Abstract
We investigated whether community-acquired acute kidney injury encountered in a tertiary hospital emergency department setting increases the risk of chronic kidney disease (CKD) and mortality, and whether plasma biomarkers could improve the prediction of those adverse outcomes. In a prospective cohort study, we enrolled 616 patients at admission to the emergency department and followed them for a median of 62.1 months. Within this cohort 130 patients were adjudicated as having acute kidney injury, 159 transient azotemia, 15 stable CKD and 312 normal renal function. Serum cystatin C and plasma neutrophil gelatinase-associated lipocalin (NGAL) were measured at index admission. After adjusting for clinical variables, the risk of developing CKD stage 3, as well as the risk of death, were increased in the acute kidney injury group (hazard ratio (HR) 5.7 (95% confidence interval, 3.8–8.7) and HR 1.9 (95% confidence interval, 1.3–2.8), respectively). The addition of serum cystatin C increased the ability to predict the risk of developing CKD stage 3, and death (HR 1.5 (1.1–2.0) and 1.6 (1.1–2.3) respectively). The addition of plasma NGAL resulted in no improvement in predicting CKD stage 3 or mortality (HR 1.0 (0.7–1.5) and 1.2 (0.8–1.8), respectively). The risk of developing CKD stage 3 was also significantly increased in the transient azotemia group (HR 2.4 (1.5–3.6). Thus, an episode of community acquired acute kidney injury markedly increases the risk of CKD, and moderately increases the risk of death. Our findings highlight the importance of follow up of patients with community acquired acute kidney injury, for potential early initiation of renal protective strategies.
Keywords: acute kidney injury, chronic kidney disease, biomarkers, cystatin C, NGAL
INTRODUCTION
Acute kidney injury (AKI) is associated with high short-term morbidity and mortality [1, 2]. The global rising incidence, and the devastating yet potentially preventable short-term outcomes, has prompted the International Society of Nephrology’s 0by25 initiative to increase AKI awareness and to change its prognosis [3–5]. Indeed, it is now widely recognized that critically ill hospitalized patients who survive an AKI episode are at considerable risk for progression to chronic kidney disease (CKD) [6–13]. However, there is still no direct evidence for a causal relationship between AKI and long-term CKD or mortality in patients with less severe forms of community-acquired AKI, which constitutes the most common setting for AKI worldwide [5]. Furthermore, there are no reliable biomarkers to predict long-term adverse outcomes of AKI, another major unmet need identified by the 0by25 initiative [5], although plasma biomarkers such as serum cystatin C [14, 15] and plasma neutrophil gelatinase-associated lipocalin [16, 17] have short-term prognostic value.
We aimed to determine whether an AKI episode encountered in a tertiary hospital emergency department setting has an impact on the long-term incidence of CKD and mortality, and whether serum cystatin C (SCysC) and plasma neutrophil gelatinase-associated lipocalin (pNGAL) measured during the index AKI episode could improve the prediction of those adverse outcomes.
RESULTS
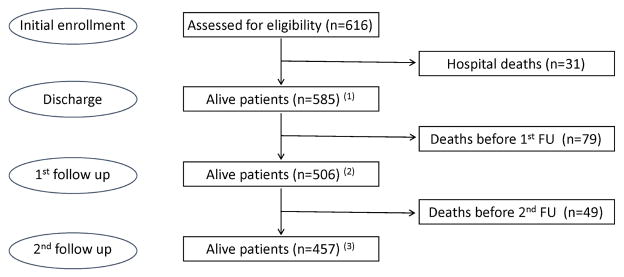
A total of 113,385 patients presented to the emergency department of Hospital Fernando Fonseca from March to November 2008. Among those, 4742 required hospital admission. Following the inclusion/exclusion criteria, 800 patients were eligible to be sequentially recruited and enrolled, and 616 consented to the initial inclusion and follow up (Supplementary Figure 1). This cohort was studied during the index hospitalization [14, 17] and classified into four clinical groups: acute kidney injury (AKI, n=130), transient azotemia (TAz, n=159), stable chronic kidney disease (sCKD, n=15) and normal function (NF, n=312). The cohort was followed after hospital discharge for a median time of 19.9 (P25–75 7.3–26.2) and 62.1 (P25–75 49.9–67.9) months (F1 and F2, respectively), as shown in Figure 1. Patient characteristics are shown in Table 1. Data regarding clinical diagnoses at discharge and the etiology of AKI and TAz are presented in Supplementary Tables 1 and 2, respectively.
Figure 1.
Study profile. Flow chart of patients during follow-up. (1)n=86, and (2)n=171 with missing GFR values.
Table 1.
Patient characteristics
| Characteristic | All Patients (n=616) | AKI 21.1% (n=130) | TAz 25.8% (n=159) | sCKD 2.4% (n=15) | NF 50.7% (n=312) | P Value |
