Abstract
Knee rotationplasty (KRP) is a type of surgery in which the rotated ankle serves as a new knee after being removed for bone tumor. Although this limb salvage surgery is rarely indicated in properly selected patients, it may offer functional advantages over transfemoral amputation, and more durable results compared with a prosthesis. The walking mechanics of adult patients after KRP is believed to be close to that of below-knee amputees. In this study, we evaluated steady-state walking of KRP patients from the viewpoint of the overall muscle power needed to keep the body centre of mass in motion. Three adult patients after KRP, all athletes, were evaluated. Ground reactions during walking were recorded during six subsequent strides on a force treadmill. The positive mechanical work and power sustaining the motion of the centre of mass and the recovery of muscle energy due to the pendulum-like mechanism of walking were computed and compared with those obtained in previous studies from above-knee, below-knee amputees and healthy individuals. In KRP patients, walking was sustained by a muscle power output which was 1.4–3.6 times lower during the step performed on the rotated limb than on the subsequent step. The recovery of muscle energy was slightly lower (0.9) or higher (1.3–1.4 times) on the affected side. In two out of the three KRP patients, our findings were more similar to those from above-knee amputees than to those from below-knee amputees. After KRP, the rotated limb does not necessarily provide the same power provided by below-knee amputation. This may have a relevance for the paralympic classification of KRP athletes.
Keywords: centre of mass, knee rotationplasty, mechanical work, Van Nes operation, walking
Introduction
Knee rotationplasty (KRP) is a rare type of limb salvage surgery, alternative to above-knee (AK) amputation, for skeletally immature children with bone sarcoma around the knee. Exceptionally, interventions after trauma in adult age have been reported (Klos et al., 2010). In the most common variant of this complex procedure (Borggreve, 1930; Fixsen, 1983) after removal of the involved segment, the distal tibia is rotated by 180° and grafted to the residual proximal femur. The neurovascular bundle is preserved. Limb length is restored by means of prosthetization (Fig. 1).
Fig. 1.
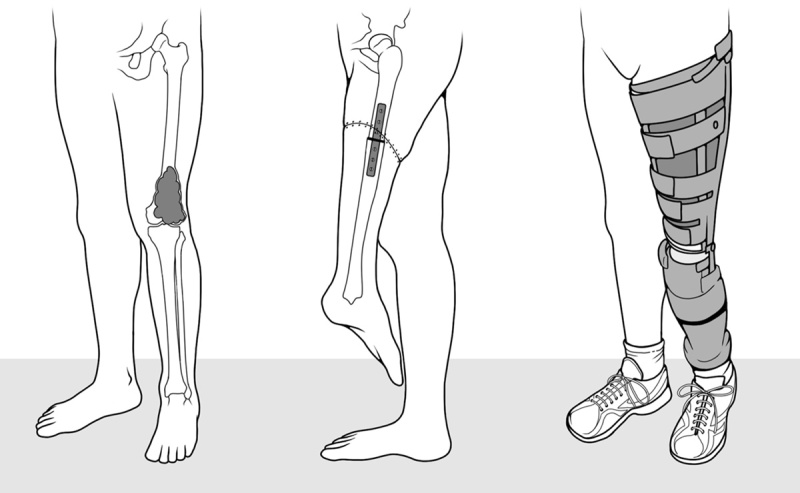
A knee rotationplasty sketch: from left to right, distal knee tumor image, reconstruction of the rotated leg image, and image of patients wearing their prosthesis, respectively.
After a few months, if not weeks, the patient will learn to skillfully use the rotated ankle as a new knee. This results in greater functionality compared with AK amputation (McClenaghan et al., 1989), in exchange for a bizarre cosmetic appearance (Fig. 1). Patient’s dexterity is surprising. This is consistent with the finding, in a single adult patient, of a normal capacity of detection of foot laterality in a mental rotation task (Curtze et al., 2010). Patients can resume walking and even running with a nearly normal appearance and get often involved in sports such as cycling and skiing (Hillmann et al., 2007). However, walking after KRP and after amputation was very rarely compared. Although KRP patients may achieve athletic performance of high level when they participate in sports competitions, it is unclear whether, in terms of power produced by the residual limb, they are most comparable to AK or below-knee (BK) amputees. Because of the presence of the pseudo-knee joint, these athletes are generally classified (http://www.paralympic.org/classification) as amputated below the knee. Actually, this might be incorrect. A paradigmatic case presented to our clinical attention pointed out that the patient’s performance during swimming races was actually more similar to AK than to BK amputees.
In this study, the effect of KRP on walking ergometrics was investigated in three patients, all professional athletes, according to a method based on mechanical work measures derived from ground-reaction forces (Cavagna, 1975; Tesio et al., 1998a), and compared with previous findings from AK and BK amputees (Tesio et al., 1998b). Results of the present study elucidate the mechanisms of motor adaptation to lower limb asymmetries, and can help in refining the paralympic classification for this condition.
