Abstract
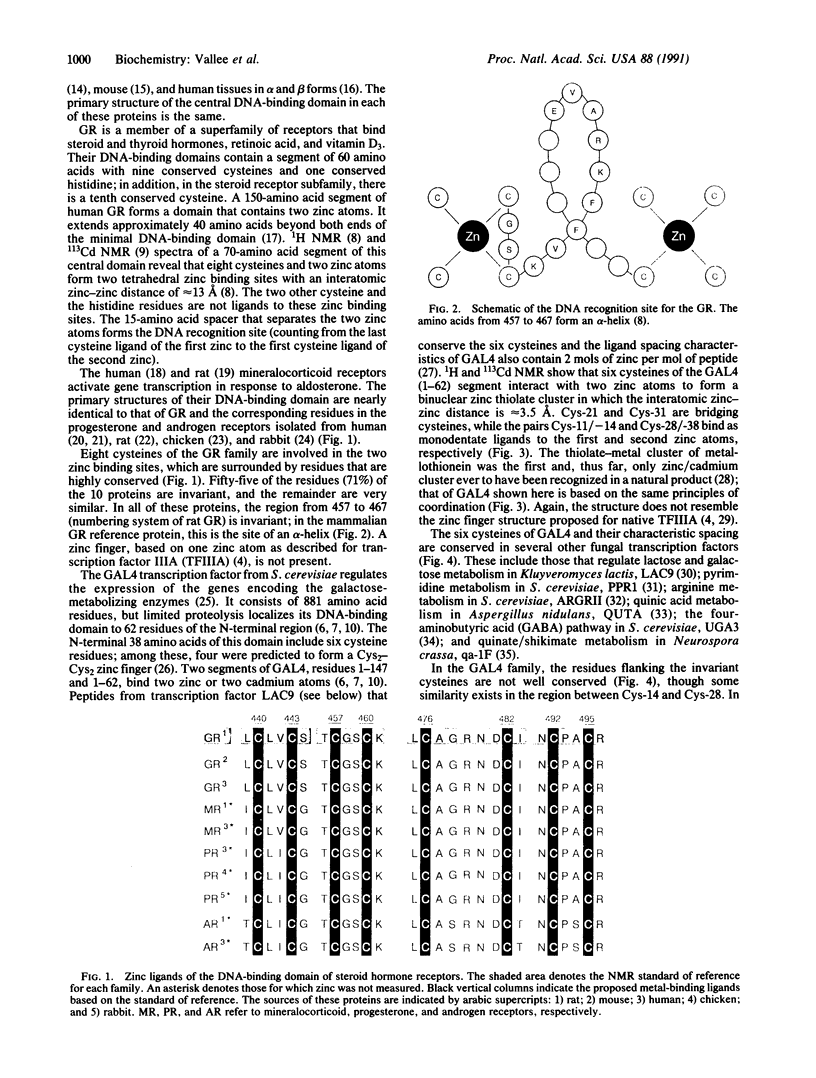
We now recognize three distinct motifs of DNA-binding zinc proteins: (i) zinc fingers, (ii) zinc clusters, and (iii) zinc twists. Until very recently, x-ray crystallographic or NMR three-dimensional structure analyses of DNA-binding zinc proteins have not been available to serve as standards of reference for the zinc binding sites of these families of proteins. Those of the DNA-binding domains of the fungal transcription factor GAL4 and the rat glucocorticoid receptor are the first to have been determined. Both proteins contain two zinc binding sites, and in both, cysteine residues are the sole zinc ligands. In GAL4, two zinc atoms are bound to six cysteine residues which form a "zinc cluster" akin to that of metallothionein; the distance between the two zinc atoms of GAL4 is approximately 3.5 A. In the glucocorticoid receptor, each zinc atom is bound to four cysteine residues; the interatomic zinc-zinc distance is approximately 13 A, and in this instance, a "zinc twist" is represented by a helical DNA recognition site located between the two zinc atoms. Zinc clusters and zinc twists are here recognized as two distinctive motifs in DNA-binding proteins containing multiple zinc atoms. For native "zinc fingers," structural data do not exist as yet; consequently, the interatomic distances between zinc atoms are not known. As further structural data become available, the structural and functional significance of these different motifs in their binding to DNA and other proteins participating in the transmission of the genetic message will become apparent.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André B. The UGA3 gene regulating the GABA catabolic pathway in Saccharomyces cerevisiae codes for a putative zinc-finger protein acting on RNA amount. Mol Gen Genet. 1990 Jan;220(2):269–276. doi: 10.1007/BF00260493. [DOI] [PubMed] [Google Scholar]
- Arriza J. L., Weinberger C., Cerelli G., Glaser T. M., Handelin B. L., Housman D. E., Evans R. M. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987 Jul 17;237(4812):268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Beri R. K., Whittington H., Roberts C. F., Hawkins A. R. Isolation and characterization of the positively acting regulatory gene QUTA from Aspergillus nidulans. Nucleic Acids Res. 1987 Oct 12;15(19):7991–8001. doi: 10.1093/nar/15.19.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr M. D., Pastore A., Gausepohl H., Frank R., Roesch P. NMR and molecular dynamics studies of the mKr2 'zinc finger'. Eur J Biochem. 1990 Mar 10;188(2):455–461. doi: 10.1111/j.1432-1033.1990.tb15423.x. [DOI] [PubMed] [Google Scholar]
- Chang C. S., Kokontis J., Chang C. T., Liao S. S. Cloning and sequence analysis of the rat ventral prostate glucocorticoid receptor cDNA. Nucleic Acids Res. 1987 Nov 25;15(22):9603–9603. doi: 10.1093/nar/15.22.9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen M., Northrop J. P., Ringold G. M. The mouse glucocorticoid receptor: mapping of functional domains by cloning, sequencing and expression of wild-type and mutant receptor proteins. EMBO J. 1986 Oct;5(10):2513–2522. doi: 10.1002/j.1460-2075.1986.tb04529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakun G. P., Fairall L., Klug A. EXAFS study of the zinc-binding sites in the protein transcription factor IIIA. Nature. 