Abstract
Hepatitis C virus (HCV)/Schistosoma mansoni coinfection is common in Egypt and other developing countries. This study aimed to investigate the influence of HCV/S. mansoni coinfection on the concentration of HCV–nonstructural protein-4 (NS4) in addition to collagen III and matrix metalloproteinase-1 (MMP-1) in different hepatic fibrosis stages. We found that coinfected patients (N = 186) showed significantly (P < 0.05, Mann–Whitney U test) higher concentrations of HCV-NS4, collagen III, and collagen III/MMP-1 ratio (CMR) than those with HCV monoinfection (N = 104) in different fibrosis stages. Conversely, coinfected patients showed significantly lower concentrations of MMP-1 when compared with HCV monoinfection. The elevated levels of CMR in case of HCV monoinfection yielded an estimated odds ratio of 1.8 and 2.6 for developing significant fibrosis (F2-F4) and cirrhosis (F4), respectively. HCV/S. mansoni coinfection increased the risk for developing F2-F4 and F4 several fold yielding an estimated odds ratio of 11.1 and 5.2, respectively. This means that coinfected patients have a 6-fold and 2-fold increased risk of developing F2-F4 and F4, respectively, over HCV-monoinfected patients. Thus, elevated levels of HCV-NS4 and CMR in HCV/S. mansoni coinfection suggest increased susceptibility of coinfected patients, compared with those with HCV monoinfection, for accelerating hepatic fibrosis progression.
Introduction
Hepatitis C virus (HCV) is one of the main causes of chronic liver disease worldwide affecting an estimated 160 million individuals.1 Strikingly, in Egypt, HCV prevalence is more than 15% among adults, and incidence is about 150,000 new cases per year.2 The prime candidate to explain high HCV prevalence in Egypt is the past practice of parenteral therapy for schistosomiasis with tartar emetic.3 This is because the injections of tartar emetic were often administered unsafely, with glass syringes and needles either improperly sterilized or used for multiple doses. Schistosomiasis represents a major tropical parasitic disease that is caused by trematode flukes of the genus Schistosoma.4 By conservative estimates, at least 230 million people worldwide are infected with this parasite.5 In Egypt, the situation is worse. During the first half of the 20th century, up to 80% of the populations of impoverished Egyptian villages were infected with schistosomes.6 Previously, Attallah and others7 had identified a schistosome antigen at 63 kDa using a specific anti–Schistosoma mansoni monoclonal antibody which was also used for the diagnosis of S. mansoni infection.
Previous studies have investigated the relation between HCV and S. mansoni, regarding HCV load, immune and treatment responses, as well as fibrosis progression rate.8,9 However, the extent to which S. mansoni could influence HCV proteins and the role of HCV proteins in stimulating extracellular-matrix (ECM) deposition were not studied. Nonstructural protein-4 (NS4) is an HCV protein that was previously proved to suppress T helper-1 (Th-1) responses.10 This viral protein has already been proposed as an alternative approach for confirmation of viremia in patients with different hepatic fibrosis stages.11
Conceptually, hepatic fibrosis results from an imbalance in the equilibrium of normal processes of synthesis and degradation of ECM, which ultimately leads to increased collagen accumulation.12 In fact, collagen degradation, which is regulated by a family of proteases called matrix metalloproteinases (MMPs), has an important role in liver fibrogenesis.13 Recently, we have proposed the ratio of collagen III and its degrading enzyme “MMP-1” as surrogate markers for liver fibrosis staging.14 Therefore, this work is concerned with identification and quantitation of both HCV-NS4 and S. mansoni antigens in addition to collagen III and MMP-1, which are directly involved in deposition and removal of ECM, in chronic hepatitis C (CHC) patients. Then, we aimed to estimate the extent to which HCV/S. mansoni coinfection could affect the concentration of HCV-NS4, collagen III, MMP-1, and the progression to different hepatic fibrosis stages.