Patients and methods
Patients
Three adult KRP patients were studied. Data were obtained from a previous study investigating the excitability of the primary motor cortex in the same patients (Tesio et al., 2014). The patients were all athletes. Their demographic and clinical characteristics are summarized in Table 1.
Table 1.
Demographic and clinical information on knee rotationplasty patients

General principles of measurement of energy changes in the centre of mass during walking
From a mechanical standpoint, the body system can be represented by its centre of mass (CoM). During walking, the CoM must be raised and lowered at each step, as well as accelerated and decelerated both forward and laterally. This entails continuous changes in the mechanical energy of the CoM due to vertical displacement, and in kinetic energy due to the forward and lateral velocity (Ev, Ef and El, respectively). The sum of these energies is the total mechanical energy of the CoM, Etot. Increments of mechanical energy represent ‘positive’ mechanical work. Similar to all legged animals (Cavagna et al., 1977; McNeill Alexander, 2003), walking in humans resembles the motion of an inverted pendulum allowing a passive transfer between vertical and horizontal energy changes. As a consequence, Etot undergoes lower increments than those occurring in the absence of this passive transfer. Nevertheless, these increments must necessarily be sustained by positive muscle work, keeping the CoM in motion with respect to the ground (‘external’ positive work, Wext). At a constant ‘optimal’ speed of about 1.4 m/s, the average saving of Wext (called ‘percent recovery’, R) at each step can trespass 60% (Cavagna et al., 1976). In asymmetric pathologic human gaits (e.g. due to unilateral lower limb amputation, unilateral hip arthritis, and hemiplegia) the pendulum-like mechanism was found to be even more efficient on the step performed by ‘pole-vaulting’ on the affected limb, and much less efficient during the subsequent step. Therefore, quite unexpectedly, the average Wext over a stride (i.e. per unit distance) usually remained within normal limits given the patients’ velocity (Cavagna et al., 1983; Tesio et al., 1985, 1998b). Not surprisingly, it was highlighted that the same may hold for the total energy expenditure (Tesio et al., 1991), which is mostly determined by Wext at low and intermediate walking speeds (Cavagna and Kaneko, 1977). In this study, the energy changes in the CoM and the muscle work and power needed at each step were measured and compared with those recorded from amputee patients in a previous study (Tesio et al., 1998b).
The technical approach to the measurement of CoM motion is based on the so-called ‘double-integration’ or ‘newtonian’ algorithms, first used by Cavagna (1975) and subsequently adapted to asymmetric gaits (Cavagna et al., 1983). In short, the patient must walk for at least two entire steps on force plates, with no contacts with the outer floor. The resulting ground reactions are thus applied to the body CoM. From force records, once the patient’s mass is known, accelerations, speed changes and 3D trajectory (Tesio et al., 2010, 2011) of the CoM can be computed. Kinetic energy (half body mass times squared speed) is easily computed from velocity; vertical displacement is easily computed from vertical speed changes; and changes in gravitational potential energy (weight times vertical displacement) are easily computed from body mass. The total mechanical energy of the COM is the sum of the kinetic and potential energy. In an ideal pendulum, the total energy would remain constant. Increments of Etot imply muscle work against the ground. The method requires long force platforms or a treadmill mounted on force sensors. Average forward speed must be measured from outside the platforms (e.g. through photocells), or it must be imposed with a treadmill. Vertical and lateral average speeds over a series of steps are assumed to be nil.
The time interval between two subsequent peaks of forward speed changes defines the step period, Sp. Step length (Sl) is determined by multiplying Sp by the average velocity during the same step.
The sum of the increments of energy into the forward, lateral and vertical directions within each step, as well as the increments of the total energy, is defined as positive work: Wf, Wl, Wv and Wext, respectively. The ‘discount’ of muscle work allowed during the step by the passive energy transfer, R, can be easily computed from the following formula:
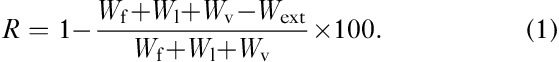 |
R represents the efficiency of the pendulum-like locomotor mechanism as a whole along the step. From Wext values, the average positive power (Ŵ) during a step, or during a given step phase, can be obtained by dividing the work by the time period considered.