|---|---|---|---|---|---|---|
| Mean Age (SD) | 59.1 (15.8) | 66.3 (12.2) | 58.4 (16.1) | 68.5 (11.5) | 56.0 (15.7) | <0.001* (a) |
| Men (%) | 386 (62.7) | 84 (64.6) | 93 (58.5) | 10 (66.7) | 199 (63.8) | 0.65** |
| Non-Black (%) | 536 (87.0) | 114 (87.7) | 137 (86.2) | 15 (100.0) | 270 (86.5) | 0.56** |
| 1st Follow Up time Median (P25–P75) | 19.9 (7.3–26.2) | 17.3 (6.2–25.9) | 21.3 (11.1–26.4) | 19.9 (8.1–22.5) | 20.1 (5.8–26.5) | 0.34* |
| 2nd Follow Up median time | 62.1 (49.9–67.9) | 57.3 (46.3–66.1) | 59.0 (48.4–66.9) | 55.6 (50.9–66.5) | 63.7 (52.0–68.7) | 0.05* |
| SCr baseline (mg/dL) Median (P25–P75) | 0.8 (0.6–0.9) | 1.0 (0.7–1.2) | 0.7 (0.6–0.9) | 1.2 (1.1–1.9) | 0.7 (0.6–0.8) | <0.001*(b) |
|
| ||||||
| Baseline eGFR (mL/min/1.73m2) Median (P25–P75) | 96.42 (82.02–111.35) | 75.58 (53.50–97.27) | 95.14 (84.00–113.99) | 54.30 (44.83–60.64) | 101.14 (91.17–114.02) | <0.001* |
|
| ||||||
| Discharge eGFR Median (P25–P75) | 89.54 (65.78–106.21) | 47.42 (29.57–72.2) | 87.45 (71.27–104.76) | 48.46 (39.96–54.68) | 99.30 (85.61–113.32) | <0.001* |
| Number (%) | 616 (100%) | 130 (100%) | 159(100%) | 15(100%) | 312 (100%) | |
|
| ||||||
| 1st Follow Up eGFR Median (P25–P75) | 83.55 (63.17–103.49) | 42.78 (23.32–70.81) | 74.82 (53.46–96.13) | 45.72 (28.70 – 51.20) | 95.31 (77.16–107.12) | <0.001* |
| Number (%) | 499 (81%) | 102 (78.5%) | 132 (83%) | 15 (100%) | 249 (79.8%) | |
|
| ||||||
| 2nd Follow Up eGFR Median (P25–P75) | 73.78 (50.67–96.54) | 40.29 (20.54–62.48) | 70.37 (53.55–91.03) | 30.47 (16.36–40.77) | 85.50 (69.70–101.80) | <0.001*(c) |
| Number (%) | 335 (54.4%) | 53 (40.8%) | 80 (50.3%) | 9 (60.0%) | 193 (61.9%) | |
|
| ||||||
| Main Comorbidities (%) | ||||||
| HT | 391 (63.6) | 101 (77.7) | 98 (62.0) | 12 (80.0) | 180 (57.7) | <0.001** |
| CVD | 219 (35.6) | 71 (54.6) | 61 (38.4) | 8 (53.3) | 79 (25.3) | <0.001** |
| DM | 192 (31.3) | 55 (42.3) | 46 (29.1) | 8 (53.3) | 83 (26.8) | 0.003 ** |
| CHF | 81 (13.1) | 39 (30) | 21 (13.2) | 4 (26.7) | 17 (5.4) | <0.001** |
| CLD | 50 (8.1) | 13 (10.0) | 11 (7.0) | 0 (0) | 26 (8.4) | 0.52 ** |
| CKD | 65 (10.6) | 43 (33.1) | 7 (4.4) | 15 (100.0) | 0 (0) | <0.001** |
| CCI | 3.0 (2.0–5.0) | 5.0 (3.0–6.0) | 3.0 (1.0–4.0) | 5.0 (3.5–6.0) | 3.0 (2.0–5.0) | <0.001*(d) |
| Susceptibility stage III–IV | 70 (11.4) | 44 (33.8) | 9 (5.7) | 11 (73.3) | 6 (1.9) | <0.001** |
|
| ||||||
| pNGAL median (ng/mL; P25–P75) T3 | 83.0 (60–138) | 167.0 (109–253) | 94.0 (60–136) | 115.0 (78–167) | 64.0 (60–93) f | <0.001* |
|
| ||||||
| Clinical evolution | ||||||
| All Sepsis (%) | 188 (30.5) | 50 (38.5) | 60 (37.7) | 3 (20) | 75 (24) | 0.002** |
| Severe sepsis (%) | 27 (4.4) | 16 (2.3) | 7 (4.4) | 0 | 4 (1.3) | <0.001** |
| ICU admission (%) | 53 (8.6) | 18 (13.8) | 11 (6.9) | 1 (6.7) | 23 (7.4) | 0.12** |
| MV (%) | 22 (3.6) | 9 (6.9) | 8 (5) | 0 | 5 (1.5) | 0.03** |
| Mean LOS (SD) | 11.3 (10) | 16.9 (15.5) | 10.5 (8.4) | 8.5 (3.8) | 9.5 (6.9) | <0.001** |
| Outcomes | ||||||
| Overall RRT (%) | 23 (3.7) | 14 (10.8) | 6 (3.8) | 1 (6.7) | 2 (0.6) | <0.001*** |
| Overall Mortality (%) | 159 (25.8) | 58 (44.6) | 36 (22.6) | 2 (13.3) | 63 (20.2) | <0.001** |
| Long-term CKD (%) | 199 (32.3) | 96 (73.8) | 49 (30.8) | 15 (100) | 39 (12.6) | <0.001** |
AKI: acute kidney injury; TAz: transient azotemia; sCKD: stable chronic kidney disease; NF: normal function; SD: standard deviation; SCr: serum creatinine; eGFR: glomerular filtration rate estimated based on serum creatinine using the CKD-EPI equation; HT: hypertension; CVD: cardiovascular disease; DM: diabetes mellitus; CHF: chronic heart failure; CLD: chronic liver disease; CKD: previous chronic kidney disease; CCI: Comorbidity Charlson Index score; T3: 12h study time [14, 20]; ICU, intensive care unit; MV, mechanical ventilation; LOS, length of stay; RRT, renal replacement therapy; CKD incidence, chronic kidney disease development during follow-up.
age p-value <0.001 comparing AKI with TAz and NF patients, AKI with CKD patients 0.496, TAz with CKD patients 0.007;
SCr baseline p value <0.001 except between TAz and NF patients (p-value= 0.797);
1st Follow Up eGFR p-value <0.001 except between TAz and NF patients (p-value=0.009), and between sCKD and TAz patients (p-value=0.002);
p<0.001 except between NF and TAz (p-value=1.000), Taz vith CKD (p-value=0.007), NF with CKD (p-value=0.015), AKI with CKD (p-value=1.000);
Kruskal-Wallis test p-values;
Chi-Square test p-values;
extension of Fisher’s Exact test p-values.