1986 Dec 18;324(6098):698–699. doi: 10.1038/324698a0. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. D., Berg J. M., Pabo C. O. Metal-dependent folding of a single zinc finger from transcription factor IIIA. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4841–4845. doi: 10.1073/pnas.84.14.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. D., Bredt D. S., Pabo C. O. Tat protein from human immunodeficiency virus forms a metal-linked dimer. Science. 1988 Apr 1;240(4848):70–73. doi: 10.1126/science.2832944. [DOI] [PubMed] [Google Scholar]
- Freedman L. P., Luisi B. F., Korszun Z. R., Basavappa R., Sigler P. B., Yamamoto K. R. The function and structure of the metal coordination sites within the glucocorticoid receptor DNA binding domain. Nature. 1988 Aug 11;334(6182):543–546. doi: 10.1038/334543a0. [DOI] [PubMed] [Google Scholar]
- Geever R. F., Huiet L., Baum J. A., Tyler B. M., Patel V. B., Rutledge B. J., Case M. E., Giles N. H. DNA sequence, organization and regulation of the qa gene cluster of Neurospora crassa. J Mol Biol. 1989 May 5;207(1):15–34. doi: 10.1016/0022-2836(89)90438-5. [DOI] [PubMed] [Google Scholar]
- Gibson T. J., Postma J. P., Brown R. S., Argos P. A model for the tertiary structure of the 28 residue DNA-binding motif ('zinc finger') common to many eukaryotic transcriptional regulatory proteins. Protein Eng. 1988 Sep;2(3):209–218. doi: 10.1093/protein/2.3.209. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H., Turcotte B., Quirin-Stricker C., Bocquel M. T., Meyer M. E., Krozowski Z., Jeltsch J. M., Lerouge T., Garnier J. M., Chambon P. The chicken progesterone receptor: sequence, expression and functional analysis. EMBO J. 1987 Dec 20;6(13):3985–3994. doi: 10.1002/j.1460-2075.1987.tb02741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorsen Y. C., Nandabalan K., Dickson R. C. LAC9 DNA-binding domain coordinates two zinc atoms per monomer and contacts DNA as a dimer. J Biol Chem. 1990 Aug 5;265(22):13283–13289. [PubMed] [Google Scholar]
- Han M. K., Cyran F. P., Fisher M. T., Kim S. H., Ginsburg A. Xenopus transcription factor IIIA. Evidence for heterogeneity of Zn2+ binding affinities and specific labeling of cysteine 287. J Biol Chem. 1990 Aug 15;265(23):13792–13799. [PubMed] [Google Scholar]
- Hanas J. S., Hazuda D. J., Bogenhagen D. F., Wu F. Y., Wu C. W. Xenopus transcription factor A requires zinc for binding to the 5 S RNA gene. J Biol Chem. 1983 Dec 10;258(23):14120–14125. [PubMed] [Google Scholar]
- Härd T., Kellenbach E., Boelens R., Maler B. A., Dahlman K., Freedman L. P., Carlstedt-Duke J., Yamamoto K. R., Gustafsson J. A., Kaptein R. Solution structure of the glucocorticoid receptor DNA-binding domain. Science. 1990 Jul 13;249(4965):157–160. doi: 10.1126/science.2115209. [DOI] [PubMed] [Google Scholar]
- Johnston M. Genetic evidence that zinc is an essential co-factor in the DNA binding domain of GAL4 protein. Nature. 1987 Jul 23;328(6128):353–355. doi: 10.1038/328353a0. [DOI] [PubMed] [Google Scholar]