Experimental setup and procedures
Ground reactions during walking were collected using a split-belt treadmill (ADAL 3D; Medical Development, Andrézieux-Bouthéon, France). The instrument is described in detail elsewhere (Tesio and Rota, 2008). Each half-treadmill is 1.26 m long and 0.3 m large, and it is mounted on four three-dimensional piezoelectric force sensors (KI 9048B; Kistler, Winterthur, Switzerland). Signals were collected at 250 Hz. In this study, force signals from both sides were summed. Patients wore their usual prosthesis and light gym shoes (Fig. 2). They were first requested to walk at their preferred velocity and cadence along a 10 m path along a 24 m long corridor, twice in each opposite direction. The average velocity was computed. They were then weighed and asked to stand quietly for 5 s on the treadmill, to provide the offset signal for vertical forces. The treadmill velocity was set at a value about 10% lower compared with average floor walking velocity, to avoid too long steps on the relatively short treadmill. The treadmill was gradually accelerated over a 1 min interval, in steps of about 0.1–0.3 m/s every 10 s. Patients were requested to look at a visual target (a black round spot 0.03 m in diameter) placed at eye level on a white wall about 2 m in front of them, thus providing a visual anchoring. At the preset speed, patients were requested to walk for 2 min for familiarization, after which six subsequent strides into one direction, with no apparent changes in velocity, nor episodes of stumbling or imbalance, were selected. This simple protocol (i.e. six strides only; one direction, only) was adopted based on unpublished findings from a previous study (Tesio and Rota, 2008), in which five strides per each opposite direction were requested for healthy controls. Because of the very high reproducibility of the results (see Fig. 2 in the cited paper), no significant (at P<0.05) reduction in variance (Bartlett test) of external work and power parameters between series of five or of 10 walks, nor an effect of step side or direction (analysis of variance) for series of 10 strides, was found. Moreover, minimizing the number of requested strides may help future comparisons with patients to whom a low number of strides can be requested (e.g. distractible children, or cases presenting with balance troubles, or fatiguing or painful conditions).
Fig. 2.
A knee rotationplasty patient walking on the split-belt treadmill during testing.
Data analysis
Data were analysed off-line. Given the small sample at hand, individual data representation was preferred to summarized data. Both within and between patients, the stride period was time-normalized. Foot-ground contact phases were determined from vertical forces exceeding 10 N (a threshold above the background noise; Tesio and Rota, 2008). Six subsequent strides beginning with the step mainly performed on the rotated lower limb were selected. In practice, the stride began when Ef reached a maximum and the rotated foot was in front position. The motion of the CoM in KRP patients was compared with the motion analysed in healthy controls and AK and BK patients studied in a previous research (Tesio et al., 1998a, 1998b).
Ethics
The study was approved by the Ethic committee of the Istituto Auxologico Italiano, Milano, Italy.
Results
The mechanical energy changes in the CoM relative to the body weight (in J/kg) during two subsequent steps, superimposed from a series of subsequent strides, are presented in Fig. 3.
Fig. 3.
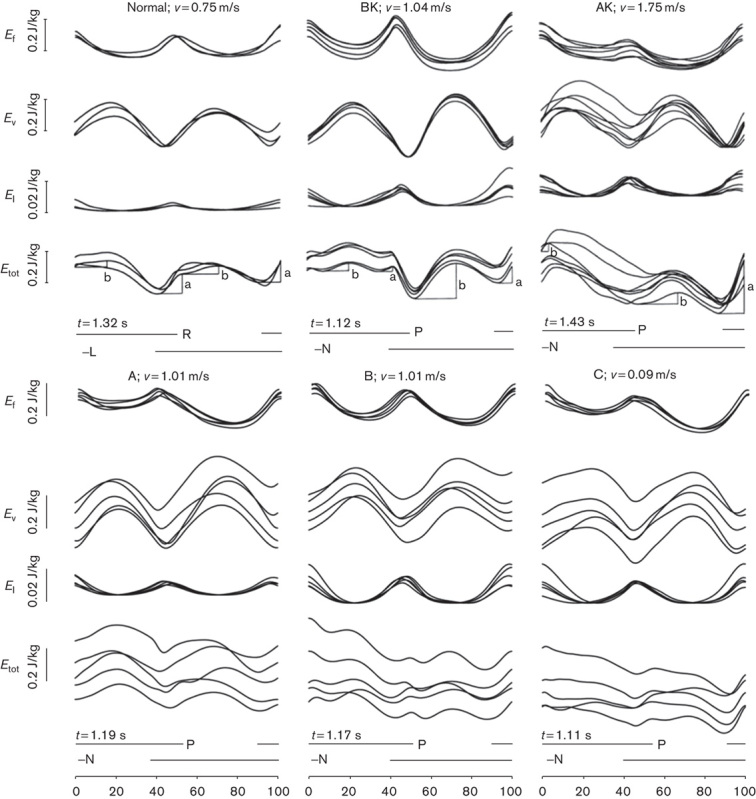
Mechanical energy changes in the CoM relative to the body weight (J/kg) during two subsequent steps. The average stride period is normalized 0–100. In each panel, the average velocity (v, in m/s) is given above the curves, right to the patients’ labels. The absolute duration of the strides is given below the Etot curves (t, in s). The interrupted horizontal segments at the bottom give the stance phase of each lower limb. In each panel the energy curves from subsequent strides are superimposed. From top to bottom, the tracings refer to Ef, Ev, El and Etot (note the different scaling for El). Panels in the upper row refer to a healthy control (a 36-year-old woman: 1.50 m and 50 kg weight), an AK amputee (a 20-year-old-man: 1.75 m and 70 kg) and a BK amputee (a 37-year-old woman: 1.66 m and 71 kg) from left to right, respectively. Graphs are reproduced from a previous study (Tesio et al., 1998a, 1998b) with the authors’ permission. Panels in the lower row refer to the three KRP patients studied here (L, left; N, normal/unaffected; P, pathologic/affected; R, right).
