, Revelsky AI, Miller B, Oriedo V. Electron ionization and atmospheric pressure photochemical ionization in gas chromatography–mass spectrometry analysis of amino acids. Eur. J. Mass Spectrom. 2003;9:497. doi: 10.1255/ejms.581. doi: http://dx.doi.org/10.1255/ejms.581. [DOI] [PubMed] [Google Scholar]
- 20.Villas-Boas SG, Delicado DG, Akesson M, Nielsen J. Simultaneous analysis of amino and nonamino organic acids as methyl chloroformate derivatives using gas chromatography–mass spectrometry. Anal. Biochem. 2003;322:134. doi: 10.1016/j.ab.2003.07.018. doi: http://dx.doi.org/10.1016/j.ab.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Zampolli MG, Basaglia G, Dondi F, Sternberg R, Szopa C, Pietrogrande MC. Gas chromatography – mass spectrometry of amino acid enantiomers as methyl chloroformate derivatives: application to space analysis. J. Chromatogr. A. 2007;1150:162. doi: 10.1016/j.chroma.2006.12.033. doi: http://dx.doi.org/10.1016/j.chroma.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 22.Kaspar H, Dettmer K, Gronwald W, Oefner P. Automated GC-MS analysis of free amino acids in biological fluids. J. Chromatogr. B. 2008;870:222. doi: 10.1016/j.jchromb.2008.06.018. doi: http://dx.doi.org/10.1016/j.jchromb.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Mudiam MKR, Ch R, Jain R, Saxena PN, Chauhan A, Murthy RC. Rapid and simultaneous determination of twenty amino acids in complex biological and food samples by solid-phase microextraction and gas chromatography–mass spectrometry with the aid of experimental design after ethyl chloroformate derivatization. J. Chromatogr. B. 2012;907:56. doi: 10.1016/j.jchromb.2012.08.035. doi: http://dx.doi.org/10.1016/j.jchromb.2012.08.035. [DOI] [PubMed] [Google Scholar]
- 24.Ohie T, Fu X-W, Iga M, Kimura M, Yamaguchi S. Gas chromatography–mass spectrometry with tertbutyldimethylsilyl derivatization: use of the simplified sample preparations and automated data system to screen organic acidemias. J. Chromatogr. B. 2000;746:63. doi: 10.1016/s0378-4347(00)00105-5. doi: http://dx.doi.org/10.1016/s0378-4347(00)00105-5. [DOI] [PubMed] [Google Scholar]
- 25.Qiu Y, Su M, Liu Y, Chen M, Gu J, Zhang J, Jia W. Application of ethyl chloroformate derivatization for gas chromatography–mass spectrometry based metabonomic profiling. Anal. Chim. Acta. 2007;583:277. doi: 10.1016/j.aca.2006.10.025. doi: http://dx.doi.org/10.1016/j.aca.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 26.Kvitvang HFN, Andreassen T, Adam T, Villas-Boas SG, Bruheim P. Highly sensitive GC-MS/MS method for quantitation of amino and nonamino organic acids. Anal. Chem. 2011;83:2705. doi: 10.1021/ac103245b. doi: http://dx.doi.org/10.1021/ac103245b. [DOI] [PubMed] [Google Scholar]
- 27.Namera A, Yashiki M, Nishida M, Kojima T. Direct extract derivatization for determination of amino acids in human urine by gas chromatography and mass spectrometry. J. Chromatogr. B. 2002;776:49. doi: 10.1016/s1570-0232(02)00075-2. doi: http://dx.doi.org/10.1016/s1570-0232(02)00075-2. [DOI] [PubMed] [Google Scholar]
- 28.Villas-Boas SG, Smart KF, Sivakumaran S, Lane GA. Alkylation or silylation for analysis of amino and non-amino organic acids by GC-MS? Metabolites. 2011;1:3. doi: 10.3390/metabo1010003. doi: http://dx.doi.org/10.3390/metabo1010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.NIST/NIH/EPA Mass Spectral Library, Standard Reference Database NIST 11, Standard Reference Data Program. Gaithersburg, Maryland: National Institute of Standards and Technology; 2014. [Google Scholar]
- 30.Knapp DR. Handbook of Analytical Derivatization Reactions. Chichester: Wiley; 1979. [Google Scholar]
