Abstract
Activation of M2 muscarinic receptors (M2Rs) in the rat anterior basolateral nucleus (BLa) is critical for the consolidation of memories of emotionally arousing events. In the present investigation immunocytochemistry at the electron microscopic level was used to determine which structures in the BLa express M2Rs. In addition, dual localization of M2R and the vesicular acetylcholine transporter protein (VAChT), a marker for cholinergic axons, was performed to determine if M2R is an autoreceptor in cholinergic axons innervating the BLa. M2R-immunoreactivity (M2R-ir) was absent from the perikarya of pyramidal neurons, with the exception of the Golgi complex, but was dense in the proximal dendrites and axon initial segments emanating from these neurons. Most perikarya of nonpyramidal neurons were also M2R-negative. About 95% of dendritic shafts and 60% of dendritic spines were M2-immunoreactive (M2R+). Some M2R+ dendrites had spines suggesting that they belonged to pyramidal cells, whereas others had morphological features typical of nonpyramidal neurons. M2R-ir was also seen in axon terminals, most of which formed asymmetrical synapses. The main targets of M2R+ terminals forming asymmetrical (putative excitatory) synapses were dendritic spines, most of which were M2R+. The main targets of M2R+ terminals forming symmetrical (putative inhibitory or neuromodulatory) synapses were unlabeled perikarya and M2R+ dendritic shafts. M2R-ir was also seen in VAChT+ cholinergic terminals, indicating a possible autoreceptor role. These findings suggest that M2R-mediated mechanisms in the BLa are very complex, involving postsynaptic effects in dendrites, as well as regulating release of glutamate, GABA and acetylcholine from presynaptic axon terminals.
Keywords: vesicular acetylcholine transporter protein, immunocytochemistry, electron microscopy, acetylcholine, autoreceptor, RRID: AB_94952, RRID: AB_572269
INTRODUCTION
The basolateral amygdala receives a remarkably robust innervation from the cholinergic basal forebrain, especially the Ch4 group in the substantia innominata (Mesulam et al., 1983a, b; Carlsen et al., 1985; Rao et al., 1987; Kordower et al., 1989), and has some of the highest levels of choline acetyltransferase (the synthetic enzyme for acetylcholine) and acetylcholinesterase (the catabolic enzyme for acetylcholine) in the brain (Ben-Ari et al., 1977; Girgis, 1980; Svendsen and Bird, 1985; Hellendall et al., 1986; Amaral and Bassett, 1989). Recent studies have shown that acetylcholine is critical for mnemonic functions performed by the basolateral amygdala (McGaugh, 2004). Post-training infusions of muscarinic cholinergic antagonists into the basolateral amygdala, or lesions of the basal forebrain cholinergic system projecting to the amygdala, produce impairments in several types of emotional/motivational learning including inhibitory avoidance, contextual fear conditioning, conditioned place preference, and drug-stimulus learning (Power et al., 2003a).
Activation of both M1 and M2 muscarinic receptors in the anterior basolateral nucleus (BLa) of the rat basolateral amygdala is required for memory consolidation functions performed by this brain region (Power et al., 2003b). Although knowledge of the cellular and subcellular localization of these receptors in the BLa is critical for understanding the actions of acetylcholine involved in consolidation of memory, previous receptor binding autoradiographic studies and in situ hybridization studies lacked the resolution necessary to identify which neurons and synapses in the BLa express different muscarinic receptor subtypes. However, the development of antibodies to specific muscarinic receptor subtypes has allowed immunohistochemical localization of these receptors at the light and electron microscopic levels (Levey et al., 1991; Mrzljak et al., 1993; Rouse et al., 1998). Pharmacological studies have identified at least 4 muscarinic receptor subtypes (designated by upper case letters M1-M4), whereas molecular biological techniques have identified 5 distinct subtypes (designated by lower case letters m1-m5) (Ehlert et al., 1995). In the present study we performed an ultrastructural analysis of the BLa using an m2 receptor subtype specific antibody. For convenience, this receptor will be abbreviated “M2R”, with the understanding that it is actually the m2 molecular subtype that was localized. In a previous study we performed an ultrastructural analysis of m1 receptor in the BLa (Muller et al., 2013). It appears that most, if not all, currently available antibodies to m3, m4 or m5 receptors do not produce specific staining when used in immunohistochemical studies (Jositsch et al., 2009).
We recently examined the distribution of M2R immunoreactivity (M2R-ir) in the amygdala using light microscopy and observed that the highest levels were in the BLa (McDonald and Mascagni, 2011). This study revealed that there appeared to be no M2R-ir in the somata of pyramidal neurons, the principal neurons of the BLa, and the overwhelming majority of interneurons. On the other hand, the neuropil of the BLa had very high levels of M2R-ir, although the types of labeled structures could not be resolved at the light microscopic level. The present investigation is the first electron microscopic investigation of the ultrastructural localization of M2R in the BLa. In addition, dual localization of M2R and the vesicular acetylcholine transporter protein (VAChT), a marker for cholinergic axons, was performed to determine if M2R is an autoreceptor in cholinergic axons innervating the BLa.
MATERIALS AND METHODS
Animals
A total of 13 adult male Sprague-Dawley rats (250-350g; Harlan, Indianapolis, IN) were used in these studies. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Use and Care Committee (IACUC) of the University of South Carolina. All efforts were made to minimize animal suffering and to use the minimum number of animals necessary to produce reliable scientific data.
Light microscopy
Localization of the m2 muscarinic cholinergic receptor (M2R) at the light microscopic level was performed in 3 rats using the avidin-biotin immunoperoxidase (ABC) technique. Rats were anesthetized with chloral hydrate (350 mg/kg, I.P.) and perfused intracardially with phosphate buffered saline (PBS; pH 7.4) containing 0.5% sodium nitrite (50 ml) followed by 3.0% paraformaldehyde in 0.1 M phosphate buffer (PB) at pH 7.4 (500 ml). Following perfusion, brains were removed and postfixed for 3 h in 3.0% paraformaldehyde. Brains were then sectioned on a vibratome at a thickness of 50 μm in the coronal plane. Sections were processed for immunohistochemistry in wells of tissue culture plates. All antibodies were diluted in 0.1M PBS containing 0.3% Triton X-100, 1% normal goat serum, and 1% bovine serum albumin (BSA).
Sections through the amygdala were incubated in a rat monoclonal antibody to m2 (1:1000; catalog #mAB367, Millipore) for 48 h at 4° C and then processed for the avidin-biotin immunoperoxidase technique using a biotinylated donkey anti-rat secondary antibody (1:200; Jackson ImmunoResearch Laboratories) and a Vectastain Standard ABC kit (Vector Laboratories, Burlingame, CA) with nickel-enhanced DAB (3, 3′-diaminobenzidine-4HCl, Sigma Chemical Co., St. Louis, MO) as a chromogen to generate a black reaction product (Hancock, 1986). Following the immunohistochemical procedures, sections were mounted on gelatinized slides, dried overnight, dehydrated in ethanols, cleared in xylene, and coverslipped in Permount (Fisher Scientific, Pittsburgh, PA). Slides were analyzed using an Olympus BX51 microscope, and digital light micrographs were taken with an Olympus DP2-BSW camera system. Brightness and contrast were adjusted using Photoshop 6.0 software.
Tissue preparation for electron microscopy
Ten rats were used for electron microscopic studies. These rats were anesthetized with chloral hydrate (350 mg/kg, I.P.), or a mixture (I.P.) of ketamine (85mg/kg), xylazine (8mg/kg) and acepromazine (4mg/kg), and perfused intracardially with PBS containing 0.5% sodium nitrite (50 ml) followed by an acrolein/paraformaldehyde mixture (2.0% paraformaldehyde-3.75% acrolein in PB for 1 minute, followed by 2.0% paraformaldehyde in PB for 30 minutes). Following removal, brains were postfixed in 2.0% paraformaldehyde for 1 hour and sectioned on a vibratome in the coronal plane at 60 μm. Sections were rinsed in 1.0% borohydride in PB for 30 minutes and then rinsed thoroughly in several changes of PB for 1 hour. Sections were then cryoprotected in 30% sucrose in PB for 3 h, followed by three cycles of freeze-thaw over liquid nitrogen in order to increase antibody penetration. They were then processed for immunocytochemistry in the wells of tissue culture plates.
Single-labeling electron microscopic immunocytochemistry for M2R
Single-label pre-embedding electron microscopic immunocytochemistry using a nickel-intensified DAB immunoperoxidase method was performed in 4 rats to analyze the ultrastructural localization of M2R in the anterior subdivision of the basolateral nucleus (BLa; bregma levels −2.1 through −2.6; Paxinos and Watson, 1997). The BLa was chosen for study because it receives the densest cholinergic innervation in the amygdala, and since M2R in the BLa is known to be critical for memory consolidation of emotionally arousing experiences (Power et al., 2003b). Sections through the BLa were incubated in the same rat monoclonal antibody used for light microscopy (1:350) for 36 h at 4° C in PBS containing 1% normal donkey serum (NDS) and 2% BSA, and then processed using a biotinylated donkey anti-rat secondary antibody (1:200; Jackson ImmunoResearch Laboratories) and a Vectastain Standard ABC kit (Vector Laboratories, Burlingame, CA) with nickel-enhanced DAB (3, 3′-diaminobenzidine-4HCl, Sigma Chemical Co.) as a chromogen to generate a black reaction product (Hancock, 1986). Immunohistochemical processing with the primary antibody omitted produced no staining at the light and electron microscopic levels.
Sections were then postfixed in 2% osmium tetroxide in 0.16 M sodium cacodylate buffer (pH 7.4) for 1 h, dehydrated in graded ethanols and acetone, and flat embedded in Polybed 812 (Polysciences, Warrington, PA) in slide molds between sheets of Aclar (Ted Pella, Redding, CA). Silver thin-sections were collected on formvar-coated slot grids, stained with uranyl acetate and lead citrate, and examined with a JEOL-200CX electron microscope. Micrographs were taken with an AMT XR40 digital camera system (Advanced Microscopy Techniques, Danvers, MA). For publication, figures were then assembled, labeled and their components’ tonal ranges adjusted and matched using Adobe Photoshop 6.0.