On the ordinate, each of the six panels gives, from top to bottom, the changes in Ef, Ev, El and Etot (in J/kg, note the different scaling for El), respectively, as a function of average duration of the strides (standardized to 0–100). The energy curves for each stride are superimposed individually, rather than being averaged, thus allowing to assess their reproducibility. The average absolute duration of the strides is given below the Etot curves. The horizontal segments mark the stance phase of the right (R) or left (L) lower limb for a representative healthy control (upper left panel) or of the stance on the affected-pathologic (P) or unaffected-normal (N) lower limb for single representative BK and AK patients (mid-upper and right-upper panels), and for each of the three KRP patients (lower row of panels), respectively. It may be noted that the average speed was not the same across patients: the control and the AK amputee walked at 0.75 m/s, whereas the AK amputee and the KRP patients walked at the imposed speed of 1 m/s (actually, 0.99–1.04 m/s). Data from controls and the amputees come from two studies (see patients and methods section) performed on ground-nested platforms so that speed could not be imposed. The ‘spontaneous speed’ was requested (see discussion section on this point). The figure shows that, thanks to the pendulum-like mechanism of gait (reflected by the mirror shapes of Ev and both Ef and El), Etot undergoes only two reduced increments in the healthy patient. The larger one occurs during the double stance phase or, more precisely, during the so-called ‘push-off’ phase mainly sustained by the plantar flexors of the rear foot (increment a). A smaller increment, b, occurs during the single stance (Cavagna and Kaneko, 1977). Both in amputees (mid and right panels, and upper row) and in KRP patients, increment a is much greater at the N–P transition compared with the P–N transition.
In Fig. 4, on the ordinate the upper panel gives the muscular work per distance unit, hence as an average between two subsequent steps (Wext, in J/kg/m), provided to sustain the increments of Etot, and thus the motion of the body CoM, as an average between two subsequent steps, as a function of the average forward velocity, given on the abscissa. The lower panel gives the relative amount of Wext saved, thanks to the pendulum-like mechanism of gait (R).
Fig. 4.
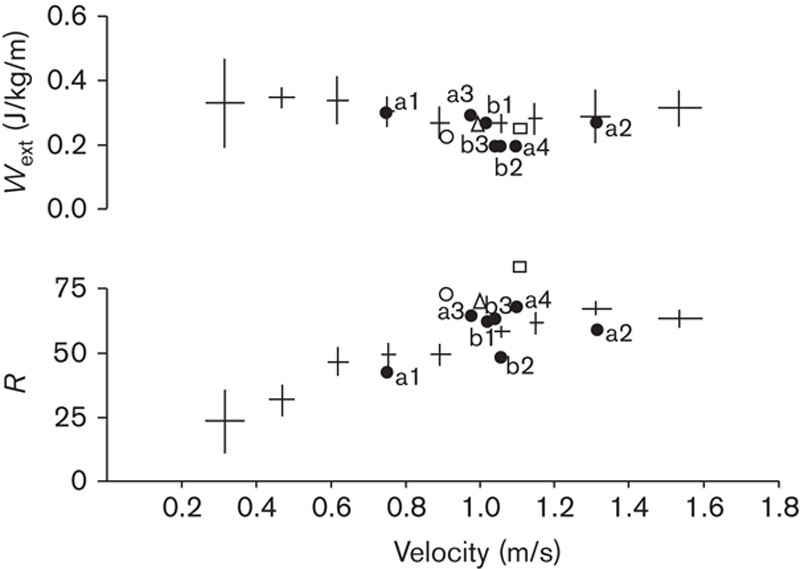
The upper ordinate shows the ‘external’ muscular work per unit distance, Wext (J/kg/m). The lower ordinate gives the recovery of Wext along the stride, R, due to the pendulum-like mechanism of gait, as a function of waking velocity (m/s), given on the abscissa. The crossed symbols give mean and 95% confidence limits on both axes of normative values recorded from 125 steps made by eight healthy controls. The filled dots refer to four AK (labeled a) and to three BK (labeled b) amputees. Normative and amputees’ data are reproduced from a previous study (Tesio et al., 1998a, 1998b) with the authors’ and publishers’ permission. The triangle, the square and the open circle refer to KRP patients A, B and C, respectively (see Table 1 for details). The Wext and R values of patients A, B, and C are as follows: 0.25 and 0.70; 0.24 and 0.86; and 0.22 and.74, respectively.
Crossed symbols give normative data with SD in either axis recorded from 125 steps made by eight healthy controls. Filled dots refer to average values across four AK (a) and three BK (b) amputees. Empty symbols refer to the three KRP patients (Table 1): A (triangle), B (square) and circle (C). Normative and amputees’ data are reproduced from previous works (Tesio et al., 1998a, 1998b), with the authors’ permission. It can be seen that per unit distance Wext and R are in line with normal values for the corresponding walking velocity. A trend can be seen with KRP patients being more efficient (higher R and lower Wext), compared with amputees and even healthy controls. Numeric values for KRP patients are given in the figure legend.