- Kammerer B., Guyonvarch A., Hubert J. C. Yeast regulatory gene PPR1. I. Nucleotide sequence, restriction map and codon usage. J Mol Biol. 1984 Dec 5;180(2):239–250. doi: 10.1016/s0022-2836(84)80002-9. [DOI] [PubMed] [Google Scholar]
- Laughon A., Gesteland R. F. Primary structure of the Saccharomyces cerevisiae GAL4 gene. Mol Cell Biol. 1984 Feb;4(2):260–267. doi: 10.1128/mcb.4.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. S., Gippert G. P., Soman K. V., Case D. A., Wright P. E. Three-dimensional solution structure of a single zinc finger DNA-binding domain. Science. 1989 Aug 11;245(4918):635–637. doi: 10.1126/science.2503871. [DOI] [PubMed] [Google Scholar]
- Loosfelt H., Atger M., Misrahi M., Guiochon-Mantel A., Meriel C., Logeat F., Benarous R., Milgrom E. Cloning and sequence analysis of rabbit progesterone-receptor complementary DNA. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9045–9049. doi: 10.1073/pnas.83.23.9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenguy F., Dubois E., Descamps F. Nucleotide sequence of the ARGRII regulatory gene and amino acid sequence homologies between ARGRII PPRI and GAL4 regulatory proteins. Eur J Biochem. 1986 May 15;157(1):77–81. doi: 10.1111/j.1432-1033.1986.tb09640.x. [DOI] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misrahi M., Atger M., d'Auriol L., Loosfelt H., Meriel C., Fridlansky F., Guiochon-Mantel A., Galibert F., Milgrom E. Complete amino acid sequence of the human progesterone receptor deduced from cloned cDNA. Biochem Biophys Res Commun. 1987 Mar 13;143(2):740–748. doi: 10.1016/0006-291x(87)91416-1. [DOI] [PubMed] [Google Scholar]
- Neuhaus D., Nakaseko Y., Nagai K., Klug A. Sequence-specific [1H]NMR resonance assignments and secondary structure identification for 1- and 2-zinc finger constructs from SW15. A hydrophobic core involving four invariant residues. FEBS Lett. 1990 Mar 26;262(2):179–184. doi: 10.1016/0014-5793(90)80184-k. [DOI] [PubMed] [Google Scholar]
- Pan T., Coleman J. E. GAL4 transcription factor is not a "zinc finger" but forms a Zn(II)2Cys6 binuclear cluster. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2077–2081. doi: 10.1073/pnas.87.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan T., Coleman J. E. Structure and function of the Zn(II) binding site within the DNA-binding domain of the GAL4 transcription factor. Proc Natl Acad Sci U S A. 1989 May;86(9):3145–3149. doi: 10.1073/pnas.86.9.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan T., Freedman L. P., Coleman J. E. Cadmium-113 NMR studies of the DNA binding domain of the mammalian glucocorticoid receptor. Biochemistry. 1990 Oct 2;29(39):9218–9225. doi: 10.1021/bi00491a016. [DOI] [PubMed] [Google Scholar]
- Patel P. D., Sherman T. G., Goldman D. J., Watson S. J. Molecular cloning of a mineralocorticoid (type I) receptor complementary DNA from rat hippocampus. Mol Endocrinol. 1989 Nov;3(11):1877–1885. doi: 10.1210/mend-3-11-1877. [DOI] [PubMed] [Google Scholar]