Electron microscopic double-label immunocytochemistry
Electron microscopic immunocytochemistry using a sequential dual-labeling immunoperoxidase method (Muller et al., 2006) was utilized in 6 rats to examine the relationships of cholinergic axon terminals to M2R+ structures, and to determine if there is expression of M2R in VAChT+ cholinergic terminals. A goat polyclonal antibody to the vesicular acetylcholine transporter (VAChT; ImmunoStar, Hudson, WI) was used to label cholinergic axons (Weihe et al., 1996; Gilmor et al., 1996; Muller et al., 2011, 2013). Sections were incubated for 36 h at 4°C in the rat M2R antibody (1:200) diluted in PBS containing 3% NDS and 1% BSA, and processed using a biotinylated donkey anti-rat secondary antibody (1:200; Jackson ImmunoResearch Laboratories) for 1 h and a Vectastain Elite ABC kit (Vector Laboratories). M2R immunoreactivity was then visualized using a Vector VIP (Very Intense Purple) peroxidase substrate kit (Vector Laboratories). This procedure yields a reaction product that appears purple in the light microscope, and granular or particulate in the electron microscope (Smiley et al., 1997; Van Haeften and Wouterlood, 2000; Muller et al., 2006). This chromogen will be termed “PUR” in the present account. After rinsing, sections were incubated in an avidin/biotin blocking solution (Avidin/Biotin Blocking Kit, Vector Laboratories). Sections were then incubated for 36 hrs at 4°C in a goat VAChT antibody (1:4,000) in PBS containing 3% NDS and 1% BSA, and processed using a biotinylated donkey anti-goat secondary antibody (1:300; Jackson ImmunoResearch Laboratories) for 1 h and a Vectastain Standard ABC kit (Vector Laboratories) with non-intensified DAB as the chromogen to produce a brown reaction product. Sections were then processed for electron microscopy as described above.
The diffuse DAB reaction product for VAChT was easily distinguished from the granular/particulate PUR reaction product for M2R at the ultrastructural level. In the smallest structures, such as spines and small axon terminals, the criterion for calling a structure “labeled” for M2R was the observation of at least 2 PUR granules of reaction product in the structure. For all other structures the criterion was three or more granules. Light and electron microscopic examination of sections processed using this dual-labeling DAB/PUR immunoperoxidase method, but with the M2R antibody omitted, produced no PUR reaction product; only single-labeled VAChT+ axons with diffuse DAB reaction product were observed (Fig. 11C). Light and electron microscopic examination of sections processed using this dual-labeling DAB/PUR immunoperoxidase method, but with the VAChT antibody omitted, produced no DAB reaction product; only single-labeled M2R+ structures with particulate PUR reaction product were observed.
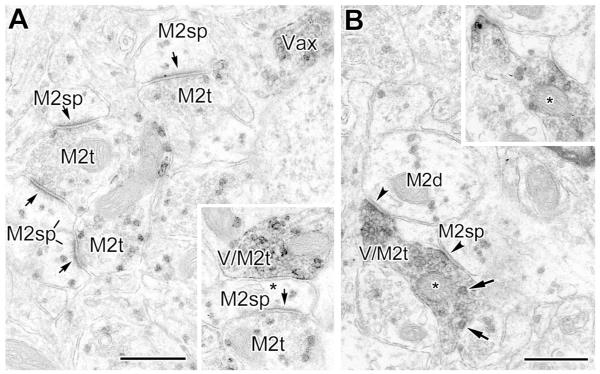
Fig. 11.
(A) Four M2R+ spines (M2sp) receive asymmetrical synaptic inputs from M2R+ terminals (M2t, arrows) (PUR as a chromogen for M2R). A VAChT+ axon stained with DAB is also indicated (Vax). Inset: A nearby VAChT+ terminal (with diffuse DAB label) that is also labeled for M2R (granular PUR label) (V/M2t) forms a symmetrical synapse with an M2R+ spine (asterisk in inset). (B) A VAChT+/M2R+ colabeled axon with two terminal swellings (V/M2t) forms symmetrical synaptic contacts (arrowheads) with an M2R+ dendrite (M2d) and an adjacent M2R+ spine (M2sp), arrowheads). The spinous nature of the latter process was more evident in adjacent serial thin sections. Two granules of PUR reaction product are indicated by arrows. Inset: A serial section of the VAChT/M2R+ colabeled axon (asterisk), where the diffuse DAB label for VAChT is paler, reveals the granular PUR label for M2R-immunoreactivity more clearly. Scale bars = 0.5 μm.
Analysis
Profiles were identified as perikarya, axon initial segments, large-caliber dendrites (>1 μm), small-caliber dendrites (<1 μm), dendritic spines, and axon terminals using established morphological criteria (Peters et al., 1991). The BLa contains pyramidal and nonpyramidal neurons that are very similar to those in the cerebral cortex (McDonald, 1992; Sah et al., 2003; McDonald 2003; Pape and Pare, 2010; Spampanato et al, 2011). In the absence of specific markers for these neurons, putative pyramidal cell perikarya were identified by their large size, round nuclei, and synaptic input from terminals forming only symmetrical synapses (Muller et al., 2006). Putative nonpyramidal cell perikarya are smaller, often have infolded nuclei, and receive both symmetrical and asymmetrical synapses, with the latter sometimes associated with perikaryal spines (Muller et al., 2005, 2011); their dendrites can be followed for a significant length without a visible spine, and they often have a high density of asymmetrical synapses.
For both single and double-labeled preparations, the two brains judged to have the best ultrastructural preservation and immunohistochemistry were selected for quantitative analysis. Since each technique was performed in different rats, the quantitative analysis involved a total of four rats. Two vibratome sections were chosen from each brain for each technique. About 4000-5000 μm2 from each section was used for quantitation. Serial sections were analyzed and often followed on consecutive grids. Serial sections were helpful for verifying label in small and lightly immunoreactive structures, and determining the synaptic nature of contacts. For each vibratome section, areas that exhibited the best morphology and uniform immunohistochemistry were chosen within a single thin section for quantitation. Areas that were very close to the tissue/plastic interface exhibited poor ultrastructure whereas areas that were too deep in the vibratome section had diminished staining. The areas selected for quantitative analysis appeared to be midway between the latter two extremes. All M2R-positive and M2R-negative dendrites and spines were counted in that area, and followed serially only to ascertain identification and label. This analysis provided an estimate of the percentage of dendrites and spines that were M2R+. Counts of M2R+ structures were similar in the single-labeled DAB preparations and the double-labeled preparations where PUR was used to stain M2R (see Results).
M2R+ axon terminals forming asymmetrical (presumptive excitatory) synapses or symmetrical (predominantly inhibitory) synapses were counted in both the single-labeled nickel-intensified DAB material, as well as in the M2R/VAChT dual-labeled material (in which M2R was visualized using the PUR chromogen), and the postsynaptic targets of these terminals, as well as whether they were M2R-positive or negative, were noted. Synapses were identified using standard criteria: 1) parallel presynaptic and postsynaptic membranes exhibiting membrane thickenings, 2) a synaptic cleft containing dense material, and 3) clustered synaptic vesicles associated with the presynaptic membrane (Peters et al., 1991). Asymmetrical and symmetrical synapses were identified based on the presence or absence, respectively, of a prominent postsynaptic density and on the relative widths of their synaptic clefts. Whereas synaptic clefts of asymmetrical synapses are typically 20 nm wide, symmetrical synapses have a narrower synaptic cleft that is generally about 12 nm wide (Peters et al., 1991). In most cases the two types of synapses were easily distinguished. However, a small number of synapses had postsynaptic densities that appeared to be of intermediate thickness. These synapses typically had wide synaptic clefts and were considered asymmetrical synapses.
Antibody Specificity
The rat monoclonal M2R primary antibody (Millipore, catalog #mAB367, RRID: AB_94952) was raised against a highly purified GST fusion protein of a part of the third intracellular loop of the human m2 receptor corresponding to amino acids 225-359 (Table 1). In immunoblots this antibody recognized the M2R fusion protein, but showed no cross-reactivity to other muscarinic receptor subtypes (Levey et al., 1995). No immunostaining with this M2R antibody was observed in M2R knockout mice (Jositsch et al., 2009). The pattern of m2R immunohistochemical labeling with this antibody in the rat brain, and the amygdala in particular (McDonald and Mascagni, 2011), is identical to that seen with polyclonal antibodies developed by the Levey laboratory (Levey et al., 1995).
Table 1.
Table of Primary Antibodies
| Antigen | Immunogen | Manufacturer | Dilution |
|---|---|---|---|
| M2 muscarinic acetylcholine receptor |
Highly purified GST fusion protein of a part of the third intracellular loop (amino acid residues 225-359) of the human m2 muscarinic acetylcholine receptor |
Millipore (Billerica, MA, USA), rat monoclonal, cat# mAB367, RRID: AB_94952 |
1:200 |
| Vesicular acetylcholine transporter |
Synthetic carboxy-terminal 20 amino acid sequence (511-530) of the cloned rat vesicular acetylcholine transporter |
ImmunoStar (Hudson, WI, USA), goat polyclonal, cat# 24286 RRID: AB_572269 |
1:4000 |
The VAChT antibody (Immunostar, catalog #2486, RRID: AB_572269) was raised in goat using a synthetic carboxy-terminal 20-amino-acid sequence (511-530: CSPPGPFDGCEDDYNYYSRS) from the cloned rat VAChT as an immunogen, and has previously been characterized (Arvidsson et al., 1997) (Table 1). The antisera recognizes VAChT, but not vesicular monoamine transporters, in transfected cells. VAChT immunoreactivity was also seen in cells that are known to express the protein, such as PC12 cells and cultured spinal motoneurons. Preadsorption of the antisera with the immunizing peptide (Arvidsson et al., 1997) or omission of the primary antibody (McDonald and Mascagni, 2011) completely abolished immunostaining in all brain areas.