Figure 5 gives Wext and R as the ratio (log graphic scale) between the steps performed on the affected (P) and the unaffected (N) lower limb. The value distribution is given in box-plot form for AK and BK patients, whereas individual symbols (Fig. 4) are given for KRP patients.
Fig. 5.
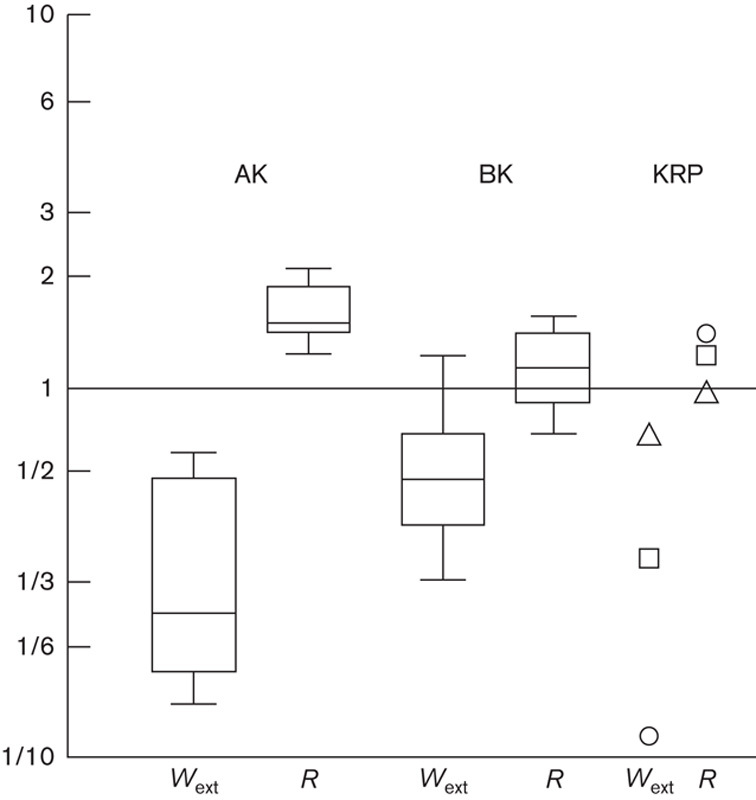
Unaffected/affected (N/P) step ratio of external muscular work (Wext) and recovery of muscle work (R) in above-knee amputees (AK), below-knee amputees (BK) and KRP patients, from left to right, respectively. In healthy individuals, the ratio is 1 with minimal variation across controls (horizontal continuous line, Tesio et al., 1985). Amputees’ data are reproduced from a previous work (Tesio et al., 1998b), with permission. Ratios were 0.9, 1.3 and 1.4 in patients A (triangle), B (square) and C (circle), respectively (see Table 1 for details).
In healthy individuals the ratio is 1 (horizontal line) with minimal variations across patients (not shown, Tesio et al., 1985). A relevant asymmetry is found in AK patients, in which much less muscular work (Wext) has to be performed during the ‘affected’ step, compared with the ‘unaffected’ step (Wext ratio «1). Correspondingly, the pendulum-like mechanism is much more efficient during the affected step (R ratios ≥1). The same pattern was found in the KRP patients and, to a minor extent, in BK patients.
Even greater asymmetries can be detected when looking at the push-off phase of the step (increment a of Etot, see Fig. 6).
Fig. 6.
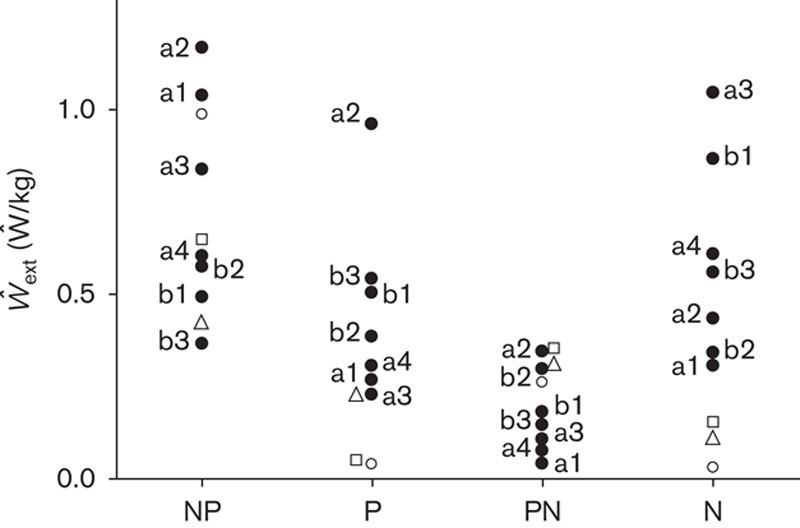
The ordinate shows the average power, Ŵext (Ŵ/kg), provided by muscles to sustain the changes in Etot during the double and the single stance phases of gait. Ŵext is computed by dividing the increments of Etot by their duration. The first and the third column from left refer to the double stance phases, when the foot of the unaffected (N) or the affected (P) lower limb are in rear position, respectively. The second and fourth columns from the left refer to the single contact phase of the affected and unaffected lower limbs, respectively. Filled dots refer to AK and BK patients labeled a and b, respectively. Amputees’ data are reproduced from a previous study (Tesio et al., 1998b) with permission. Open circles refer to KRP patients labeled A, B and C, respectively (see Table 1 for details).