- Párraga G., Horvath S., Hood L., Young E. T., Klevit R. E. Spectroscopic studies of wild-type and mutant "zinc finger" peptides: determinants of domain folding and structure. Proc Natl Acad Sci U S A. 1990 Jan;87(1):137–141. doi: 10.1073/pnas.87.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmeron J. M., Jr, Johnston S. A. Analysis of the Kluyveromyces lactis positive regulatory gene LAC9 reveals functional homology to, but sequence divergence from, the Saccharomyces cerevisiae GAL4 gene. Nucleic Acids Res. 1986 Oct 10;14(19):7767–7781. doi: 10.1093/nar/14.19.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze P., Wörgötter E., Braun W., Wagner G., Vasák M., Kägi J. H., Wüthrich K. Conformation of [Cd7]-metallothionein-2 from rat liver in aqueous solution determined by nuclear magnetic resonance spectroscopy. J Mol Biol. 1988 Sep 5;203(1):251–268. doi: 10.1016/0022-2836(88)90106-4. [DOI] [PubMed] [Google Scholar]
- Shang Z., Liao Y. D., Wu F. Y., Wu C. W. Zinc release from Xenopus transcription factor IIIA induced by chemical modifications. Biochemistry. 1989 Dec 12;28(25):9790–9795. doi: 10.1021/bi00451a037. [DOI] [PubMed] [Google Scholar]
- Smith R. F., Smith T. F. Automatic generation of primary sequence patterns from sets of related protein sequences. Proc Natl Acad Sci U S A. 1990 Jan;87(1):118–122. doi: 10.1073/pnas.87.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South T. L., Blake P. R., Sowder R. C., 3rd, Arthur L. O., Henderson L. E., Summers M. F. The nucleocapsid protein isolated from HIV-1 particles binds zinc and forms retroviral-type zinc fingers. Biochemistry. 1990 Aug 28;29(34):7786–7789. doi: 10.1021/bi00486a002. [DOI] [PubMed] [Google Scholar]
- Tilley W. D., Marcelli M., Wilson J. D., McPhaul M. J. Characterization and expression of a cDNA encoding the human androgen receptor. Proc Natl Acad Sci U S A. 1989 Jan;86(1):327–331. doi: 10.1073/pnas.86.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Active-site zinc ligands and activated H2O of zinc enzymes. Proc Natl Acad Sci U S A. 1990 Jan;87(1):220–224. doi: 10.1073/pnas.87.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Short and long spacer sequences and other structural features of zinc binding sites in zinc enzymes. FEBS Lett. 1989 Oct 23;257(1):138–140. doi: 10.1016/0014-5793(89)81805-8. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990 Jun 19;29(24):5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Falchuk K. H. Zinc and gene expression. Philos Trans R Soc Lond B Biol Sci. 1981 Aug 14;294(1071):185–197. doi: 10.1098/rstb.1981.0098. [DOI] [PubMed] [Google Scholar]
- Vallee B. L. Zinc biochemistry in normal and neoplastic growth processes. Experientia. 1977 May 15;33(5):600–601. doi: 10.1007/BF01946521. [DOI] [PubMed] [Google Scholar]
- Weinberger C., Hollenberg S. M., Rosenfeld M. G., Evans R. M. Domain structure of human glucocorticoid receptor and its relationship to the v-erb-A oncogene product. Nature. 1985 Dec 19;318(6047):670–672. doi: 10.1038/318670a0. [DOI] [PubMed] [Google Scholar]
- Yarbrough W. G., Quarmby V. E., Simental J. A., Joseph D. R., Sar M., Lubahn D. B., Olsen K. L., French F. S., Wilson E. M. A single base mutation in the androgen receptor gene causes androgen insensitivity in the testicular feminized rat. J Biol Chem. 1990 May 25;265(15):8893–8900. [PubMed] [Google Scholar]