Materials and Methods
Samples.
Two-hundred and ninety CHC Egyptian individuals with a mean age of 43.5 years were enrolled from the Tropical Medicine department, Mansoura University hospitals, Mansoura, Egypt. Informed consents were obtained from all participants, and they were fully informed concerning the diagnostic procedures involved and disease nature. The study protocol conformed to ethical guidelines of the 1975 Helsinki Declaration. Blood samples were collected by vein puncture within 2 weeks of liver biopsy. Needle liver-biopsy specimens were obtained using a 18-gauge or larger needle. To be considered as adequate for scoring, the liver biopsies had to measure at least 15 mm and/or contain at least five portal tracts, except for cirrhosis for which no limitation was required. Biopsies were interpreted according to the METAVIR scoring system.15 In the METAVIR system, the stage score represents the amount of fibrosis based on a 5-point scale: F0, no fibrosis; F1, portal fibrosis alone; F2, portal fibrosis with rare septae; F3, portal fibrosis with many septae; and F4, cirrhosis. Patients were further classified according to S. mansoni status into two groups. The first group included 104 patients with HCV monoinfection. This cohort comprised 76 men and 28 women with a mean age of 42.7 years. The second group included 186 patients with HCV/S. mansoni coinfection. This group comprised 121 men and 65 women with a mean age of 43.9 years.
Laboratory tests.
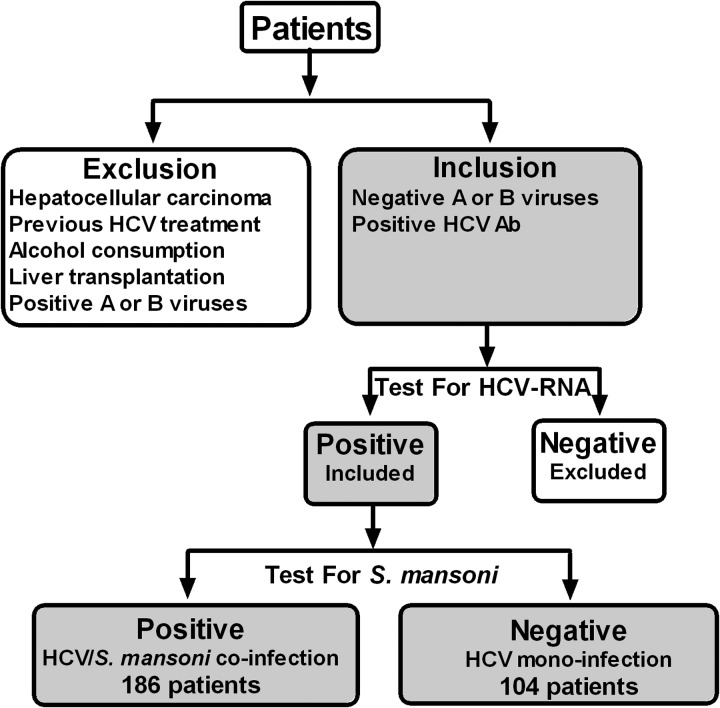
Complete blood count was performed on blood treated with ethylenediaminetetraacetic acid-K3 using KX-21 Sysmex automated hematology analyzer (Sysmex Corporation, Kobe, Japan), and another portion was treated with citrate solution for prothrombin time–international normalized ratio. Liver function tests were all measured on fresh serum on an automated biochemistry analyzer (Hitachi 917; Roche Diagnostics, Mannheim, Germany). HCV diagnosis was based on a positive test for anti-HCV antibodies (Sorin Biomedica, Diagnostic, Vercelli, Italy). Patients were then confirmed for the presence of HCV-RNA using polymerase chain reaction assay (COBAS Ampliprep/ COBAS TaqMan, Roche Diagnostics, Pleasanton, CA). HCV-monoinfected patients had no history or laboratory evidence of previous or current S. mansoni infection. The diagnosis of S. mansoni was based on detecting vital or dead schistosomal ova in stools or rectal biopsy with seropositivity to schistosomal antibodies. A flowchart diagram showing the selection criteria and process is depicted in Figure 1 .