The average power, Ŵext (in Ŵ/kg), provided by muscles during the double and the single stance phases of gait is reported on the ordinate. Power is computed by dividing the sum of the positive increments of Etot by their cumulative duration. Filled dots refer to AK patients (a1–a4) and BK patients (b1–b3). Empty symbols refer to the three KRP patients (see the legend of Fig. 4 for labels).
It is evident that most of the positive power needed to keep the body in motion is provided at push-off by muscles of the trailing leg, similar to healthy individuals (presumably mostly because of the calf muscles) (Tesio et al., 1998b). However, in both the amputees and the KRP patients this only holds for the unaffected lower limb (see also the increment a of Etot in Fig. 3). When the affected lower limb is in rear position, much less power is provided. The dominant propulsive role of the unaffected lower limb is even more evident for KRP compared with amputee patients. During both of the single stance phases, the power output is much lower compared with push-off, for all patients.
Discussion
Perhaps a relevant weakness of this study resides in its small sample size. It must be recalled, however, that KRP is a very rare procedure. To the Authors’ knowledge, in Italy (some 60 million inhabitants) there are presently 42 survivors, all operated at the same hospital. Perhaps the variability of results might have been reduced by requesting more than six strides to each patient. The reasons for limiting the number of strides are given in the patients and methods section. Here, it is of relevance that a potentially higher error variance reinforces any significant difference that is nonetheless emerging between KRP and amputee patients. A potential source of systematic error in comparing diagnostic groups is the different speed adopted by controls and AK patients (0.75 m/s), versus BK and amputee patients (around 1 m/s, Fig. 3). This is justified by the different method adopted for controls and amputees – for example, walking on ground-nested platforms at the preferred speed, versus walking on a force treadmill at an imposed speed, respectively. This error is presumably much lower than that suspected. As far as the mechanics of the centre of mass is concerned, for the same speed no substantial differences exist between the two modalities (Tesio and Rota, 2008, discussion section). With respect to the different speeds, it must be considered that the external work and the efficiency of the pendulum-like energy transfer shows a plateau between about 0.7 and 1.4 m/s (Cavagna and Kaneko, 1977; see their figure 3, left panels).
Compared with transfemural amputation, it is believed that rotationplasty provides better clinical results by preserving the ankle as a new knee with flexion–extension capability and the foot proprioception (Jacobs, 1984; Krajbich, 1991; Merkel et al., 1991). Several studies have documented the good functional outcome of patients after rotationplasty in the long-term follow-up (Hillmann et al., 1999; Fuchs et al., 2003; Hopyan et al., 2006; Ginsberg et al., 2007; Benedetti et al., 2016), with a mild restriction of daily activities, and great ability to perform sports exercises, even at competitive level (Hillmann et al., 2007). Gait analysis of KRP patients revealed only slight asymmetries as regards stride duration, stride length, cadence, velocity, and stance–swing ratio, compared with healthy individuals. Although the gait kinematics was similar to that of patients with distal AK amputation, the rotated knee excursion was higher in KRP patients (Fuchs et al., 2003). Furthermore, the muscles of the rotated leg showed good adaptation to their new function, as revealed by their temporal activation detected through surface electromyography recordings along the gait cycle (Hillmann et al., 2000).
Despite the kinematic symmetry achieved after KRP, significant asymmetries here were detected in ground-reaction forces, which were much lower during the ‘affected’ step. One suggested cause was the hypotrophy of the muscles on the rotated limb, possibly caused by their partial detachment and/or denervation, and forcing an overuse of the unaffected lower limb (Catani et al., 1993). Nevertheless, the cause–effect relationship might be circular. A previous study (Steenhoff et al., 1993) found that the strength of the plantar flexors and extensors of the ankle in isometric conditions averaged about 70% of those of the unaffected side, thus leaving a potential, despite hypotrophy, for a remarkable muscle power at the ‘new’ knee (Hillmann et al., 2000). Moreover, the ‘extensor’ (i.e. plantar flexor) moment arms at the rotated ankle are more favourable than those at a normal knee (Steenhoff et al., 1993). Previous experimental measurements suggest that the patellar tendon moment arm decreases remarkably from 15° to 0° knee flexion (Tsaopoulos et al., 2006), whereas estimated Achilles tendon moment arm is relatively stable (Clarke et al., 2015) and it tends to be greater in plantarflexed position, which is the same adopted by KRP patients during early stance. There seems to be room, therefore, for speculating that muscle atrophy might well be also the effect, not only the cause, of the decreased power output. Stated otherwise, central inhibition might sustain a disuse atrophy (see below).