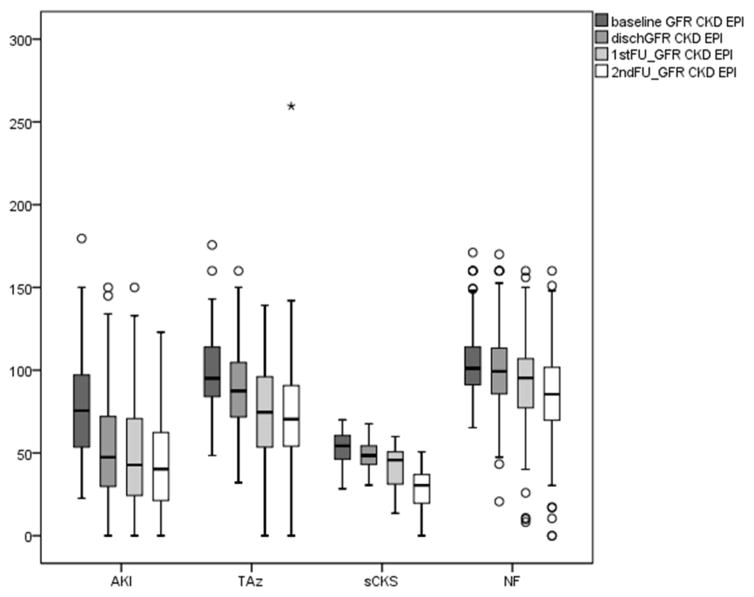
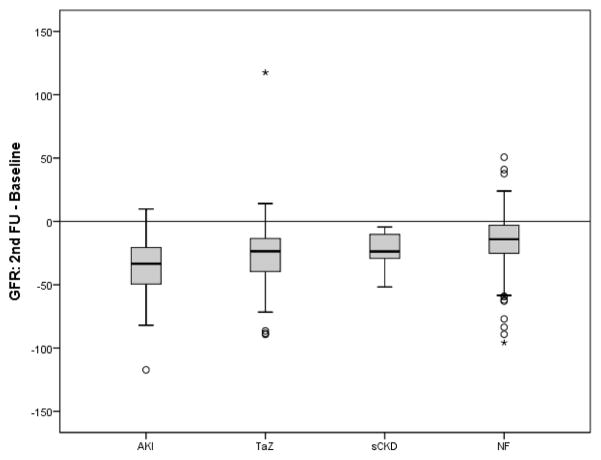
The median estimated glomerular filtration rate (eGFR) values at different study times are shown in Table 1 and Figure 2A. Kidney function decreased in all four clinical groups, most marked in AKI patients, with a decrease from 75.6 at baseline to 42.8 at F1, and 40.3 mL/min/1.73m2 at F2. The TAz group displayed a decrease from 95.1 at baseline to 74.8 at F1, and 70.4 mL/min/1.73m2 at F2. The sCKD group showed a progressive decrease in eGFR at both times of follow-up (54.3 at baseline, 45.7 at F1, and 30.5 mL/min/1.73m2 at F2). The NF group showed very minimal decreases in kidney function, with eGFR median levels of 101.1, 95.3 and 85.5 mL/min/1.73m2 at baseline, F1 and F2 respectively. Within all four groups, the eGFR change was significant comparing baseline with F2 (p<0.001). When analysed as change in eGFR between baseline and F2, the ΔeGFRs in the AKI and TAz groups were significantly greater when compared to the NF group (p<0.001), as shown in Figure 2B. There were no significant differences in ΔeGFR between NF and sCKD groups.
Figure 2.
Figure 2A. Evolution of chronic kidney disease by estimated GFR. Kidney function by estimated GFR at baseline, discharge, 1st follow-up (median 19.9 months), and 2nd follow-up (median 62.1 months), in each clinical group. AKI, acute kidney injury; TAz, transient azotemia; sCKD, stable chronic kidney disease; NF, normal function. p<0.05 for all groups, comparing baseline to 2nd follow up.
Figure 2B. Change in eGFR between baseline and last follow-up time (F2). AKI, acute kidney injury; TAz, transient azotemia; sCKD, stable chronic kidney disease; NF, normal function. p<0.001 when comparing AKI and TAz with NF.
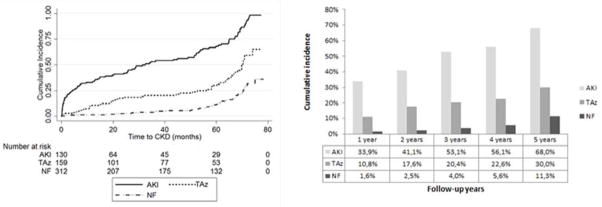
In the AKI group, the incidence of CKD stage ≥3 (defined as eGFR ≤ 60mL/min/1.73m2) increased significantly during the follow-up period, with 64.7% (66/102) and 71.7% (38/53) reaching this outcome at F1 and F2 respectively (p<0.001 comparing F1 and F2 with baseline) (Figure 3). The incidence also increased significantly in the TAz group to 30.3% (40/132) and 33.8% (27/80) at F1 and F2 respectively (p<0.001 comparing F1 and F2 with baseline). In the NF group, only 6.8% (17/249) and 16.1% (31/193) developed CKD stage ≥ 3 at F1 and F2, respectively (p<0.001 comparing F2 with baseline) (Table 2). Analysis of the cumulative incidence of CKD stage ≥ 3 demonstrated that 68% of AKI patients developed CKD by five years of follow up, in contrast to 30% in the TAz group and 11.3% in the NF group (Figure 4).
Figure 3.
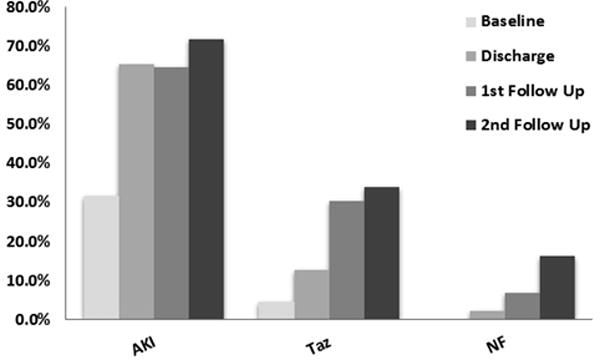
Percent of eGFR ≤60 mL/min/1.73m2 at baseline, discharge, 1st follow-up (median 19.9 months), and 2nd follow-up (median 62.1 months), in each clinical group. AKI, acute kidney injury; TAz, transient azotemia; NF, normal function. *p<0.001 compared to baseline.
Table 2.
Patients with eGFR≤60mL/min by group classification and follow-up time
| Follow-up Time | Number* (%) | AKI 21.1% (130) | TAz 25.8% (159) | NF 50.7% (312) | p Value |
|---|---|---|---|---|---|
| Baseline | 616 (100.0) | 41/130 (31.5) | 7/159 (4.4) | 0 (0) | <0.001a |
| Discharge | 616 (100.0) | 85/130 (65.4) | 20/159 (12.6) | 6/312 (1.9) | <0.001a |
| 1st Follow Up (F1) | 499 (81.0) | 66/102 (64.7) | 40/132 (30.3) | 17/249 (6.8) | <0.001a |
| 2nd Follow Up (F2) | 335 (54.4) | 38/53 (71.7) | 27/80 (33.8) | 31/193 (16.1) | <0.001a |
| P Value | <0.001 b | <0.001b | <0.001b |
Number (%) of patients with eGFR ≤60 mL/min/1.73m2 at discharge, and both follow-up time points (F1 and F2).
p-value obtained by Pearson’s Chi-squared test, referring to the comparison between classification categories at each study time;
p-value obtained by mixed-effects logistic regression model, referring to the comparison of the odds of having eGFR≤60 at study times, for each classification category;
AKI, acute kidney injury; TAz, transient azotemia; NF, normal function;
total number of patients at each study time.
Figure 4.
Cumulative incidence of chronic kidney disease during follow-up time. The figure on the left shows Kaplan Meier curves estimates (p < 0.001 for both Taz and AKI compared to normal function) for the entire follow up period. Figure on the right shows cumulative incidence rates during the five years of follow-up. AKI, acute kidney injury; TAz, transient azotemia; NF, normal function.