Figure 1.
A flowchart showing the selection process of patients included in this study. Hepatitis C virus (HCV) diagnosis was based on a positive test for anti-HCV antibodies. Patients were then confirmed for the presence of HCV-RNA using polymerase chain reaction assay. HCV-monoinfected patients had no history or laboratory evidence of previous or current Schistosoma mansoni infection. The diagnosis of S. mansoni was based on detecting vital or dead schistosomal ova in stools or rectal biopsy with seropositivity to schistosomal antibodies. Patients with serological evidence of active hepatitis A or B viruses, history of habitual alcohol consumption, hepatocellular carcinoma, previous interferon treatment, and liver transplantation were excluded from the present study.
Western blot, Gel electroelution, and enzyme-linked immunosorbent assay.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was carried out in 0.75 mm thick, 12% vertical slab gels according to the method of Laemmli.16 Western blot was then used to transfer the separated bands onto a nitrocellulose membrane (0.45 mm pore size, Sigma, St. Louis, MO) in a protein transfer unit according to Towbin and others.17 They were immunostained using respective antibodies (ABC Diagnostics, New Damietta, Egypt) corresponding to HCV-NS4, S. mansoni antigen, collagen III, and MMP-1, separately.7,11,14
Next, bands of the aforementioned markers were cut and electroeluted separately from preparative polyacrylamide gels at 200 V for 3 hours in a dialysis bag (Sigma). The protein content of the purified bands was determined,18 and the remainder was stored at −20°C until use in the concentration curve. The aforementioned markers were then quantified in patients' sera using enzyme-linked immunosorbent assay (ELISA) according to Attallah and others.7,11,14
Statistical analysis.
All statistical analyses were done by a Statistical Package for the Social Sciences (SPSS) software (v.15.0; SPSS Inc., Chicago, IL) and GraphPad Prism package (v.5.0; (GraphPad Software, San Diego, CA). Statistical analyses were done for groups of F1, F2-F3, and F4. Stages F2 and F3 were combined together in one group due to their small size and to reduce sample bias. However, statistical analysis was also done for significant fibrosis (F2-F4) because this group is of great clinical interest and has been adopted as a target for most clinicians. This is because the presence of significant fibrosis is widely accepted as an indication to commence treatment.19–21 Variables were expressed as mean ± standard deviation. Correlation was evaluated by Spearman's rank correlation coefficients. Statistically significant differences between groups were determined using the Student's t test in case the Kolmogorov–Smirnov test results were not significant. The nonparametric Mann–Whitney U test was used as an alternative to t test to determine differences between groups for continuous nonnormally distributed variables. P values were corrected for multiple comparisons using Bonferroni correction. Findings with P < 0.05 after correction were considered significant. The adjusted odds ratio (with 95% confidence intervals) was derived from logistic regression analysis to estimate the risk of a target disorder from subjects without it.
Results
Patients' characteristics.