Findings indicate that KRP patients present mechanical energy changes in the CoM similar to those found in amputees. During the push-off phase the rear unaffected lower limb is overloaded, whereas during the next push-off the rear rotated lower limb is underloaded, compared with healthy controls. In other words, the body system ‘pole-vaults’ almost passively (nearly like an ideal pendulum) over the rotated leg, whereas it requires a strong muscular power during the oscillation on the unaffected leg.
This asymmetry is less marked in BK, compared with AK patients, indicating that the stump muscles in BK do provide some of the muscle power needed to keep the CoM in motion. In two out of the three patients studied here (Figs 5 and 6), the asymmetry in work and muscle power was superimposable to the one found in BK patients, whereas for the third KRP patient the asymmetry was superimposable to the one found in AK patients. This suggests that the method applied in this study may help to fine-tune the paralympic classification of ‘limb deficiency’ of KRP patients. These can be functionally akin to an AK, rather than a BK, amputee athlete, despite the presence of a pseudo-knee joint.
As a final consideration, one should note that KRP patients show a high level of motor skill on the rotated leg and one would expect that the rotated leg muscles become hypertrophic, to compensate for the lost muscle mass. Contrary to this expectation, both the stumps of amputees and the rotated limb of KRP patients invariably become hypotrophic: as suggested by Tesio et al. (2014), this might represent an example of ‘learned-nonuse’ (Taub et al., 2014) involving the affected lower limb in case of unilateral impairments, whichever the cause (Tesio et al., 1998b). Consistently enough, in the same three KRP patients the rotated plantar flexors were found to be under-represented in the contralateral motor cortex in a study based on brain mapping through transcranial magnetic stimulation (Tesio et al., 2014). The usefulness of maintaining an active muscular control over the former ankle joint has been highlighted (Hillmann et al., 2000). Nevertheless, based on the findings of the present study, strengthening of the rotated leg appears a challenging task, working against the spontaneous tendency towards an adaptive recovery.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- Alexander RM. (2003). Principles of animal locomotion. Princeton, NJ: Princeton University Press. [Google Scholar]
- Benedetti MG, Okita Y, Recubini E, Mariani E, Leardini A, Manfrini M. (2016). How much clinical and functional impairment do children treated with knee rotationplasty experience in adulthood? Clin Orthop Relat Res 474:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borggreve J. (1930). Kniegelenkersatz durch das in der Beinlängsachse um 180° gedrehte Fuβgelenk. Arch Orthop Trauma Surg 28:175–178. [Google Scholar]
- Catani F, Capanna R, Benedetti MG, Battistini A, Leardini A, Cinque G, Giannini S. (1993). Gait analysis in patients after Van Nes rotationplasty. Clin Orthop Relat Res 296:270–277. [PubMed] [Google Scholar]
- Cavagna GA. (1975). Force platforms as ergometers. J Appl Physiol 39:174–179. [DOI] [PubMed] [Google Scholar]
- Cavagna GA, Kaneko M. (1977). Mechanical work and efficiency in level walking and running. J Physiol 268:467–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavagna GA, Thys H, Zamboni A. (1976). The sources of external work in level walking and running. J Physiol 262:639–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavagna GA, Heglund NC, Taylor CR. (1977). Mechanical work in terrestrial locomotion: two basic mechanisms for minimizing energy expenditure. Am J Physiol 233:R243–R261. [DOI] [PubMed] [Google Scholar]
- Cavagna GA, Tesio L, Fuchimoto T, Heglund NC. (1983). Ergometric evaluation of pathological gaits. J Appl Physiol 55:606–613. [DOI] [PubMed] [Google Scholar]
- Clarke EC, Martin JH, d'Entremont AG, Pandy MG, Wilson DR, Herbert RD. (2015). A non-invasive, 3D, dynamic MRI method for measuring muscle moment arms in vivo: demonstration in the human ankle joint and Achilles tendon. Med Eng Phys 37:93–99. [DOI] [PubMed] [Google Scholar]
- Curtze C, Otten B, Postema K. (2010). Effects of lower limb amputation on the mental rotation of feet. Exp Brain Res 201:527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fixsen JA. (1983). Rotation-plasty. J Bone Joint Surg Br 65:529–530. [DOI] [PubMed] [Google Scholar]
- Fuchs B, Kotajarvi BR, Kaufman KR, Sim FH. (2003). Functional outcome of patients with rotationplasty about the knee. Clin Orthop Relat Res 415:52–58. [DOI] [PubMed] [Google Scholar]