To explore the risk factors for developing CKD stage ≥3, several multivariable Cox regression models were fitted to the data (Table 3). Model 1 is the clinical model. In this model, following univariable analysis of gender, categorized age (<63 years is the reference category), race, categorized susceptibility [14, 17, 18], all studied comorbidities, and the Charlson Comorbidity Index Score (CCI) [19], the following multivariable model was obtained: age: HR 2.4 (95% CI:1.7–3.4; p<0.001), cardiovascular disease (CVD): HR 1.9 (95% CI, 1.4–2.6; p<0.001), CCI: HR 1.9 (95% CI, 1.4–2.6; p<0.001), and susceptibility: HR 2.2 (95% CI,1.5–3.0; p<0.001). In Model 2, the clinical classification of patient groups (AKI, TAz, and NF) was added to Model 1, yielding significant HR for age, CVD and CCI, but not for susceptibility (p<0.001; p=0.007; p=0.003, and p=0.75, respectively). Patients with AKI had a 6-fold risk of developing CKD stage ≥3, when compared with NF (HR 5.7; 95% CI, 3.8–8.7; p<0.001). Patients with TAz had 2.4-fold risk of CKD stage ≥3 (HR 2.4; 95% CI, 1.5–3.6; p<0.001).
Table 3.
Multivariable Cox regression models to identify risk factors for CKD stage 3.
| MODEL 1 | MODEL 2 | MODEL 3 | MODEL 4 | |
|---|---|---|---|---|
| Variables | Clinical AIC=1908.4 | Clinical + Classification AIC= 1841.9 | MODEL 2 + SCysC AIC=1720.0 | MODEL 2 + pNGAL AIC=1774.6 |
| (a)Age | 2.4 (1.7–3.4) | 2.3 (1.6 – 3.2) | 2.1 (1.5 – 3.1) | 2.2 (1.5 – 3.2) |
|
| ||||
| CVD | 1.9 (1.4–2.6) | 1.5 (1.1 – 2.1) | 1.7 (1.2 – 2.4) | 1.5 (1.1 – 2.1) |
|
| ||||
| (b) CCI | 1.9 (1.4–2.6) | 1.6 (1.2 – 2.2) | 1.6 (1.1 – 2.2) | 1.7 (1.2 – 2.3) |
|
| ||||
| (c) Susceptibility | 2.2 (1.5–3.0) | 1.1 (0.7 – 1.5) | 0.8 (0.5 – 1.3) | 1.1 (0.7 – 1.6) |
|
| ||||
| (d)Classification | ||||
|
| ||||
| AKI | 5.7 (3.8 – 8.7) | 4.7 (2.9 – 7.7) | 5.6 (3.5 – 8.8) | |
|
| ||||
| TAz | 2.4 (1.5 – 3.6) | 2.3 (1.5 – 3.5) | 2.3 (1.5 – 3.5) | |
|
| ||||
| SCys | 1.5 (1.1 – 2.0) | |||
|
| ||||
| (e)pNGAL | 1.0 (0.7 – 1.5) | |||
Results are expressed as hazard ratios (95% confidence interval); SCysC, serum cystatin C; pNGAL, plasma NGAL; AIC, Akaike’s Information Criterion; CVD, cardiovascular disease; CCI, Charlson Comorbidity Index; AKI, Acute Kidney Injury; TAz, Transient Azotemia.
The cut point 63 years for age was obtained by the analysis of Martingale residuals, age < 63 years old was considered as the reference category;
reference category: CCI≤3;
Susceptibility stage I–II considered as the reference category;
reference category: NF;
reference category: pNGAL ≤ 133 ng/mL.
Model 1: p<0.001 for all variables; Model 2: CVD p=0.007, CCI p=0.003, Susceptibility p=0.749, p<0.001 for the remaining variables; Model 3: CVD p=0.002, CCI p=0.011, Susceptibility p=0.399, SCysC p=0.011, p<0.001 for the remaining variables; Model 4: CVD p=0.010, CCI p=0.002, Susceptibility p=0.779, pNGAL p=0.809, p<0.001 for the remaining variables.
Biomarkers measured during the index admission were added, separately, to Model 2 to explore their ability to improve prediction of CKD stage ≥3. In Model 3, addition of SCysC yielded a HR of 1.5 (95% CI, 1.1–2.0; p=0.011). In Model 4, addition of pNGAL did not increase the risk of developing CKD, with a HR of 1.0 (95% CI, 0.7–1.5; p=0.809).
In order to quantify the improvement resulting from adding clinical classification (Model 2) to Model 1, and from adding SCysC to Model 2 (Model 3), or pNGAL to Model 2 (Model 4), continuous net reclassification improvement (NRI) and integrated discrimination improvement (IDI) measures for censored data were calculated. The NRI quantifies the correctness of upward and downward movement of predicted probabilities as a result of adding a new marker to an existing baseline model. The IDI quantifies the magnitudes of changes in those probabilities. Adding clinical classification to Model 1 resulted in an improvement in predicting CKD stage ≥3 according to NRI events and non-events (40.2%, 95% CI, 25.8–54.7 and 34.5%, 95% CI, 24.8–44.1, respectively), and to IDI events and non-events (0.07, 95% CI, 0.04–0.09 and 0.03, 95% CI, 0.02–0.05, respectively). The change in predicting CKD stage ≥3 from adding SCysC to Model 2 resulted in an improvement of NRI non-events (41.0%, 95% CI, 31.3–50.7), and in a negligible change of NRI events and IDI (events and non-events) (Table 4). The change in predicting CKD stage ≥3 from adding pNGAL to Model 2 resulted in a worsening of NRI non-events (−31.5%, 95% CI, −41.3–−21.8), and in a negligible change in NRI events and IDI (events and non-events) (Table 4). Additional measures of the performance of each of the four multivariable models are showed in Table 4. For all four models, the C statistic value for predicting CKD stage ≥3 was in the 0.74–0.81 range.
Table 4.