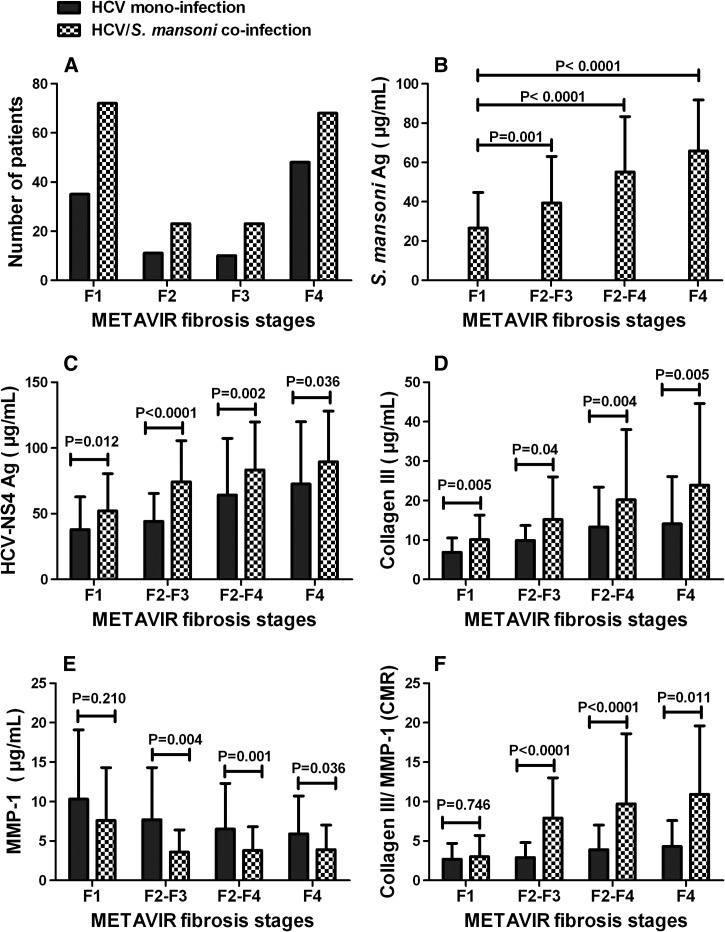
Laboratory characteristics of CHC patients with and without S. mansoni infection are summarized in Table 1. Overall, there were 107/290 (36.9%) with F1, 34/290 (11.7%) with F2, 33/290 (11.4%) with F3, 67/290 (23.1%) with moderate/sever fibrosis (F2-F3), 183/290 (63.1%) with significant fibrosis (F2-F4), and 116/290 (40.0%) with liver cirrhosis (F4) as seen in Figure 2A .
Table 1.
Characteristics of patients with HCV monoinfection and HCV/Schistosoma mansoni coinfection
| Variables | Group I (N = 104) | Group II (N = 186) | P value |
|---|---|---|---|
| HCV monoinfection | HCV/S. mansoni coinfection | ||
| Age (years) | 42.7 ± 8.6 | 43.9 ± 7.2 | NS† |
| ALT (U/L)* | 73.3 ± 37.3 | 75.0 ± 43.9 | NS† |
| AST (U/L)* | 55.5 ± 34.2 | 63.0 ± 34.5 | NS† |
| ALP (U/L)* | 88.0 ± 33.8 | 94.0 ± 38.5 | NS‡ |
| PT-INR* | 1.1 ± 0.1 | 1.2 ± 0.07 | NS† |
| Total bilirubin (mg/dL)* | 0.84 ± 0.4 | 0.88 ± 0.5 | NS† |
| Albumin (g/L)* | 43.0 ± 0.3 | 41.0 ± 0.4 | NS† |
| Platelet count (109/L)* | 193.5 ± 50.2 | 175.3 ± 49.8 | NS‡ |
ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase; HCV = hepatitis C virus; NS = nonsignificant; PT-INR = prothrombin time–international normalized ratio. Variables were expressed as mean ± standard deviation. P values were corrected for multiple comparisons using Bonferroni correction. Findings with P < 0.05 after correction were considered significant.
Reference values: ALT = up to 41 U/L in males, up to 31 U/L in females; AST = up to 37 U/L in males, up to 31 U/L in females; ALP = 22–92 U/L; PT-INR = 1; total bilirubin = up to 1 mg/dL; albumin = 3.8–5.4 g/dL; platelet count = 150–400 × 109/L.
Student t test.
Mann–Whitney U test.
Figure 2.