- Ginsberg JP, Rai SN, Carlson CA, Meadows AT, Hinds PS, Spearing EM, et al. (2007). A comparative analysis of functional outcomes in adolescents and young adults with lower-extremity bone sarcoma. Pediatr Blood Cancer 49:964–969. [DOI] [PubMed] [Google Scholar]
- Hillmann A, Hoffmann C, Gosheger G, Krakau H, Winkelmann W. (1999). Malignant tumor of the distal part of the femur or the proximal part of the tibia: endoprosthetic replacement or rotationplasty. Functional outcome and quality-of-life measurements. J Bone Joint Surg Am 81:462–468. [DOI] [PubMed] [Google Scholar]
- Hillmann A, Rosenbaum D, Schroter J, Gosheger G, Hoffmann C, Winkelmann W. (2000). Electromyographic and gait analysis of forty-three patients after rotationplasty. J Bone Joint Surg Am 82:187–196. [DOI] [PubMed] [Google Scholar]
- Hillmann A, Weist R, Fromme A, Volker K, Rosenbaum D. (2007). Sports activities and endurance capacity of bone tumor patients after rotationplasty. Arch Phys Med Rehabil 88:885–890. [DOI] [PubMed] [Google Scholar]
- Hopyan S, Tan JW, Graham HK, Torode IP. (2006). Function and upright time following limb salvage, amputation, and rotationplasty for pediatric sarcoma of bone. J Pediatr Orthop 26:405–408. [DOI] [PubMed] [Google Scholar]
- Jacobs PA. (1984). Limb salvage and rotationplasty for osteosarcoma in children. Clin Orthop Relat Res 188:217–222. [PubMed] [Google Scholar]
- Klos K, Mückley T, Gras F, Hofmann GO, Schmidt R. (2010). Early posttraumatic rotationplasty after severe degloving and soft tissue avulsion injury: a case report. J Orthop Trauma 24:e1–e5. [DOI] [PubMed] [Google Scholar]
- Krajbich JI. (1991). Modified Van Nes rotationplasty in the treatment of malignant neoplasms in the lower extremities of children. Clin Orthop Relat Res 262:74–77. [PubMed] [Google Scholar]
- McClenaghan BA, Krajbich JI, Pirone AM, Koheil R, Longmuir P. (1989). Comparative assessment of gait after limb-salvage procedures. J Bone Joint Surg Am 71:1178–1182. [PubMed] [Google Scholar]
- McNeill Alexander R. (2003). Principles of animal locomotion. Princeton, NJ: Princeton University Press. [Google Scholar]
- Merkel KD, Gebhardt M, Springfield DS. (1991). Rotationplasty as a reconstructive operation after tumor resection. Clin Orthop Relat Res 270:231–236. [PubMed] [Google Scholar]
- Steenhoff JR, Daanen HA, Taminiau AH. (1993). Functional analysis of patients who have had a modified Van Nes rotationplasty. J Bone Joint Surg Am 75:1451–1456. [DOI] [PubMed] [Google Scholar]
- Taub E, Uswatte G, Mark VW. (2014). The functional significance of cortical reorganization and the parallel development of CI therapy. Front Hum Neurosci 8:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesio L, Rota V. (2008). Gait analysis on split-belt force treadmills: validation of an instrument. Am J Phys Med Rehabil 87:515–526. [DOI] [PubMed] [Google Scholar]
- Tesio L, Civaschi P, Tessari L. (1985). Motion of the center of gravity of the body in clinical evaluation of gait. Am J Phys Med 64:57–70. [PubMed] [Google Scholar]
- Tesio L, Roi GS, Moller F. (1991). Pathological gaits: inefficiency is not a rule. Clin Biomech 6:47–50. [DOI] [PubMed] [Google Scholar]
- Tesio L, Lanzi D, Detrembleur C. (1998a). The 3-D motion of the centre of gravity of the human body during level walking. I. Normal subjects at low and intermediate walking speeds. Clin Biomech 13:77–82. [DOI] [PubMed] [Google Scholar]
- Tesio L, Lanzi D, Detrembleur C. (1998b). The 3-D motion of the centre of gravity of the human body during level walking. II. Lower limb amputees. Clin Biomech 13:83–90. [DOI] [PubMed] [Google Scholar]
- Tesio L, Rota V, Chessa C, Perucca L. (2010). The 3D path of body centre of mass during adult human walking on force treadmill. J Biomech 43:938–944. [DOI] [PubMed] [Google Scholar]
- Tesio L, Rota V, Perucca L. (2011). The 3D trajectory of the body centre of mass during adult human walking: evidence for a speed–curvature power law. J Biomech 44:732–740. [DOI] [PubMed] [Google Scholar]
- Tesio L, Benedetti MG, Rota V, Manfrini M, Perucca L, Caronni A. (2014). Surgical leg rotation: cortical neuroplasticity assessed through brain mapping using transcranial magnetic stimulation. Int J Rehabil Res 37:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaopoulos DE, Baltzopoulos V, Maganaris CN. (2006). Human patellar tendon moment arm length: measurement considerations and clinical implications for joint loading assessment. Clin Biomech 21:657–667. [DOI] [PubMed] [Google Scholar]