RESULTS
Distribution of M2R-immunoreactivity
Light microscopic examination of the BLa revealed that this nucleus had the highest levels of M2R-immunoreactivity (M2R-ir) in the amygdala (Fig. 1A). The rostral part of the lateral nucleus also had robust M2R-ir (Fig. 1A), but staining diminished at more caudal levels. Almost all cell bodies in the BLa, most of which correspond to the large cell bodies of BLa pyramidal cells, had no M2R-ir. In contrast, the neuropil in the BLa was very densely stained, consisting of M2R+ puncta, neuronal processes, and diffuse staining (Fig. 1B). However, an occasional intensely-stained nonpyramidal neuron was encountered, consistent with previous light microscopic studies of M2R in the amygdala (McDonald and Mascagni, 2011).
Fig. 1.
Light micrographs of M2R-ir in the BLa. (A) Low power photomicrograph of a section through the BLa and adjacent amygdalar nuclei at the bregma −2.3 level. Note dense staining in the BLa and adjacent portion of the lateral nucleus (Lat), but much lighter staining in the main intercalated nucleus (IN), lateral central nucleus (CL), and caudatoputamen (CP). (B) High power photomicrograph of the BLa. Note dense neuropilar staining and little or no M2R-ir in neuronal cell bodies (asterisks). Also note that there are M2R+ puncta in apparent contact with some of the cell bodies (e.g., in the neuron near the bottom of the field). Scale bars = 150 μm in A, 20 μm in B.
Electron microscopic analysis of the BLa also confirmed that M2R-ir was dense in the neuropil of the BLa, but usually not observable in neuronal perikarya (Figs. 2-4). M2R-ir in putative pyramidal cell perikarya, when present, was only found associated with the Golgi complex (Figs. 3, 4). Because M2R-ir was never seen in other portions of these perikarya, including along the plasma membrane, these perikarya were termed “unlabeled” despite the presence of M2R-ir in the Golgi complex observed in some of these cells. It is assumed that the M2R-ir in the Golgi complex in these neurons indicates that M2R is being packaged for transport to dendritic and/or axonal processes of these neurons. Whereas most perikarya of putative nonpyramidal cells appeared free of M2R-ir, a few were densely labeled and probably correspond to previously described M2R+ somatostatin/neuropeptide-Y-containing nonpyramidal neurons (not shown) (McDonald and Mascagni, 2011).
Fig. 2.
Electron micrographs showing lack of M2R-ir in putative pyramidal cell perikarya, identified on the basis of synaptology (see Materials and Methods), but dense M2R-ir in processes arising from these perikarya (PUR as chromogen for M2R). (A) An unlabeled pyramidal cell perikaryon (Upk) gives rise to a densely-labeled proximal dendrite (M2d, center), with other M2R+ dendrites indicated. (B) An unlabeled pyramidal cell perikaryon (Upk) gives rise to an M2R+ process (M2p) that may be a small-caliber primary dendrite or an axon initial segment. Its identification as a putative axon initial segment is based on the fact that: (1) there appears to be some fasciculation of microtubules, (2) the conical shape of its origin from the perikaryon resembles that of an axon hillock, and (3) ribosomes and rough endoplasmic reticulum of the perikaryon do not extent into the putative axon hillock (Peters et al., 1991). M2R+ terminals (M2t) are also indicated, one forming a symmetrical synapse with the M2R+ process (arrowhead), and another forming an asymmetrical synapse with an M2R+ spine (asterisk). A DAB-labeled VAChT+ axon is shown to the right of the field (Vax). Scale bars = 0.5 μm.
Fig. 4.
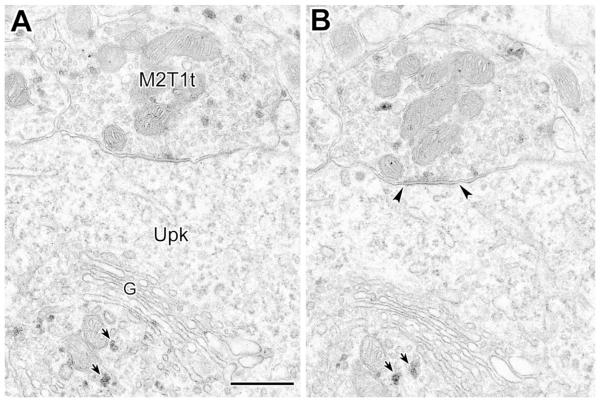
(A, B) Serial electron micrographs of an M2R+ putative GABAergic type 1 terminal from the basal forebrain forming a symmetrical synapse with a pyramidal cell perikaryon (Upk, arrowheads) (PUR as chromogen for M2R). In the perikaryon several grains are visible in what appears to be the trans-Golgi network of the Golgi complex (G, short arrows), but not in other portions of the perikaryon. Also note that some PUR particles are adjacent to the plasma membrane of the axon terminal. Scale bar = 0.5 μm.
Fig. 3.
M2R+ synaptic inputs to a perikaryon, spines and dendrites (PUR as chromogen for M2R). An unlabeled putative pyramidal cell perikaryon (Upk) receives symmetrical synaptic input from an M2R+ terminal (M2t, arrowheads), and has a single PUR grain (short arrow) associated with a tubular profile that may be part of the trans-Golgi network (see Fig. 4). A nearby M2R+ dendrite (M2d) and its spine, each receive synaptic inputs from M2R+ terminals (M2t, center and right). The presence of the spine suggests that this dendrite may belong to a pyramidal cell. The dendrite, where the spine emerges, receives a symmetrical synapse (M2t, center, arrowheads) and the spine head receives an asymmetrical synapse (M2t, right, arrow). A DAB-labeled VAChT+ axon is also indicated (Vax). Scale bar = 0.5 μm.
Unlabeled perikarya gave rise to dendrites that exhibited dense M2R-ir. This was seen in putative pyramidal cells (Fig. 2) as well as in most putative nonpyramidal cells. Axon initial segments exhibiting M2R-ir were also observed (Fig. 5A, and perhaps Fig. 2B). Overall, the great majority of dendrites were M2R+ in both PUR (96.1%; 174/181) and Ni-DAB-labeled preparations (94.7%; 142/150) (Figs. 2-3, 7-10), with only a few small-caliber dendrites unlabeled. Some M2R+ dendrites had features consistent with pyramidal cell dendrites, including giving off spines (Figs. 3, 9) and having mainly symmetrical synapses. Other M2R+ dendrites had features consistent with nonpyramidal cell dendrites, with a high density of synaptic inputs from terminals with asymmetrical (putative glutamatergic) synapses, and in longitudinal view, a substantial length free of spines (Figs. 7, 8B). Approximately half of the dendritic spines surveyed were M2R+ in both PUR (61.6%; 85/138) and Ni-DAB-labeled preparations (48.7%; 75/154) (Figs. 7, 9, 11). The higher frequency of M2R+ spines in PUR preparations was probably due to the fact that M2R-ir in lightly1labeled smaller profiles may be easier to recognize with a granular label. Particulate PUR label for M2R was sometimes found adjacent to dendritic plasma membranes, including plasma membranes at or along the edges of synapses formed by VAChT+ cholinergic terminals and unlabeled or M2R+ non-cholinergic terminals (Figs. 7-10).
Fig. 5.
M2R+ structures labeled using Ni-DAB as a chromogen. (A) An M2R+ terminal (M2t) forms a symmetrical synapse with a lightly-immunoreactive axon initial segment (M2ais) identified on the basis of its decorated fasciculated microtubules. The M2ais also receives a symmetrical synapse from an unlabeled terminal (Ut, arrowheads). Two nearby M2R+ spines (M2sp, top and bottom right) receive asymmetrical synapses from unlabeled terminals (Ut, asterisks). (B) A lightly-immunoreactive M2R+ dendrite (M2d) receives symmetrical synaptic input from an M2R+ terminal (M2t, arrowhead) and asymmetrical synaptic input from an adjacent unlabeled terminal (Ut, arrow). Scale bars =0.5 μm.
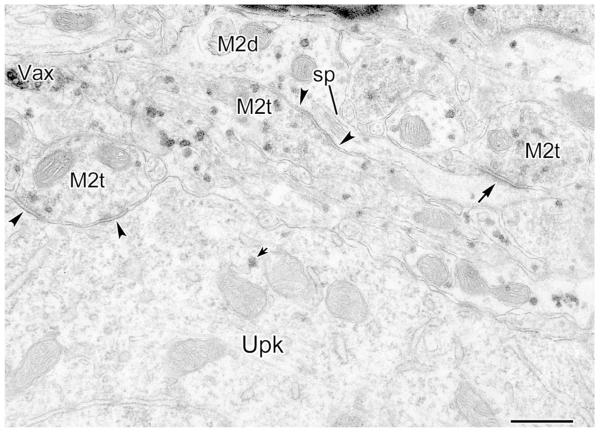
Fig. 7.