- 31.Zaikin VG, Halket J. Derivatization in mass spectrometry. 2. Acylation. Eur. J. Mass Spectrom. 2003;9:421. doi: 10.1255/ejms.576. doi: http://dx.doi.org/10.1255/ejms.576. [DOI] [PubMed] [Google Scholar]
- 32.Zaikin VG, Halket J. Derivatization in mass spectrometry. 3. Alkylation(arylation) Eur. J. Mass Spectrom. 2004;10:1. doi: 10.1255/ejms.619. doi: http://dx.doi.org/10.1255/ejms.619. [DOI] [PubMed] [Google Scholar]
- 33.Zaikin VG, Halket J. Derivatization in mass spectrometry. 6. Formation of mixed derivatives of polyfunctional compounds. Eur. J. Mass Spectrom. 2005;11:611. doi: 10.1255/ejms.773. doi: http://dx.doi.org/10.1255/ejms.773. [DOI] [PubMed] [Google Scholar]
- 34.Todua NG, Tretyakov KV, Borisov RS, Zhilyaev DI, Zaikin VG, Stein SE, Mikaia AI. Electron ionization mass spectra of alkylated sulfabenzamides. Rapid Commun. Mass Spectrom. 2011;25:750. doi: 10.1002/rcm.4918. doi: http://dx.doi.org/10.1002/rcm.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leimer KR, Rice RH, Gehrke CW. Complete mass spectra of N-trifluoroacetyl-n-butyl esters of amino acids. J. Chromatogr. A. 1977;141:121. doi: 10.1016/s0021-9673(00)99131-3. doi: http://dx.doi.org/10.1016/s0021-9673(00)99131-3. [DOI] [PubMed] [Google Scholar]
- 36.Irikura KK, Todua NG. Facile Smiles-type rearrangement in radical cations of N-acyl arylsulfonamides and analogs. Rapid Commun. Mass Spectrom. 2014;28:829. doi: 10.1002/rcm.6850. doi: http://dx.doi.org/10.1002/rcm.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Awad T, Maher H, DeRuiter J, Clark C. Studies on the formation of N-methylperfluoroalkylnitrile cations from perfluoroacylphenethylamines in electron ionization mass spectrometry: unique marker ion fragments in methamphetamine analysis. Eur. J. Mass Spectrom. 2012;18:287. doi: 10.1255/ejms.1185. doi: http://dx.doi.org/10.1255/ejms.1185. [DOI] [PubMed] [Google Scholar]
- 38.Talaty ER, Young SM, Dain RP, Van Stipdonk MJ. A study of fragmentation of protonated amides of some acylated amino acids by tandem mass spectrometry: observation of an unusual nitrilium ion. Rapid Commun. Mass Spectrom. 2011;25:1119. doi: 10.1002/rcm.4965. doi: http://dx.doi.org/10.1002/rcm.4965. [DOI] [PubMed] [Google Scholar]
- 39.Manhas MS, Hsieh RS, Bose AK. Mass spectral studies. Part VII. Unusual fragmentation of some N-trifluoroacetyl amino acid methyl esters. J. Chem. Soc. C. 1970:116. doi: http://dx.doi.org/10.1039/j39700000116. [Google Scholar]
- 40.Bonnet J-Y, Thissen R, Frisan M, Vuitton V, Quirico É, Orthous-Daunay F-R, Dutuit O, Le Roy L, Fray N, Cottin H, Hörst SM, Yelle RV. Compositional and structural investigation of HCN polymer through high resolution mass spectrometry. Int. J. Mass Spectrom. 2013;354–355:193. doi: http://dx.doi.org/10.1016/j.ijms.2013.06.015. [Google Scholar]
- 41.Andersen JL, Andersen TM, Flamm C, Hanczyc MM, Merkle D, Stadler P. Navigating the chemical space of HCN polymerization and hydrolysis: guiding graph grammars by mass spectrometry data. Entropy. 2013;15:4066. doi: http://dx.doi.org/10.3390/e15104066. [Google Scholar]
- 42.Tamura K, Alexander RW. Peptide synthesis through evolution. Cell. Molec. Life Sci. 2004;61:1317. doi: 10.1007/s00018-004-3449-9. doi: http://dx.doi.org/10.1007/s00018-004-3449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banerjee A, Ganguly G, Tripathi R, Nair NN, Paul A. Unearthing the mechanism of prebiotic nitrile bond reduction in hydrogen cyanide through a curious association of two molecular radical anions. ChemEur. J. 2014;20:6348. doi: 10.1002/chem.201304627. doi: http://dx.doi.org/10.1002/chem.201304627. [DOI] [PubMed] [Google Scholar]