Performance of the multivariable Cox regression models for CKD
| MODEL 1 | MODEL 2 | MODEL 3 | MODEL 4 | |
|---|---|---|---|---|
| Performance measure | Clinical | Clinical + Classification | MODEL 2 + SCysC | MODEL 2 + pNGAL |
| OVERALL | ||||
|
| ||||
| (a) LR statistic | 70.56 | 123.85 | 69.29 | |
| (b) explained variation (%) | 20.0% | 28.6% | 30.1% | 28.5% |
|
| ||||
| DISCRIMINATION | ||||
|
| ||||
| C statistic | 0.739 | 0.794 | 0.810 | 0.794 |
|
| ||||
| CALIBRATION | ||||
|
| ||||
| slope shrinkage estimate | 0.965 | 0.956 | 0.937 | 0.952 |
|
| ||||
| ADDED VALUE | (c1) | (c2) | (c3) | |
|
| ||||
| NRI events % (CI) | 40.2 (25.8 – 54.7) | 9.2 (−5.7 – 24.1) | 12.4 (−2.3 – 27.1) | |
| NRI non-events % (CI) | 34.5 (24.8 – 44.1) | 41.0 (31.3 – 50.7) | −31.5 (−41.3 – −21.8) | |
|
| ||||
| IDI | 0.10 (0.07 – 0.13) | 0.009 (0.001 – 0.017) | 0.001 (−0.001 – 0.002) | |
| IDI events | 0.07 (0.04 – 0.09) | 0.005 (−0.002 – 0.013) | 0.002 (0.000 – 0.003) | |
| IDI non-events | 0.03 (0.02 – 0.05) | 0.004 (0.000 – 0.007) | −0.001 (−0.002 – 0.000) | |
For all likelihood-ratio tests: MODEL 1 nested in MODEL 2, and MODEL 2 nested in MODEL 3 and MODEL 4, p<0.001 was obtained;
Nagelkerke R2;
(c1) corresponds to the added value to the clinical model attained by Classification, on the developed sample; (c2) and (c3) correspond, respectively, to the added value to the clinical + Classification model attained by SCysC and pNGAL, on the developed sample.
Overall, 39.4% of patients in our cohort (n=132/335) displayed an eGFR loss of >25% at F2 from the pre-AKI baseline. In univariable analysis, clinical variables of risk for eGFR loss of >25% were age (OR 1.04; 95% CI, 1.02–1.06; p<0.001); CVD (OR 3.15, 95% CI, 1.97–5.01; p<0.001); hypertension (OR 4.59, 95%CI, 2.62–8.04; p<0.001) and chronic heart failure (OR 2.72, 95% CI, 1.39–5.31; p=0.004). Plasma NGAL measured at 12 hours of index admission was associated with an OR for eGFR loss >25% of 1.04 (95% CI, 1.01–1.07; p=0.003) for each 10 ng/mL increase, whereas SCysC displayed an OR 3.38 (95% CI, 2.25–5.06; p< 0.001) for each 0.5 mg/L rise. The pNGAL concentrations within the grey zone [17] showed an OR 1.67 (95% CI, 0.86–3.22; p=0.013); while levels in the high-risk zone displayed an OR 4.24 (95% CI, 2.44–7.35; p<0.001). Categorical SCysC (≤0.98 or >98 mg/L) showed an OR 5.22 (95% CI, 3.11–8.75; p<0.001).
The selected variables by the univariable analysis that remained in the multivariable model for prediction of an eGFR loss of >25% were CVD (OR 2.24, 95% CI, 1.29–3.88; p= 0.004); hypertension (OR 3.22, 95% CI, 1.66–6.26; p=0.001); SCysC (OR 2.62, 95% CI, 1.44–4.79; p= 0.002); grey zone pNGAL (OR 1.54, 95% CI, 0.74–3.18; p=0. 25); and high-risk pNGAL (OR 2.96, 95% CI, 1.51–5.81, p=0.002). This multivariable model presented a Hosmer-Lemeshow goodness of fit test p-value=0.947, and an AUC-ROC curve of 0.78 (95% CI, 0.73–0.83).
In the overall cohort, 8.1% (50/616) of patients developed the composite endpoint of CKD stage 5/ESRD (end stage renal disease), and dialysis requirement during the follow up period: 26.9% (35/130) corresponded to AKI group, 5.7% (9/159) to TAz group, 20% (3/15) were in the sCKD group, and only 0.64% (2/312) were in the NF group. AKI and TAz group developed the composite endpoint significantly more frequently when compared with the NF group (p<0.001). The discriminative ability of SCysC for predicting this composite outcome was represented by a ROC-AUC of 0.90 (95% CI, 0.87–0.93); for pNGAL, the corresponding ROC-AUC was 0.79 (95% CI, 0.73–0.86).
The overall mortality rate in the cohort was 25.8% (n=159), with 19.5% of the deaths occurring during the index hospitalization (31/159), 39.6% (34/159) during the first two years after discharge, 32.1% (51/159) between the second and fifth years of follow-up; and 8.8% (14/159) up to the end of follow-up. The majority of deaths occurred in the AKI group. Overall mortality in the AKI group was 44.6% (58/130), with rates of 22.6% (36/159), 13.3% (2/15) and 20.2% (63/312) in TAz, sCKD and NF groups, respectively (Table 1).
To explore the risk factors for death, several multivariable Cox regression models were also fitted to the data (Table 5). The clinical model (Model 1) identified the following mortality risk factors: cancer (HR 3.3, 95% CI, 2.1–5.1; p<0.001); CCI score (HR 2.2, 95% CI, 1.5–3.1 p<0.001); chronic liver disease (HR 1.7, 95% CI, 1.0–2.7; p=0.035; age (HR 1.0, 95% CI, 1.0–1.1; p<0.001); and female gender (HR 0.6, 95% CI, 0.5–0.9, p=0.015). Results from the multivariable Model 2 showed that patients in the AKI group displayed a 2-fold greater risk of death, when compared with NF patients (HR 1.9, 95% CI, 1.3–2.8; p<0.001). The ability of biomarkers to predict mortality during the follow up period showed a HR 1.6 (95% CI, 1.1–2.3; p<0.001) for SCysC (Model 3), and HR 1.2 (95% CI, 0.8–1.8; p=0.269) for pNGAL (Model 4).
Table 5.