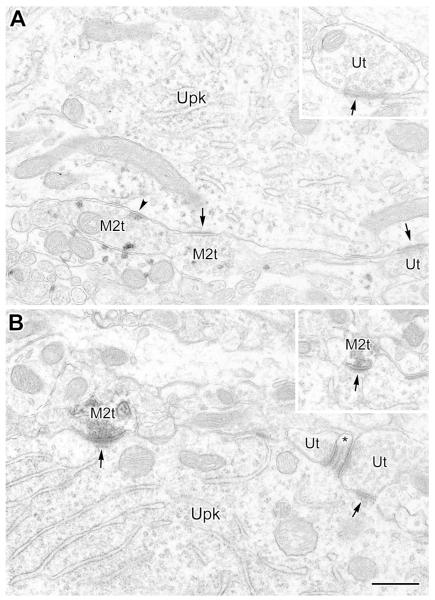
Variety of synaptic inputs onto an M2R+ dendrite and nearby spines (PUR as chromogen for M2R). The multiple asymmetrical synapses with this dendrite suggests that it may belong to a nonpyramidal neuron. The M2R+ dendrite (M2d) receives asymmetrical synaptic inputs (arrows) from an M2R+ terminal (M2t), an M2R+ axon (M2ax) with two M2R+ terminals, and one unlabeled terminal (Ut), as well as symmetrical synaptic inputs (arrowheads) from two M2R+ terminals (M2t). In addition, a large spine head (M2sp, left) and a small spine neck (M2sp, top) receive asymmetrical synaptic inputs from M2R+ terminals (M2t, arrows). Inset shows a serial section of three of the terminals (asterisks) and the M2d. Note the accumulation of PUR grains along the dendritic plasma membrane at the edge of the synapses (double arrows in inset); an additional symmetrical synaptic site on the M2t on the right is indicated (arrowhead). Scale bar = 0.5 μm.
Fig. 10.
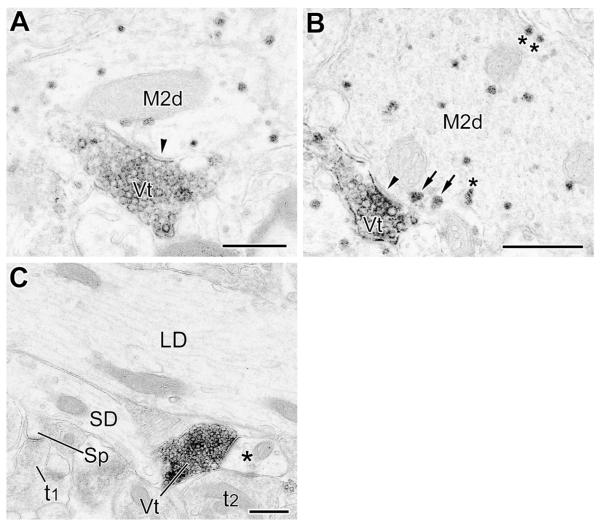
(A and B) VAChT+ terminals (Vt) form symmetrical synapses (arrowheads) with small-caliber (A) and large-caliber (B) M2R+ dendritic shafts (M2d) (PUR as a chromogen for M2R). Arrows in B indicate two granules of M2R PUR reaction product near the plasma membrane just outside the active zone of the synapse. This configuration was seen in about half of synapses involving VAChT+ terminals, and some non-cholinergic terminals. Additional granules of plasma membrane-associated M2R PUR reaction are indicated by asterisks. (C) Representative control section in which the M2R antibody was omitted. Note total absence of M2R granular PUR reaction product in a large-caliber dendrite (LD), small-caliber dendrite (SD), and a spine (Sp) and its presynaptic terminal (t1). On the right a DAB-labeled VAChT+ axon terminal (Vt) and an unlabeled axon terminal (t2) contact a small-caliber dendrite (asterisk); the VAChT+ terminal forms a symmetrical synapse whereas the contact of the unlabeled terminal appears to be a puncta adherens. Scale bars = 0.5 μm.
Fig. 9.
(A) An M2R+ dendrite (M2d) gives rise to a large unlabeled mushroom-shaped spine (Usp) that receives asymmetrical synaptic input from an M2R+ terminal (M2t, arrows). (B) An M2R+ spine (M2sp) emerges from a M2R+ dendrite (M2d) and receives asymmetrical synaptic input from an M2R+ terminal (M2t, arrow). The presence of the spines associated with these dendrites in A and B suggests that they may belong to pyramidal cells. Note that some PUR particles are adjacent to the plasma membrane of the dendrite. Scale bar (for panels A and B) = 0.5 μm.
Fig. 8.
M2R+ synaptic inputs to M2R+ dendrites (PUR as a chromogen for M2R). (A) An M2R+ dendrite (M2d) receives asymmetrical synaptic input from an M2R+ terminal (M2t, arrow). Note that some PUR particles are adjacent to the plasma membrane of this axon terminal. Two nearby unlabeled terminals are indicated (Ut). (B) An M2R+ dendrite (M2d) receives asymmetrical synaptic inputs from three M2R+ terminals (M2t, arrows) and symmetrical synaptic input from another M2R+ terminal (M2t, arrowhead). Multiple asymmetrical synaptic inputs suggests this dendrite may arise from a nonpyramidal cell. Note that some PUR particles are adjacent to the plasma membrane of the dendrite and axon terminals. Scale bars = 0.5 μm.
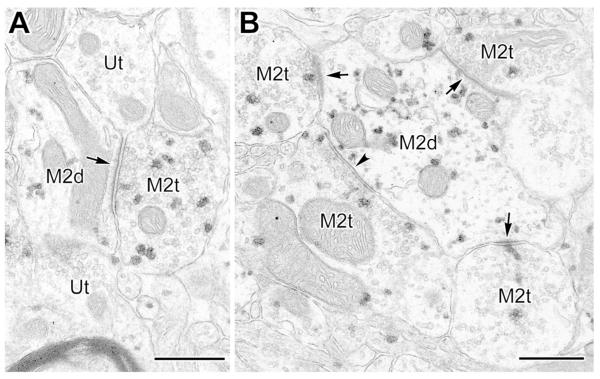
Many M2R+ terminals were found in both PUR and Ni-DAB preparations (Figs. 2-9, 11). For both chromogens, approximately two-thirds of the M2R+ terminals formed asymmetrical synapses (66.5% [189/284] with PUR, and 68.8% [84/122] with Ni-DAB), and one-third formed symmetrical synapses (33.5% (95/284) with PUR, and 31.1% (38/122) with Ni-DAB. Granular PUR label for M2R-ir could be seen among synaptic vesicles, as well as along plasma membranes, including near synapses (Figs. 3, 4, 6-9, 11). The great majority of terminals forming symmetrical synapses were of the size (up to 1 μm in diameter) and morphology typical of those belonging to local GABAergic interneurons (McDonald et al., 2002; Muller et al., 2003, 2005, 2006, 2007). However, some terminals forming symmetrical synapses were large (approximately 2 μm in diameter) and contained many mitochondria and synaptic vesicles (Fig. 4), typical of terminals that originate from GABAergic projection neurons in the basal forebrain (McDonald et al., 2011).
Fig. 6.
M2R+ synaptic inputs to the perikarya of nonpyramidal neurons. (A) Electron micrograph (with PUR as a chromogen for M2R) of an unlabeled perikaryon (Upk) receiving a symmetrical synapse from an M2R+ terminal (M2t, left, arrowhead) and asymmetrical synaptic inputs from an M2R+ terminal (M2t, center, arrow) and two unlabeled terminals (Ut, bottom right and inset, arrows). Only perikarya of nonpyramidal neurons receive asymmetrical (putative glutamatergic) synaptic inputs. (B) An unlabeled nonpyramidal perikaryon (Upk) receives asymmetrical synaptic inputs from M2R+ terminals (M2t, left and inset, arrows; Ni-DAB as chromogen for M2R) and an unlabeled terminal (Ut, right, arrow). The unlabeled terminal is forming one of two asymmetrical synapses with a small perikaryal spine (Ut, right, asterisk) typical of a subset of nonpyramidal neurons. Note subsynaptic bodies in the spine, as well as postsynaptic to the M2R+ terminal on the left. Scale bar (for panels A and B) = 0.5 μm.
In the M2R/VAChT double-labeled preparations, the great majority of VAChT+ axon terminals that formed synapses targeted M2R+ dendritic shafts or spines (Figs. 10, 11). However, some of the VAChT+ axon terminals targeted M2R-negative dendrites and spines. All of these synapses were symmetrical. VAChT+ cholinergic terminals were often in close proximity to M2R+ non-cholinergic terminals forming asymmetrical or symmetrical synapses. In most cases the PUR reaction product in M2R+ dendritic shafts and spines that were postsynaptic to VAChT+ axons terminals was not found directly beneath the postsynaptic membrane. It was common, however, for postsynaptic M2R-ir to be located just outside of the active zone of the synapse (Fig. 10B). VAChT+ axon terminals that were lightly labeled by the DAB chromogen were frequently observed to also contain particulate M2R-ir from the PUR chromogen (Fig. 11). In terminals where VAChT-ir was dense, it was difficult to detect particulate PUR reaction product. All of these M2R+/VAChT+ terminals formed symmetrical synapses, typical of cholinergic VAChT+ terminals in the BLa (Muller et al., 2011, 2013). M2R+/VAChT+ terminals were observed forming symmetrical synapses with PUR-labeled M2R+ dendrites and spines (Fig. 11), as well as with M2R-negative dendrites and spines. Terminals containing both DAB and PUR reaction products were never observed in control sections in which one of the two primary antibodies was omitted (see Fig. 10C for an M2R antibody omission control).
Targets of M2R+ terminals
In general, the main postsynaptic targets of M2R+ terminals making asymmetrical synaptic contacts were spines, most of which were also M2R+ (Table 2). The main postsynaptic targets of M2R+ terminals making symmetrical synaptic contacts were M2R+ dendrites and unlabeled perikarya (Table 2). One criterion for identifying pyramidal cell perikarya ultrastructurally is that they receive exclusively symmetrical synaptic inputs (see Materials and Methods). Thus all M2R+ terminals that made synaptic contact with putative pyramidal cells formed symmetrical synapses (Figs. 3, 4). Unlabeled putative nonpyramidal cell perikarya received synaptic inputs from M2R+ terminals forming asymmetrical as well as symmetrical synapses (Fig. 6). Large M2R+ putative GABAergic (type 1) terminals from the basal forebrain were found to make symmetrical synaptic contacts with putative pyramidal cell perikarya (Fig. 4) as well as with putative nonpyramidal cell perikarya (not shown), consistent with the targets of type 1 terminals in the BLa (McDonald et al., 2011).
Table 2.