Impact of hepatitis C virus (HCV)/Schistosoma mansoni coinfection on HCV–nonstructural protein-4 (NS4) concentration and extracellular-matrix deposition. (A) Number of patients, (B) levels of S. mansoni antigens in different hepatic fibrosis stages, (C) levels of HCV-NS4, (D) levels of collagen III, (E) levels of matrix metalloproteinase-1 (MMP-1), and (F) levels of collagen/MMP-1 ratio (CMR) in different hepatic fibrosis stages in HCV-infected patients with and without schistosomiasis. Statistically significant differences between groups were determined using the nonparametric Mann–Whitney U test.
Western-blot analysis.
The target HCV-NS4 and S. mansoni antigens in addition to collagen III and MMP-1 were identified in CHC patients based on SDS-PAGE followed by Western blot. As a result, a single immunoreactive band was shown at 27 kDa, 63 kDa, 70 kDa, and 245 kDa corresponding to HCV-NS4, S. mansoni antigen, collagen III, and MMP-1, respectively (Supplemental Figure 1), as previously published by Attallah and others.7,11,14
Quantification of S. mansoni antigen in different hepatic fibrosis stages.
The concentration of S. mansoni antigen was found to increase significantly (P < 0.05, Mann–Whitney U test) with the progression of liver fibrosis as presented in Figure 2B. Our results showed that patients with F4 displayed a 2.5-fold and 1.7-fold increase in S. mansoni antigen concentration over those who developed F1 and F2-F3, respectively. On the other hand, patients with F2-F3 and F2-F4 had a 1.5-fold and 2.1-fold increase, respectively, in S. mansoni antigen concentration over patients who have F1.
Impact of HCV/S. mansoni coinfection on nonstructural viral protein.
The levels of HCV-NS4 in relation to different fibrosis stages in CHC patients with and without S. mansoni are presented in Figure 2C, and the differences were found statistically significant. Importantly, F1 patients coinfected with HCV and S. mansoni showed significantly (P < 0.05, Mann–Whitney U test) higher concentration of HCV-NS4 compared with F1 patients monoinfected with HCV. The same results were obtained with significant difference for HCV-NS4 in other fibrosis stages.
Influence of HCV/S. mansoni coinfection on ECM deposition.
Levels of collagen III, MMP-1, and collagen III/MMP-1 ratio (CMR) were determined in CHC patients with and without S. mansoni as depicted in Figure 3D –F. The results demonstrated that each METAVIR fibrosis group was accompanied by a significant increase in the concentration of collagen III in case of coinfection compared with patients at the same stage in monoinfection. The same goes for CMR, but the differences were statistically not significant for patients with early fibrosis stage (F1). The higher collagen content in patients with HCV/S. mansoni coinfection may indicate their increased susceptibility for progressing to subsequent fibrosis stage faster than those with HCV monoinfection. On the contrary, each fibrosis group was associated with a significant drop in MMP-1 concentration in case of coinfection compared with HCV monoinfection, but the difference was statistically not significant for patients with early fibrosis stage (F1).