Multivariable Cox regression models for death
| Variable | MODEL 1 | MODEL 2 | MODEL 3 | MODEL 4 |
|---|---|---|---|---|
| Clinical AIC=1772.0 | Clinical + Classification AIC=1757.7 | MODEL 2 + SCysC AIC=1718.7 | MODEL 2 + pNGAL AIC=1735.2 | |
| Age | 1.0 (1.0 – 1.1) | 1.1 (1.0 – 1.1) | 1.0 (1.0 – 1.1) | 1.0 (1.0 – 1.1) |
|
| ||||
| (a)Sex | 0.6 (0.5 – 0.9) | 0.6 (0.4 – 0.9) | 0.6 (0.4 – 0.8) | 0.6 (0.4 – 0.9) |
|
| ||||
| CLD | 1.7 (1.0 – 2.7) | 1.6 (1.0 – 2.5) | 1.4 (0.9 – 2.4) | 1.5 (0.9 – 2.5) |
|
| ||||
| (b)CCI | 2.2 (1.5 – 3.1) | 2.2 (1.6 – 3.2) | 1.9 (1.3 – 2.7) | 2.0 (1.4 – 2.9) |
|
| ||||
| Cancer | 3.3 (2.1 – 5.1) | 3.7 (2.4 – 5.7) | 4.5 (2.8 – 7.0) | 4.2 (2.7 – 6.5) |
|
| ||||
| (c)Classification | ||||
|
| ||||
| AKI | 1.9 (1.3 – 2.8) | 1.4 (0.9 – 2.3) | 1.8 (1.1 – 2.7) | |
| TAz | 1.1 (0.7 – 1.7) | 1.0 (0.7 – 1.6) | 1.1 (0.7 – 1.7) | |
| sCKD | 0.3 (0.1 – 1.1) | 0.2 (0.1 – 0.8) | 0.3 (0.1 – 0.9) | |
|
| ||||
| SCys | 1.6 (1.1 – 2.3) | |||
|
| ||||
| (d)pNGAL | 1.2 (0.8 – 1.8) | |||
Results are expressed as hazard ratios (95% confidence interval); SCysC, serum cystatin C; pNGAL, plasma NGAL; AIC, Akaike’s Information Criterion; AKI, acute kidney injury; TAz, transient azotemia; sCKD, stable chronic kidney disease; CLD, Chronic Liver Disease; CCI, Comorbidity Charlson Index score;
reference category: male;
reference category: CCI≤3;
reference category: NF (normal function);
reference category: pNGAL ≤ 133 ng/mL;
Model 1: Sex p=0.015, CLD p=0.035 and p<0.001 for the remaining variables; Model 2: sex p=0.009, CLD p=0.076, Taz p=0.551, sCKD p=0.062, p<0.001 for the remaining variables; Model 3: sex p=0.002, CLD p=0.149, AKI p=0.150, Taz p=0.935, sCKD p=0.023, p<0.001 for the remaining variables; Model 4: sex p=0.006, CLD p=0.091, AKI p=0.010, Taz p=0.720, sCKD p=0.038, NGAL p=0.269, p<0.001 for the remaining variables.
Adding clinical classification to Model 1 resulted in an improvement of both NRI non-events (50.3%, 95% CI, 41.1–59.5) and IDI (0.034, 95% CI, 0.017–0.050), and in a worsening, although without statistical significance, of NRI events (−13.2, 95% CI, −28.8–2.3). The change in predicting death from adding SCysC to Model 2 was negligible based on NRI and IDI. Adding pNGAL to Model 2 resulted in an improvement of NRI events (26.6%, 95% CI, 11.0–42.2), and in negligible changes in both NRI non-events and IDI. Additional measures of the performance of each of the four multivariable models are showed in Table 6. For all four models, the C statistic value for predicting mortality was in the 0.74–0.76 range.
Table 6.
Performance of the multivariable Cox regression models for death
| MODEL 1 | MODEL 2 | MODEL 3 | MODEL 4 | |
|---|---|---|---|---|
| Performance measure | Clinical | Clinical + Classification | MODEL 2 + SCysC | MODEL 2 + pNGAL |
| OVERALL | ||||
|
| ||||
| (a) LR statistic | 20.24 | 41.00 | 24.53 | |
| (b)% of explained variation | 17.0% | 18.98% | 19.87% | 19.72% |
|
| ||||
| DISCRIMINATION | ||||
|
| ||||
| C statistic | 0.738 | 0.755 | 0.758 | 0.753 |
|
| ||||
| CALIBRATION | ||||
|
| ||||
| slope shrinkage estimate | 0.956 | 0.925 | 0.906 | 0.915 |
|
| ||||
| ADDED VALUE | (c1) | (c2) | (c3) | |
|
| ||||
| NRI events % (CI) | −13.2 (−28.8 – 2.3) | 12.1 (−3.5 – 27.7) | 26.6 (11.0 – 42.2) | |
| NRI non-events % (CI) | 50.3 (41.1 – 59.5) | 8.4 (−0.1 – 17.8) | 4.8 (−4.6 – 14.1) | |
|
| ||||
| IDI | 0.034 (0.017 – 0.050) | 0.014 (0.002 – 0.027) | 0.009 (0.003 – 0.014) | |
| IDI events | 0.026 (0.011 – 0.041) | 0.017 (0.006 – 0.029) | 0.012 (0.007 – 0.017) | |
| IDI non-events | 0.008 (0.000 – 0.015) | −0.003 (−0.008 – 0.002) | −0.004 (−0.006 – −0.001) | |
For all likelihood-ratio tests: MODEL 1 nested in MODEL 2, and MODEL 2 nested in MODEL 3 and MODEL 4, p<0.001 was obtained;
Nagelkerke R2;
(c1) corresponds to the added value to the clinical model attained by Classification, on the developed sample; (c2) and (c3) correspond, respectively, to the added value to the clinical + Classification model attained by SCysC and pNGAL, on the developed sample.
DISCUSSION
The results of this prospective observational long-term follow-up study demonstrate that community-acquired AKI in the setting of a tertiary care inner city emergency department is associated with a substantially increased risk of death and of developing CKD stage 3 or greater. Our findings significantly extend the findings from recent analyses of administrative databases suggesting that AKI in critically ill hospitalized patients leads to CKD, increased risk of end-stage renal disease (ESRD), and excess mortality [7–11]. To our knowledge, this is the first report of a rigorous prospective follow-up of all subjects who survived an AKI episode acquired in the heterogeneous community setting.
At approximately 5 years of follow up, patients who initially presented with community-acquired AKI [14, 17] exhibited the highest incidence of CKD, with 40.2% of the patients in this group developing CKD stage ≥3, 39.4% exhibiting an eGFR loss of >25%, and 27% reaching the composite endpoint of CKD stage 5, ESRD, and dialysis requirement. Furthermore, the mortality rate in the AKI group was the highest, at 44.6%. The incidence of adverse outcomes demonstrated herein is higher than those reported in previous retrospective studies. In hospitalized American Medicare beneficiaries, the likelihood of initiating end stage renal disease (ESRD) treatment was 6.96% at the end of 2 years of follow-up in patients with AKI, and 14.29% in patients with both AKI and CKD [7]. In a Canadian population-based cohort study of patients with acute kidney injury who required in-hospital dialysis but survived without dialysis, the risk of ESRD at a median follow up of three years was 8.5% [8]. Observational studies of administrative databases may be biased with respect to patient selection and ascertainment [10, 20], which may account for some of the discrepancy in results. Furthermore, our prospective follow up period exceeds that of previously reported retrospective studies.
Our rigorous adjudication process [14, 17] allowed for the identification of subjects with transient azotemia, a condition that has traditionally been considered physiologic and fully reversible. However, our long term follow up of these subjects yielded an unexpectedly high incidence of CKD, with 29.4% developing CKD stage 3, and 5.7% reaching the composite endpoint of CKD stage 5, ESRD, and dialysis requirement. Our findings lend support to the emerging notion that even transient AKI is not necessarily benign, especially since it is associated with short-term adverse outcomes, including dialysis requirement and death [21, 22].