Postsynaptic targets of M2R+ axon terminals forming asymmetrical (AS) or symmetrical (S) synapses in the BLa. PUR and Ni-DAB data were similar and were pooled (406 synapses total). M2R-positive and M2R-negative structures are indicated with a plus sign (+) or a minus sign (−), respectively. Abbreviations: Pk, perikarya; LD, large-caliber dendrite (>1.0 μm wide); SD, small-caliber dendrite (<1.0 μm wide); Sp; spine.
| Synapse Type |
Pk+ | Pk−1 | LD+ | LD− | SD+ | SD− | Sp+ | Sp− | Total |
|---|---|---|---|---|---|---|---|---|---|
| AS | 0% | 0.3% 1/273 |
4.8%, (13/273) |
0% | 17.6%, (48/273) |
0% | 55.3%, (151/273) |
21.9%, (60/273) |
273 (100%) |
| S | 3.0% (4/133) |
25.6% (34/133) |
21.8% (29/133) |
0.7% (1/133) |
29.3%, (39/133) |
1.5% (2/133) |
10.4%, (14/133) |
7.5%, (10/133) |
133 (100%) |
The term “unlabeled perikarya” (Pk−) refers to perikarya with no M2R-ir, or M2R-ir only in the Golgi complex.
Although twice as many M2R+ terminals, overall, made asymmetrical as compared to symmetrical synaptic contacts, dendritic shafts were postsynaptic to approximately equal numbers of M2R+ asymmetrical (n = 61) and symmetrical (n = 68) terminals. Most of the asymmetrical synapses from M2R+ terminals were onto small-caliber dendrites (n = 48), many of which probably belonged to nonpyramidal cells (Fig. 8). Symmetrical synaptic inputs were common onto both small-caliber (n = 39) and large-caliber dendrites (n = 29).
Over half of the synapses formed by M2R+ terminals were asymmetrical synaptic inputs to spines (Figs. 2, 3, 7, 9, 11), and 72% (151/211) of these postsynaptic spines were M2R+. Targets of symmetrical synapses from M2R+ terminals also included spine stalks, where the spine heads received asymmetrical synaptic input (Fig. 3). In addition, terminals co-labeled for VAChT-ir and M2R-ir mainly targeted M2R+ spines and small-caliber dendrites (Fig. 11).
DISCUSSION
The results of the present study demonstrate that there is robust expression of the m2 muscarinic cholinergic receptor (M2R) in dendritic shafts and spines of pyramidal neurons, and the dendritic shafts of some nonpyramidal cells in the BLa (Fig. 12). In addition, axon terminals forming asymmetrical (putative excitatory) synapses or symmetrical (putative inhibitory or neuromodulatory) synapses also express M2R. Some of the symmetrical synapses are formed by cholinergic VAChT+/M2R+ terminals in which M2R may act as an autoreceptor regulating acetylcholine release (Rouse et al., 2000). The ubiquity and high levels of M2R-ir in the neuropil of the BLa at the ultrastructural level of analysis is consistent with light microscopic observations (Fig. 1) and autoradiographic receptor binding studies (Mash and Potter, 1986; Spencer et al., 1986) showing dense neuropilar M2R immunoreactivity and receptor binding, respectively. The dense M2R-ir in the BLa seen at the ultrastructural level also matches the robust innervation of this amygdalar nucleus by cholinergic axons originating in the basal forebrain (Carlsen et al., 1985; Muller et al., 2011).
Fig. 12.
Schematic diagram illustrating the main findings of this study in relation to the basic circuitry of pyramidal neurons (blue) in the BLa. Inputs forming asymmetrical and symmetrical synapses are shown in green and red, respectively. M2R+ structures are indicated in black. All synaptic relationships that represent at least 7.5% of asymmetrical or symmetrical synapses involving pre- or postsynaptic M2R-ir are illustrated. In addition, since only a subset of each type of synaptic contact involved M2R+ structures, M2R-negative synapses that involve discrete pre- and postsynaptic elements are also illustrated. It is important to recall that M2R+ terminals forming asymmetrical synapses actually outnumber M2R+ terminals forming symmetrical synapses by approximately 2 to 1, which is not represented in this diagram.
In stark contrast to the extensive expression of M2R in the neuropilar components of the BLa, all pyramidal neuronal perikarya and virtually all nonpyramidal neuronal perikarya were unlabeled, except in the trans-Golgi network of the Golgi complex, which presumably reflects M2Rs that will be transported to the plasma membranes of dendritic and/or axonal processes of these neurons (Nathanson, 2008). In addition, it is important to point out that only a subset of each type of synaptic contact involved M2R+ structures. Thus, many synapses of each type did not involve M2R+ structures, or only one of the two components of the synapse (either the presynaptic axon terminal or the postsynaptic structure) exhibited M2R-ir (Fig. 12). These findings suggest the intriguing possibility that only specific inputs to BLa pyramidal neurons and interneurons are modulated by M2Rs when acetylcholine is released in the BLa, and that this modulation may be presynaptic, postsynaptic, or both, depending on the input. Elucidation of these mechanisms should contribute to an understanding of the role of M2Rs in the modulation of memory consolidation by the BLa (Power et al., 2003a, b).
M2R+ dendrites and spines in the BLa: Evidence for M2R-mediated postsynaptic cholinergic modulation of pyramidal and nonpyramidal neurons
In contrast to the m1 muscarinic receptor, which is found at high levels in BLa pyramidal neuronal perikarya (Muller et al., 2013), immunoreactivity for the m2 receptor was only observed in the vicinity of the Golgi complex of pyramidal neurons. This suggests that it is being packaged for transport to the plasma membranes of dendritic and axonal processes of pyramidal neurons, both of which contained high levels of the receptor starting at their initiation points from the cell body. Since pyramidal neurons comprise about 85% of BLa neurons, and have longer and more extensively branched dendrites than most nonpyramidal cells, the finding that almost all dendritic shafts are M2R+ indicates that the great majority of M2R+ dendrites belong to pyramidal neurons. Since it is also well established that most dendritic spines in the BLa arise from pyramidal neuronal distal dendrites (Muller et al., 2006), the great majority of M2R+ spines observed in this study must be associated with pyramidal neuronal dendrites. In addition, some of the M2R+ dendritic shafts of nonpyramidal cells seen in this study, identified by their characteristic multiple asymmetrical synapses, were also M2R-ir.
Particulate PUR label for M2R found adjacent to or contacting plasma membranes of dendritic shafts suggests that the excitability of dendrites may be modulated through M2R-mediated mechanisms after release and diffusion of acetylcholine from cholinergic terminals. However, since the extent to which the particles of PUR chromogen can diffuse from the antigenic binding site has yet to be ascertained, the precise localization of M2R-ir cannot be determined with certainty using PUR (or DAB) as immunoperoxidase chromogens. Cytoplasmic M2R-ir in dendritic shafts most likely represents receptor protein that is in the process of being transported to or from the plasma membrane (Bernard et al., 1998). The finding that cytoplasmic M2R-ir is found in some spines and not others suggests the likelihood that M2R-ir in spines represents receptor that is in the process of being transported to or from the spine that contains it. Thus although the use of PUR and Ni-DAB as chromogens in immunoperoxidase studies is a far more sensitive technique than the silver-intensified immunogold technique, further work using the latter high-resolution method is critical for more precise localization of M2Rs.
M2R+ axon terminals in the BLa: Evidence for M2R-mediated presynaptic cholinergic modulation of glutamatergic and intrinsic GABAergic inputs
Axon terminals in the BLa exhibiting M2R-ir formed asymmetrical or symmetrical synapses suggesting M2R modulation of synaptic release. M2R+ terminals forming asymmetrical synapses mainly contacted dendritic spines, the majority of which were also M2R+. M2R+ terminals forming symmetrical synapses mainly contacted unlabeled pyramidal neuronal perikarya and M2R+ dendrites.
Previous electron microscopic studies of the basolateral amygdala indicate that axon terminals forming asymmetrical synapses represent glutamatergic inputs from the cerebral cortex (Smith and Paré, 1994; Brinley-Reed et al., 1995; Farb and LeDoux, 1999; Smith et al., 2000), midline/intralaminar thalamus (Carlsen and Heimer, 1988; LeDoux et al., 1991), and internuclear and intranuclear amygdalar connections arising from basolateral amygdalar pyramidal neurons (Stefanacci et al., 1992; Smith and Paré, 1994; Paré et al., 1995; Smith et al., 2000). Many of the symmetrical synapses onto BLa pyramidal neurons are formed by several distinct subpopulations of GABAergic interneurons (Carlsen, 1988; Aylward and Totterdell, 1993; Smith et al., 2000; McDonald et al., 2002; Muller et al., 2003, 2006, 2007). However, monoaminergic projections from the brainstem (Asan, 1998, Muller et al., 2007, 2009; Farb and LeDoux, 2010; Zhang, 2013) and cholinergic projections from the basal forebrain (Carlsen and Heimer, 1986; Muller et al., 2011) also form symmetrical synapses. The use of a dual-labeling immunoperoxidase technique to study M2/VAChT localization in the present investigation allowed us to distinguish cholinergic versus non-cholinergic terminals.
VAChT+ terminals are usually about 1-3 μm apart (Muller et al., 2013). Because of the very high density of VAChT+ terminals in the BLa, the great majority of non-cholinergic M2R+ terminals are probably within 1-2 μm of a cholinergic terminal. The short distance between cholinergic terminals and M2R+ terminals suggests that synaptic spillover of acetylcholine during high rates or bursts of cholinergic activity, or possible diffusion from non-synaptic cholinergic terminals, could modulate the release of glutamate or GABA from neighboring M2R+ terminals (Vizi and Kiss, 1998, 2010; Sarter et al., 2009). For comparison, it has been suggested that in the striatum and substantia nigra the sphere of influence of released dopamine is about 2 μm for activation of low-affinity dopamine receptors and 7-8 μm for high-affinity receptors (Rice and Cragg, 2008).
The anatomical findings of the present study are consistent with electrophysiological studies of the BLa that have demonstrated modulation of glutamate and GABA release from axon terminals by muscarinic cholinergic mechanisms. Thus, Washburn and Moises (1992) reported that muscarinic agonists produced reductions in the amplitude of synaptically-evoked EPSPs and IPSPs in pyramidal neurons of the BLa via a presynaptic mechanism, although the subtype of muscarinic receptor was not determined. Yajeya and coworkers used methoctramine and gallamine to block M2Rs and estimated that approximately 30% of the reduction in the amplitude of synaptically-evoked glutamatergic EPSPs in BLa pyramidal neurons with external capsule stimulation was mediated by presynaptic M2Rs (Yajeya et al., 2000). Similar results were obtained in the cortex and hippocampus (Wang and Yuan, 2009; Amar et al., 2010; Zheng et al., 2012). Since the majority of the postsynaptic targets of M2R+ axons forming excitatory asymmetrical synapses in the present study were M2R+ spines or distal dendrites, this receptor may actually mediate both pre- and postsynaptic effects at these synapses.
The results of the present study suggest that GABA release is modulated by activation of M2Rs in the axon terminals of GABAergic interneurons targeting both perisomatic (somata, proximal dendrites, and axon initial segments) and distal dendritic compartments (thin distal dendrites and spines) of BLa pyramidal neurons via symmetrical synapses. It is well established that interneurons targeting the perisomatic compartment are critical for determining the firing pattern of pyramidal cells, whereas those targeting the distal dendritic compartment are important for regulating synaptic plasticity (Freund and Buzsáki, 1996). The three main interneuronal subpopulations targeting the perisomatic compartment are: (1) parvalbumin (PV) containing basket cells innervating somata/proximal dendrites, (2) PV+ axo-axonic (chandelier) cells innervating axon initial segments, and (3) CCK+ basket cells innervating somata of BLa pyramidal neurons via axon terminals forming distinctive invaginating synapses (McDonald and Betette, 2001; Muller et al., 2006; Yoshida et al., 2011; Trouche et al., 2013). The morphology of the numerous flat, non-invaginating, symmetrical perisomatic synapses formed by M2R+ terminals in the present study suggests that these terminals may be PV+ (Yoshida et al., 2001). In our previous immunofluorescence studies of M2R-ir in the amygdala we observed little or no localization of M2R in PV+ (or CCK+) axon terminals contacting pyramidal neuronal perikarya, but as in the present study perisomatic axon terminals were seen in immunoperoxidase preparations (McDonald and Mascagni, 2011), suggesting that there are low levels of M2R-ir in these terminals that could not be detected with the low sensitivity of the immunofluorescence technique. This interpretation is consistent with the findings of Szabo et al. (2009), who reported that an M2R antagonist partially reversed the carbachol-induced reduction of GABA release onto pyramidal neurons in PV-pyramidal neuron pairs. Similar results were obtained in the hippocampus, where it was shown that GABA release by both PV+ basket cells and PV+ axo-axonic cells was modulated by M2R activation (Szabo et al. 2010). As in our previous immunofluorescence study (McDonald and Mascagni, 2011), immunofluorescence studies of the hippocampus, using the same M2R antibody that was used in the present study, demonstrated no M2R-ir in PV+ structures (Hajos et al., 1998). However, colocalization of PV+/MR2+ was observed in axon terminals forming perisomatic synapses with hippocampal pyramidal neurons when M2Rs were localized using immunoperoxidase and PV was localized using a silver-intensified immunogold procedure (Hajos et al., 1998). Since the perisomatic region is where sodium-mediated action potentials are generated, M2R mediated modulation of GABAergic PV+ inputs to this region of BLa pyramidal neurons would be expected to regulate the firing of these neurons and also contribute to network oscillations (Woodruff and Sah, 2007b).
In the present study almost half of M2R+ terminals forming symmetrical synapses contacted thin distal dendritic shafts and spines, most of which were M2R+. The large number of thin dendrites contacted suggest that most of these are distal dendrites of pyramidal neurons, and most of the spines probably arise from pyramidal neuronal distal dendrites (Muller et al., 2006). Although some of these M2R+ terminals may belong to a subclass of PV+ interneurons that innervate non1perisomatic portions of pyramidal neurons (Muller et al., 2006; Woodruff and Sah, 2007a), others probably contain somatostatin (SOM) or neuropeptide Y (NPY). Thus, in our previous immunofluorescence study many M2R+ axon terminals in the neuropil exhibited SOM-ir or NPY-ir (McDonald and Mascagni, 2011). There are at least three possible sources for these SOM+/M2R+ and NPY+/M2R+ axons: (1) interneurons in the BLa that express SOM-ir and/or NPY-ir and synapse with pyramidal neuronal distal dendrites and spines (McDonald 1989; McDonald et al., 1995; Muller et al., 2007), (2) long-range SOM+ or SOM+/NPY+ nonpyramidal neurons in the entorhinal cortex that project to the BLa (McDonald and Zaric, 2015), or (3) SOM+/NPY+/M2R+ neurons surrounding the amygdalar intercalated nuclei that project to the BLa (“SPIN neurons” of McDonald and Mascagni, 2011; McDonald and Zaric, 2015).
M2R+ cholinergic terminals and M2R+ GABAergic basal forebrain afferent terminals in the BLa: Evidence for M2R-mediated presynaptic modulation of basal forebrain inputs
In the present study, M2R localization in both VAChT+ cholinergic terminals and putative type 1 GABAergic basal forebrain afferents in the BLa provides evidence for M2R-mediated modulation of release of acetylcholine and GABA, respectively, from these basal forebrain inputs. Pharmacological studies had detected a presynaptic receptor that inhibited the release of acetylcholine in the hippocampus, but the exact identity of this receptor was controversial until immunoelectron microscopic studies identified this autoreceptor as M2R (Rouse et al., 2000). The hippocampus receives its cholinergic inputs from the medial septal area. Our studies suggest that the M2R is also an autoreceptor in the cholinergic projections from the nucleus basalis region (Ch4) to the basolateral amygdala. These results are consistent with immunohistochemical studies which found colocalization of M2R and choline acetyltransferase, the synthetic enzyme for acetylcholine, in the nucleus basalis and medial septal area (Levey et al., 1995).
Some of the M2R+ terminals forming symmetrical synapses were very large and had numerous synaptic vesicles and mitochondrial profiles, characteristic of the distinctive type 1 axon terminals of GABAergic basal forebrain (BF) afferents to the BLa (McDonald et al., 2011). These BF GABAergic projection neurons are intermingled with cholinergic neurons of the BF, but constitute only about 10-15% of BF amydalopetal neurons (Mascagni and McDonald, 2009). The innervation of the BLa by M2R+ GABAergic axons of BF type 1 axons is consistent with studies which have demonstrated that non-cholinergic BF neurons express M2R protein and mRNA (Vilaro et al., 1992; Levey et al., 1995). The main targets of BF type 1 axons in the BLa are perikarya of pyramidal and nonpyramidal neurons (McDonald et al., 2011). Likewise, M2R+ type 1 axons observed in the present study appeared to synapse with presumptive pyramidal and nonpyramidal neurons, identified on the basis of their distinctive morphological features. It is of interest that many putative type 1 BF terminals in the BLa are also M1R+ (Muller et al., 2013). Since presynaptic muscarinic receptors generally inhibit neurotransmitter release, the expression of M2R-ir and M1R-ir in these type 1 BF terminals suggests that phasic release of acetylcholine by BF cholinergic axons, regulated by M2R+ autoreceptors, might be followed by a rapid attenuation of GABA release from type 1 BF axons via M2R+ and M1R+ heteroceptors. This postulated coordinated action of BF cholinergic and GABAergic axons may play an important role in regulating oscillatory activity in the BLa, as in the hippocampus (Freund and Buzsaki, 1996; Borhegyi et al., 2004).
Comparison of M2R-ir and M1R-ir in the BLa
Light and electron microscopic studies have shown that the BLa contains very high levels of both M2Rs (McDonald and Mascagni, 2011; present study) and M1Rs (McDonald and Mascagni, 2011; Muller et al., 2013). It is therefore not surprising that injections of either M1R or M2R antagonists into the rat BLa block a key function of this nucleus, the consolidation of memory (Power et al., 2003a, b). One major difference in the distribution of these receptors is that there are high levels of M1Rs, but not M2Rs, in pyramidal neuronal perikarya, suggesting that only M1Rs can modulate the excitability of pyramidal neurons in this domain. However, separate ultrastructural studies (M2R: present study; M1R: Muller et al., 2013) have shown that each receptor is found in more than 90% of dendritic shafts and about 60% of dendritic spines. These findings indicate that the vast majority of dendrites express both receptor subtypes, but that many spines might express only one subtype. The main inputs to spines are axon terminals forming asymmetrical synapses. Since only some of these terminals were M2R+ (present study) or M1R+ (Muller et al., 2013), these findings regarding spines and their inputs suggest that specific glutamatergic inputs to spines from distinct functional areas such as the prefrontal cortex, hippocampus, entorhinal cortex, intralaminar/midline thalamic nuclei, and lateral/basolateral amygdalar nuclei may be associated, either presynaptically and/or postsynaptically, with only one of these muscarinic receptor subtypes. This would permit selective modulation of distinct excitatory inputs by release of acetylcholine in the BLa. Likewise, the finding that only some of the terminals forming symmetrical (mainly inhibitory) synapses were M2R+ (present study) or M1R+ (Muller et al., 2013) leaves open the possibility that inputs to pyramidal neurons from different interneuronal subpopulations may be selectively modulated. Finally, whereas putative GABAergic inputs from the basal forebrain express both M1Rs and M2Rs, cholinergic basal forebrain inputs only express M2Rs as autoreceptors. Thus, the release of acetylcholine in the BLa could orchestrate a complex array of coordinated effects due to the selective expression of distinct muscarinic receptors expressed in specific components of BLa circuitry. Future studies are required to clarify the electrophysiological and behavioral roles of M1Rs and M2Rs, as well as other muscarinic receptor subtypes and nicotinic receptor subunits in the BLa.