Another surprising finding in our study pertains to the fact that even patients who displayed normal kidney function at index admission did develop a small but significant drop in eGFR over time. Forty patients initially adjudicated to the normal function group (12.8% of the total subjects in this group) had developed CKD stage 3 or greater at the five year follow up time. However, upon close examination, it became apparent that 24 out of these 40 patients developed at least one episode of intrinsic AKI during the follow up period, which might explain their progression to CKD. In addition, among the cohort of 40 subjects initially judged to have normal kidney function by serum creatinine criteria, 20 of them displayed an elevation in plasma biomarkers of AKI [17]. Thus, in retrospect, a substantial proportion of the patients had biomarker-positive creatinine-negative subclinical AKI at index hospitalization, which is known to portend poor outcomes [23, 24].
We examined whether plasma AKI biomarkers pNGAL and SCysC measured during the acute episode provide added value to predict CKD and mortality beyond the clinical model. In the acute setting, SCysC outperforms serum creatinine as a marker of AKI severity [14, 23]. Plasma NGAL is also an early predictive marker of AKI severity [16, 17] that detects patients who have an increased risk of acute adverse outcomes even in the absence of increases in serum creatinine [24]. Since the severity of AKI is known to determine CKD progression [6], it is plausible that these biomarkers of AKI severity may improve our ability to predict CKD and mortality after an AKI episode. However, while addition of SCysC slightly increased the prediction of CKD and mortality, the addition of pNGAL resulted in no improvement.
This study has important limitations. First, it is a single center study, and the results must be validated in larger multicenter studies. Second, serum creatinine measurements were used to define the different patient categories at index admission, which has well-known limitations. Third, proteinuria and microalbuminuria, classical markers of CKD progression, were not measured during initial presentation or during follow up. Fourth, we excluded patients with severe CKD from the index cohort, and recent meta-analyses have identified underlying CKD as one of the strongest risk factors for AKI [25, 26].
In conclusion, this study ascertains a previously unrecognized and alarmingly high rate of CKD and death five years after an episode of community-acquired AKI in the setting of a tertiary care inner city emergency department. We also identify transient azotemia as a novel risk factor for CKD. Serum cystatin C measured at the time of the initial AKI episode provides moderate improvement in the prediction of these adverse outcomes. Thus, we provide the first prospective results that validate and support the International Society of Nephrology’s global 0by25 initiative to increase awareness of the long-term consequences of community-acquired AKI, a disease with enormous health and financial burden. Our findings highlight the importance of close follow up of patients with community acquired AKI, for potential initiation of early, widely available, and inexpensive renal protective strategies.
METHODS
Patients
This is a prospective observational study involving follow up of a cohort of 616 patients who were enrolled following presentation to the emergency department of the Fernando Fonseca Hospital in Portugal during March to November 2008 [14, 17]. This is a tertiary acute care hospital serving a population of 700,000 from the Lisbon inner city area, with a high emergency department admission rate and an open door policy of providing care to all comers. Exclusion criteria were: age under 18 or over 80 years of age, established CKD stage 4 or greater, severe AKI at admission (defined as an increase in serum creatinine >300% over baseline or >4 mg/dl for more than 48 hours or needing dialysis therapy), and cytotoxic therapy. Patients who were discharged alive from the index presentation were prospectively followed for a median time of 62.1 months. All hospital admission data as well as data related to CKD, dialysis, and death were prospectively collected. All patients signed an informed consent to participate, and the study protocol was approved by the institutional Ethics Committee.
Procedures
At index admission, baseline renal function by SCr, medical history, and demographic characteristics were obtained from hospital electronic records for 1–6 months before ED presentation. A prospective renal function assessment was carried out by measuring serum creatinine (SCr), serum Cystatin C (SCysC), and plasma NGAL (pNGAL) at 0, 6, 12, 24, and 48 hours (T0, T6, T12, T24, and T48, respectively) from admission [14, 17]. Kidney function was measured by SCr at discharge (D, n=585), 1st follow-up (F1 at median time of 19.9 months, P25=7.3, P75=26.2; n=506), and 2nd follow-up (F2 at median time of 62.1 months, P25=49.9, P75=67.9; n=457). The estimated glomerular filtration rate (eGFR) was determined using the CKD-Epidemiology Collaboration (CKD-EPI) formula [27] and compared to the baseline eGFR obtained approximately 6 months prior to index admission in 2008. CKD was defined as eGFR < 60 mL/min/1.73m2, documented for more than 3 months, and CKD stages were according to K/DOQI guidelines [28]. We adjudicated the renal status at index admission [14, 17]. AKI was defined according to RIFLE [29] and AKIN [30] criteria, as a new increase in SCr that did not resolve in 72 hours. Transient azotemia (TAz) was defined as a new increase in SCr that resolved in less than 72 hours. Normal function (NF) was defined as a baseline eGFR greater than 60 mL/min per 1.73 m2 and no increases in SCr during the hospital stay. Stable CKD (sCKD) was defined as a stably reduced eGFR <60 mL/min per 1.73 m2 before admission and <25% change from baseline during the hospitalization.
To analyze factors that might predispose to AKI, nature and timing of the inciting event, and the response of the kidney to the insult, variables included in the “Multidimensional Criteria” [18] were recorded, including “susceptibility” (pre-existing kidney disease and risk morbidities of developing acute injury, nature and timing of the ”insult” on the basis of the specific insult and the time interval from the insult to the point of evaluation (in this case admission to the emergency department), “response” corresponding to the RIFLE classification, and non-renal organ dysfunction.
Serum creatinine, SCysC and pNGAL measured at 12 hours following index admission were used as predictive markers of death and long-term kidney outcomes. Patients were classified into 3 grades of AKI risk according to pNGAL concentrations [17]: low (pNGAL <97 ng/ml), grey zone (pNGAL 97–133 ng/ml), and high (pNGAL >133 ng/ml). A cut-off point for SCysC concentration of 0.98 mg/l was used to identify patients at high risk for AKI [14]. The Charlson Comorbidity Index (CCI) score was used for comorbidity evaluation [20]. The score values were added in all the multivariable analyses.
The primary outcomes were the development of CKD Stage ≥3 (eGFR <60 ml/min/1.73m2) and mortality. The secondary outcome was an eGFR loss of >25% from the pre-AKI baseline. The tertiary outcome was a composite endpoint of CKD stage 5, ESRD (end stage renal disease), and dialysis requirement.