Acknowledgments
Grant Sponsor: National Institutes of Health Grants R01MH104638 and R01DA027305.
Footnotes
Conflict of interest statement: None of the authors have a conflict of interest.
LITERATURE CITED
- Amar M, Lucas-Meunier E, Baux G, Fossier P. Blockade of different muscarinic receptor subtypes changes the equilibrium between excitation and inhibition in rat visual cortex. Neuroscience. 2010;169:1610–1620. doi: 10.1016/j.neuroscience.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Bassett JL. Cholinergic innervation of the monkey amygdala: an immunohistochemical analysis with antisera to choline acetyltransferase. J Comp Neurol. 1989;281:337–361. doi: 10.1002/cne.902810303. [DOI] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Elde R, Meister B. Vesicular acetylcholine transporter (VAChT) protein: a novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J Comp Neurol. 1997;378:454–467. [PubMed] [Google Scholar]
- Asan E. The catecholaminergic innervation of the rat amygdala. Adv Anat Embryol Cell Biol. 1998;142:1–118. doi: 10.1007/978-3-642-72085-7. [DOI] [PubMed] [Google Scholar]
- Aylward RL, Totterdell S. Neurons in the ventral subiculum, amygdala and entorhinal cortex which project to the nucleus accumbens: their input from somatostatin-immunoreactive boutons. J Chem Neuroanat. 1993;6:31–42. doi: 10.1016/0891-0618(93)90005-o. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Zigmond RE, Shute CC, Lewis PR. Regional distribution of choline acetyltransferase and acetylcholinesterase within the amygdaloid complex and stria terminalis system. Brain Res. 1977;120:435–444. doi: 10.1016/0006-8993(77)90397-3. [DOI] [PubMed] [Google Scholar]
- Bernard V, Laribi O, Levey AI, Bloch B. Subcellular redistribution of m2 muscarinic acetylcholine receptors in striatal interneurons in vivo after acute cholinergic stimulation. J Neurosci. 1998;18:10207–10218. doi: 10.1523/JNEUROSCI.18-23-10207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borhegyi Z1, Varga V, Szilágyi N, Fabo D, Freund TF. Phase segregation of medial septal GABAergic neurons during hippocampal theta activity. J Neurosci. 2004;24:8470–8479. doi: 10.1523/JNEUROSCI.1413-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley-Reed M, Mascagni F, McDonald AJ. Synaptology of prefrontal cortical projections to the basolateral amygdala: an electron microscopic study in the rat. Neurosci Lett. 1995;202:45–48. doi: 10.1016/0304-3940(95)12212-5. [DOI] [PubMed] [Google Scholar]
- Carlsen J, Zaborszky L, Heimer L. Cholinergic projections from the basal forebrain to the basolateral amygdaloid complex: a combined retrograde fluorescent and immunohistochemical study. J. Comp. Neurol. 1985;234:155–167. doi: 10.1002/cne.902340203. [DOI] [PubMed] [Google Scholar]
- Carlsen J, Heimer L. A correlated light and electron microscopic immunocytochemical study of cholinergic terminals and neurons in the rat amygdaloid body with special emphasis on the basolateral amygdaloid nucleus. J. Comp. Neurol. 1986;244:121–136. doi: 10.1002/cne.902440110. [DOI] [PubMed] [Google Scholar]
- Carlsen J, Heimer L. The basolateral amygdaloid complex as a cortical-like structure. Brain Res. 1988;441:377–380. doi: 10.1016/0006-8993(88)91418-7. [DOI] [PubMed] [Google Scholar]
- Drake CT, Bausch SB, Milner TA, Chavkin C. GIRK1 immunoreactivity is present predominantly in dendrites, dendritic spines, and somata in the CA1 region of the hippocampus. Proc Natl Acad Sci U S A. 1997;94:1007–1012. doi: 10.1073/pnas.94.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert FJ, Roeske WR, Yamamura HI. Molecular biology, pharmacology, and brain distribution of subtypes of the muscarinic receptor. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the Fourth Generation of Progress. Raven Press; New York: 1995. pp. 111–124. [Google Scholar]
- Farb CR, Ledoux JE. Afferents from rat temporal cortex synapse on lateral amygdala neurons that express NMDA and AMPA receptors. Synapse. 1999;33:218–229. doi: 10.1002/(SICI)1098-2396(19990901)33:3<218::AID-SYN6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Farb CR, Chang W, Ledoux JE. Ultrastructural characterization of noradrenergic axons and Beta-adrenergic receptors in the lateral nucleus of the amygdala. Front Behav Neurosci. 2010;4:162. doi: 10.3389/fnbeh.2010.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gilmor ML, Nash NR, Roghani A, Edwards RH, Yi H, Hersch SM, Levey AI. Expression of the putative vesicular acetylcholine transporter in rat brain and localization in cholinergic synaptic vesicles. J. Neurosci. 1996;16:2179–2190. doi: 10.1523/JNEUROSCI.16-07-02179.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis M. Acetylcholinesterase enzyme localization in the amygdala: a comparative histochemical and ultrastructural study. Acta Anat. (Basel) 1980;106:192–202. doi: 10.1159/000145181. [DOI] [PubMed] [Google Scholar]
- Hájos N, Papp EC, Acsády L, Levey AI, Freund TF. Distinct interneuron types express m2 muscarinic receptor immunoreactivity on their dendrites or axon terminals in the hippocampus. Neuroscience. 1998;82:355–376. doi: 10.1016/s0306-4522(97)00300-x. [DOI] [PubMed] [Google Scholar]
- Hancock MB. Two-color immunoperoxidase staining: visualization of anatomic relationships between immunoreactive neural elements. Am J Anat. 1986;175:343–352. doi: 10.1002/aja.1001750216. [DOI] [PubMed] [Google Scholar]
- Hellendall RP, Godfrey DA, Ross CD, Armstrong DM, Price JL. The distribution of choline acetyltransferase in the rat amygdaloid complex and adjacent cortical areas, as determined by quantitative micro-assay and immunohistochemistry. J Comp Neurol. 1986;249:486–498. doi: 10.1002/cne.902490405. [DOI] [PubMed] [Google Scholar]
- Jositsch G, Papadakis T, Haberberger RV, Wolff M, Wess J, Kummer W. Suitability of muscarinic acetylcholine receptor antibodies for immunohistochemistry evaluated on tissue sections of receptor gene-deficient mice. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:389–395. doi: 10.1007/s00210-008-0365-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Bartus RT, Marciano FF, Gash DM. Telencephalic cholinergic system of the New World monkey (Cebus apella): morphological and cytoarchitectonic assessment and analysis of the projection to the amygdala. J Comp Neurol. 1989;279:528–545. doi: 10.1002/cne.902790403. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Farb CR, Milner TA. Ultrastructure and synaptic associations of auditory thalamo-amygdala projections in the rat. Exp Brain Res. 1991;85:577–586. doi: 10.1007/BF00231742. [DOI] [PubMed] [Google Scholar]
- Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Edmunds SM, Hersch SM, Wiley RG, Heilman CJ. Light and electron microscopic study of m2 muscarinic acetylcholine receptor in the basal forebrain of the rat. J Comp Neurol. 1995;351:339–356. doi: 10.1002/cne.903510303. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. Parvalbumin-immunoreactive neurons and GABAergic neurons of the basal forebrain project to the rat basolateral amygdala. Neuroscience. 2009;160:805–812. doi: 10.1016/j.neuroscience.2009.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash DC, Potter LT. Autoradiographic localization of M1 and M2 muscarine receptors in the rat brain. Neuroscience. 1986;19:551–564. doi: 10.1016/0306-4522(86)90280-0. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Coexistence of somatostatin with neuropeptide Y, but not with cholecystokinin or vasoactive intestinal peptide, in neurons of the rat amygdala. Brain Res. 1989;500:37–45. doi: 10.1016/0006-8993(89)90297-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cell types and intrinsic connections of the amygdala. In: Aggleton JP, editor. The Amygdala. Wiley-Liss; New York: 1992. pp. 67–96. [Google Scholar]
- McDonald AJ. Is there an amygdala and how far does it extend? : An anatomical perspective. Ann. N.Y. Acad. Sci. 2003;985:1–21. doi: 10.1111/j.1749-6632.2003.tb07067.x. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Augustine JR. Neuropeptide Y and somatostatin-like immunoreactivity in the monkey amygdala: distribution, morphology, and differential coexistence. Neuroscience. 1995;66:959–982. doi: 10.1016/0306-4522(94)00629-j. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Betette R. Parvalbumin containing neurons in the rat basolateral amygdala: morphology and colocalization of calbindin D-28k. Neuroscience. 2001;102:413–425. doi: 10.1016/s0306-4522(00)00481-4. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Muller JF, Mascagni F. GABAergic Innervation of alpha type II calcium/calmodulin-dependent protein kinase immunoreactive pyramidal neurons in the rat basolateral amygdala. J. Comp. Neurol. 2002;446:199–218. doi: 10.1002/cne.10204. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Neuronal localization of m2 muscarinic receptor immunoreactivity in the rat amygdala. Neuroscience. 2011;196:49–65. doi: 10.1016/j.neuroscience.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Muller JF, Mascagni F. Postsynaptic targets of GABAergic basal forebrain projections to the basolateral amygdala. Neuroscience. 2011;183:144–159. doi: 10.1016/j.neuroscience.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, Zaric V, McDonald AJ. Muscarinic cholinergic receptor M1 in the rat basolateral amygdala: ultrastructural localization and synaptic relationships to cholinergic axons. J Comp Neurol. 2013;521:1743–1759. doi: 10.1002/cne.23254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Zaric V. Extrinsic origins of the somatostatin and neuropeptide Y innervation of the rat basolateral amygdala. Neuroscience. 2015;294:82–100. doi: 10.1016/j.neuroscience.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983a;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983b;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Levey AI, Goldman-Rakic PS. Association of m1 and m2 muscarinic receptor proteins with asymmetric synapses in the primate cerebral cortex: morphological evidence for cholinergic modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A. 1993;90:5194–5198. doi: 10.1073/pnas.90.11.