Statistical analysis
Categorical data are presented as frequencies and percentages, and continuous variables as mean or median, standard deviation (SD) or inter-quartile range (IQR: 25th – 75th percentile), as appropriate. Nonparametric Chi-Square and Kruskal-Wallis tests were used. Mixed-effects regression models were used to study eGFR changes over time and to compare the odds of being CKD stage 3, between study times, for each classification category. Univariable and multivariable Cox regression models were applied to study time until death and until CKD development. Hazard ratios (HR) and corresponding 95% confidence intervals (CI) were obtained. Proportional hazards assumption of Cox regression was tested with a formal significance test based on standardized Schoenfeld residuals. As this assumption was not verified for age, Martingale residuals were obtained to identify a cut point for this variable. Martingale residuals were also used to obtain a cut point for the CCI score.
Several overall measures of model performance were obtained such as the likelihood ratio test statistic, Nagelkerke R2 (assumes values between 0 and 1), discrimination C statistic, (the c-index ranges from 0 to 1) and calibration slope (assumes values between −1 and 1). These measures were internally validated with bootstrapping techniques (200 replicates). Model choice also considered Akaike’s Information Criterion (AIC) (lower values correspond to better model performances) [31, 32]. In order to quantify the improvement resulting from adding clinical classification (Model 2) to a clinical model, and from adding SCysC or pNGAL to Model 2, continuous net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were calculated [33, 34].
A logistic regression model was used to identify variables that could influence the loss of kidney function related to the baseline. The model’s performance was quantified in terms of calibration using the Hosmer-Lemeshow goodness-of-fit test and for discrimination using the area under the Receiver Operating Characteristic (ROC) curve [35]. Bootstrap 95% bias-corrected confidence intervals were obtained for these areas. A level of significance α=0.05 was considered.
All data were analysed using STATA 13.0 (StataCorp 2013, Stata Statistical Software: Release 13. College Station, TX: StataCorp LP) and R software (R: A Language and Environment for Statistical Computing, R Core Team, R Foundation for Statistical Computing, Vienna, Austria, 2014).
Supplementary Material
Supplemental Figure 1. Flow chart of patients at presentation and inclusion in the prior study [14, 17].
Acknowledgments
PD is supported by grants from the NIH (P50 DK096418). KS is supported by grants from the Portuguese Nephrology Society, and the Hospital Fernando Fonseca (HFF). We would like to acknowledge the help and support of the Emergency Department physicians and nursing staff at HFF. We are also grateful to AH Marques of the DPCG of HFF.
Footnotes
DISCLOSURE
PD is a co-inventor on submitted patents on the use of NGAL as a biomarker of kidney injury. All other authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lameire NH, Bagga A, Cruz D, et al. Acute kidney injury: an increasing global concern. Lancet. 2013;382:170–79. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 2.Remuzzi G, Horton R. Acute renal failure: an unacceptable death sentence globally. Lancet. 2013;382:2041–42. doi: 10.1016/S0140-6736(13)62193-5. [DOI] [PubMed] [Google Scholar]
- 3.Remuzzi G, Benigni A, Finkelstein FO, et al. Kidney failure: aims for the next 10 years and barriers to success. Lancet. 2013;382:353–62. doi: 10.1016/S0140-6736(13)60438-9. [DOI] [PubMed] [Google Scholar]
- 4.Horton R, Berman P. Eliminating acute kidney injury by 2025: an achievable goal. Lancet. 2015;385:2551–52. doi: 10.1016/S0140-6736(15)60269-0. [DOI] [PubMed] [Google Scholar]
- 5.Mehta RL, Cerdáa, Burdmann EA, et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385:2616–43. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 6.Chawla LS, Amdur RL, Amodeo S, et al. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79:1361–69. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–28. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179–85. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 9.Amdur RL, Chawla LS, Amodeo S, et al. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int. 2009;76:1089–97. doi: 10.1038/ki.2009.332. [DOI] [PubMed] [Google Scholar]
- 10.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney diseases as interconnected syndromes. N Engl J Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo LJ, Go AS, Chertow GM, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76:893–99. doi: 10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–48. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kellum JA, Bellomo R, Ronco C. Kidney attack. JAMA. 2012;307:2265–66.14. doi: 10.1001/jama.2012.4315. [DOI] [PubMed] [Google Scholar]
- 14.Soto K, Coelho S, Rodrigues B, et al. Cystatin C as a marker of acute kidney injury in the emergency department. Clin J Am Soc Nephrol. 2010;5:1745–54. doi: 10.2215/CJN.00690110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Y, Zhang Y, Li G, Wang L. Relationship of cystatin-C change and the prevalence of death or dialysis need after acute kidney injury: a meta-analysis. Nephrology (Carlton) 2014;19:679–684. doi: 10.1111/nep.12312. [DOI] [PubMed] [Google Scholar]
- 16.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–38. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 17.Soto K, Papoila AL, Coelho S, et al. Plasma NGAL for the diagnosis of AKI in patients admitted from the emergency department setting. Clin J Am Soc Nephrol. 2013;8:2053–63. doi: 10.2215/CJN.12181212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta RL, Chertow GM. Acute renal failure definitions and classification: time for change? J Am Soc Nephrol. 2003;14:2178–87. doi: 10.1097/01.asn.0000079042.13465.1a. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82:516–24. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 21.Uchino S, Bellomo R, Bagshaw SM, Goldsmith D. Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transplant. 2010;25:1833–39. doi: 10.1093/ndt/gfp624. [DOI] [PubMed] [Google Scholar]
- 22.Nejat M, Pickering JW, Devarajan P, et al. Some biomarkers of acute kidney injury are increased in pre-renal acute injury. Kidney Int. 2012;81:1254–62. doi: 10.1038/ki.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z, Lu B, Sheng X, Jin N. Cystatin C in prediction of acute kidney injury: a systemic review and meta-analysis. Am J Kidney Dis. 2011;58(3):356–65. doi: 10.1053/j.ajkd.2011.02.389. [DOI] [PubMed] [Google Scholar]
- 24.Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–61. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grams ME, Sang Y, Ballew SH, et al. A meta-analysis of the association of estimated GFR, albuminuria, age, race, and sex with acute kidney injury. Am J Kidney Dis. 2015;66:591–601. doi: 10.1053/j.ajkd.2015.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James MT, Grams ME, Woodward M, et al. A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis. 2015;66:602–12. doi: 10.1053/j.ajkd.2015.02.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 29.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative workgroup: Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for some traditional and novel measures. Epidemiology. 2010;21:128–38. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pepe MS, Feng Z, Huang Y, et al. Integrating the Predictiveness of a Marker with Its Performance as a Classifier. Am J of Epidemiology. 2008;167:362–8. doi: 10.1093/aje/kwm305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pencina MJ, D’Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Statistics in Medicine. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in Medicine. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 35.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Flow chart of patients at presentation and inclusion in the prior study [14, 17].