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Synaptic connections of distinct interneuronal subpopulations in the rat basolateral amygdalar nucleus. J. Comp. Neurol. 2003;456:217–236. doi: 10.1002/cne.10435. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Coupled networks of parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. J Neurosci. 2005;25:7366–7376. doi: 10.1523/JNEUROSCI.0899-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comp Neurol. 2006;494:635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. J. Comp. Neurol. 2007;500:513–529. doi: 10.1002/cne.21185. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Dopaminergic innervation of pyramidal cells in the rat basolateral amygdala. Brain Struct Funct. 2009;213:275–288. doi: 10.1007/s00429-008-0196-y. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Cholinergic innervation of pyramidal cells and parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. J. Comp. Neurol. 2011;519:790–805. doi: 10.1002/cne.22550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, Zaric V, McDonald AJ. Muscarinic cholinergic receptor M1 in the rat basolateral amygdala: ultrastructural localization and synaptic relationships to cholinergic axons. J Comp Neurol. 2013;521:1743–1759. doi: 10.1002/cne.23254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson NM. Synthesis, trafficking, and localization of muscarinic acetylcholine receptors. Pharmacol Ther. 2008;19:33–43. doi: 10.1016/j.pharmthera.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Smith Y, Paré JF. Intra-amygdaloid projections of the basolateral and basomedial nuclei in the cat: Phaseolus vulgaris-leucoagglutinin anterograde tracing at the light and electron microscopic level. Neuroscience. 1995;69:567–583. doi: 10.1016/0306-4522(95)00272-k. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1997. [Google Scholar]
- Power AE, Vazdarjanova A, McGaugh JL. Muscarinic cholinergic influences in memory consolidation. Neurobiol Learn Mem. 2003a;80:178–193. doi: 10.1016/s1074-7427(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Power AE, McIntyre CK, Litmanovich A, McGaugh JL. Cholinergic modulation of memory in the basolateral amygdala involves activation of both m1 and m2 receptors. Behav Pharmacol. 2003b;14:207–213. doi: 10.1097/00008877-200305000-00004. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HD. The fine structure of the nervous system. Oxford University Press; New York: 1991. [Google Scholar]
- Rao ZR, Shiosaka S, Tohyama M. Origin of cholinergic fibers in the basolateral nucleus of the amygdaloid complex by using sensitive double-labeling technique of retrograde biotinized tracer and immunocytochemistry. J Hirnforsch. 1987;28:553–560. [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain Res Rev. 2008;58:303–313. doi: 10.1016/j.brainresrev.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse ST, Gilmor ML, Levey AI. Differential presynaptic and postsynaptic expression of m1-m4 muscarinic acetylcholine receptors at the perforant pathway/granule cell synapse. Neuroscience. 1998;86:221–232. doi: 10.1016/s0306-4522(97)00681-7. [DOI] [PubMed] [Google Scholar]
- Rouse ST, Edmunds SM, Yi H, Gilmor ML, Levey AI. Localization of M(2) muscarinic acetylcholine receptor protein in cholinergic and non-cholinergic terminals in rat hippocampus. Neurosci Lett. 2000;284:182–186. doi: 10.1016/s0304-3940(00)01011-9. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nat Rev Neurosci. 2009;10:383–390. doi: 10.1038/nm2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Pare’ J-F, Pare’ D. Differential innervation of parvalbumin immunoreactive interneurons of the basolateral amygdaloid complex by cortical and intrinsic inputs. J Comp Neurol. 2000;416:496–508. [PubMed] [Google Scholar]
- Spampanato J, Polepalli J, Sah P. Interneurons in the basolateral amygdala. Neuropharmacology. 2011;60:765–773. doi: 10.1016/j.neuropharm.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Spencer DG, Jr, Horváth E, Traber J. Direct autoradiographic determination of M1 and M2 muscarinic acetylcholine receptor distribution in the rat brain: relation to cholinergic nuclei and projections. Brain Res. 1986;380:59–68. doi: 10.1016/0006-8993(86)91429-0. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Farb CR, Pitkänen A, Go G, LeDoux JE, Amaral DG. Projections from the lateral nucleus to the basal nucleus of the amygdala: a light and electron microscopic PHA-L study in the rat. J Comp Neurol. 1992;323:586–601. doi: 10.1002/cne.903230411. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Morrell F, Mesulam MM. Cholinergic synapses in human cerebral cortex: an ultrastructural study in serial sections. Exp Neurol. 1997;144:361–368. doi: 10.1006/exnr.1997.6413. [DOI] [PubMed] [Google Scholar]
- Svendsen CN, Bird ED. Acetylcholinesterase staining of the human amygdala. Neurosci Lett. 1985;54:313–318. doi: 10.1016/s0304-3940(85)80097-5. [DOI] [PubMed] [Google Scholar]
- Szabo G, Holderith N, Erdelyi G, Szabo G, Hajos N. Program No. 423.11 , 2009, Neuroscience meeting planner. Society for Neuroscience; Washington, DC: 2009. Cholinergic receptor activation differentially regulates GABA release from parvalbumin- and cholecystokinin-expressing perisomatic inhibitory cells in the basolateral amygdala. Online. [Google Scholar]
- Szabó GG, Holderith N, Gulyás AI, Freund TF, Hájos N. Distinct synaptic properties of perisomatic inhibitory cell types and their different modulation by cholinergic receptor activation in the CA3 region of the mouse hippocampus. Eur J Neurosci. 2010 Jun;31(12):2234–46. doi: 10.1111/j.1460-9568.2010.07292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouche S, Sasaki JM, Tu T, Reijmers LG. Fear extinction causes target-specific remodeling of perisomatic inhibitory synapses. Neuron. 2013;80:1054–1065. doi: 10.1016/j.neuron.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haeften T, Wouterlood FG. Neuroanatomical tracing at high resolution. J Neurosci Methods. 2000;103:107–116. doi: 10.1016/s0165-0270(00)00300-9. [DOI] [PubMed] [Google Scholar]
- Vilaró MT, Wiederhold KH, Palacios JM, Mengod G. Muscarinic M2-selective ligands also recognize M4 receptors in the rat brain: evidence from combined in situ hybridization and receptor autoradiography. Synapse. 1992;11:171–183. doi: 10.1002/syn.890110302. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Kiss JP. Neurochemistry and pharmacology of the major hippocampal transmitter systems: synaptic and nonsynaptic interactions. Hippocampus. 1998;8:566–607. doi: 10.1002/(SICI)1098-1063(1998)8:6<566::AID-HIPO2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Fekete A, Karoly R, Mike A. Non-synaptic receptors and transporters involved in brain functions and targets of drug treatment. Br J Pharmacol. 2010;160:785–809. doi: 10.1111/j.1476-5381.2009.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yuan LL. Activation of M2 muscarinic receptors leads to sustained suppression of hippocampal transmission in the medial prefrontal cortex. J Physiol. 2009;587:5139–5147. doi: 10.1113/jphysiol.2009.174821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MS, Moises HC. Muscarinic responses of rat basolateral amygdaloid neurons recorded in vitro. J Physiol. 1992;449:121–154. doi: 10.1113/jphysiol.1992.sp019078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihe E, Tao-Cheng JH, Schäfer MK, Erickson JD, Eiden LE. Visualization of the vesicular acetylcholine transporter in cholinergic nerve terminals and its targeting to a specific population of small synaptic vesicles. Proc Natl Acad Sci U S A. 1996;93:3547–5352. doi: 10.1073/pnas.93.8.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womble MD, Moises HC. Muscarinic inhibition of M-current and a potassium leak conductance in neurones of the rat basolateral amygdala. J Physiol. 1992;457:93–114. doi: 10.1113/jphysiol.1992.sp019366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womble MD, Moises HC. Muscarinic modulation of conductances underlying the afterhyperpolarization in neurons of the rat basolateral amygdala. Brain Res. 1993;621:87–96. doi: 10.1016/0006-8993(93)90301-3. [DOI] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Networks of parvalbumin-positive interneurons in the basolateral amygdala. J Neurosci. 2007a;27:553–563. doi: 10.1523/JNEUROSCI.3686-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Inhibition and synchronization of basal amygdala principal neuron spiking by parvalbumin-positive interneurons. J Neurophysiol. 2007b;98:2956–2961. doi: 10.1152/jn.00739.2007. [DOI] [PubMed] [Google Scholar]
- Yajeya J, De La Fuente A, Criado JM, Bajo V, Sánchez-Riolobos A, Heredia M. Muscarinic agonist carbachol depresses excitatory synaptic transmission in the rat basolateral amygdala in vitro. Synapse. 2000;38:151–160. doi: 10.1002/1098-2396(200011)38:2<151::AID-SYN6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Uchigashima M, Yamasaki M, Katona I, Yamazaki M, Sakimura K, Kano M, Yoshioka M, Watanabe M. Unique inhibitory synapse with particularly rich endocannabinoid signaling machinery on pyramidal neurons in basal amygdaloid nucleus. Proc Natl Acad Sci U S A. 2011;108:3059–3064. doi: 10.1073/pnas.1012875108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Muller JF, McDonald AJ. Noradrenergic innervation of pyramidal cells in the rat basolateral amygdala. Neuroscience. 2013;228:395–408. doi: 10.1016/j.neuroscience.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Wess J, Alzheimer C. M2 muscarinic acetylcholine receptors regulate long-term potentiation at hippocampal CA3 pyramidal cell synapses in an input-specific fashion. J Neurophysiol. 2012;108:91–100. doi: 10.1152/jn.00740.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]