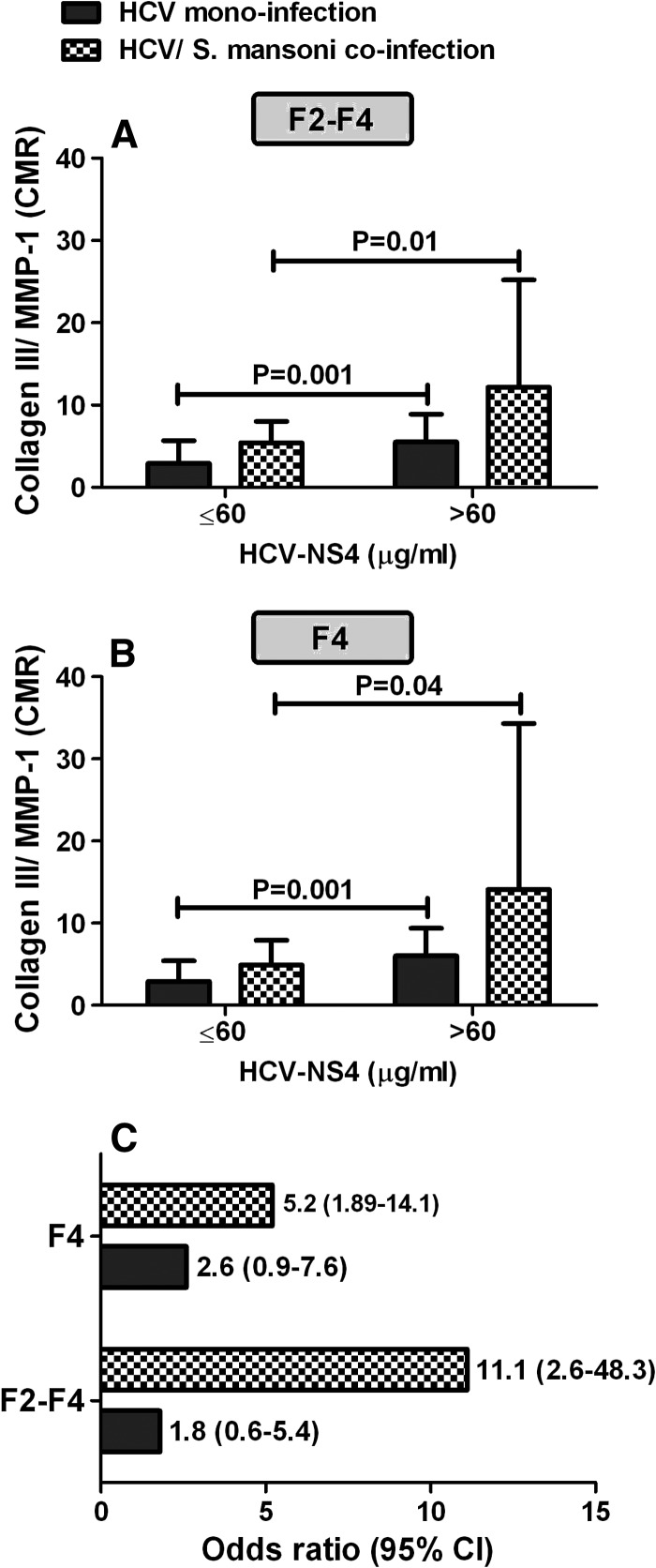
Figure 3.
Levels of collagen/matrix metalloproteinase-1 (MMP-1) ratio (CMR) in relation to hepatitis C virus–nonstructural protein-4 (HCV-NS4) concentration in absence and presence of schistosomiasis and its impact on the progression rates of liver fibrosis. (A) Levels of CMR in patients with significant fibrosis (F2-F4), (B) levels of CMR in patients with cirrhosis (F4), and (C) the risk of elevated CMR on the progression rates of liver fibrosis. Statistically significant differences between groups were determined using the nonparametric Mann–Whitney U test.
Effect of HCV protein concentration on ECM deposition.
The distribution of CMR levels in relation to HCV-NS4 concentration in patients with significant fibrosis (F2-F4) and cirrhosis (F4) is presented in Figure 3A–B and the differences were found to be statistically significant (P < 0.05, Mann–Whitney U test). As seen in Figure 3A–B, patients with HCV-NS4 concentration greater than 60 μg/mL were associated with a significant increase in CMR when compared with those with lower concentration of HCV-NS4. This finding was obtained in HCV monoinfection or HCV/S. mansoni coinfection. However, the levels of CMR tended to be elevated in case of HCV/S. mansoni coinfection in comparison with HCV-monoinfected patients at the same HCV-NS4 concentration.
In case of HCV-monoinfected patients with HCV-NS4 concentration > 60 μg/mL, the mean value for CMR in F2-F4 was 5.5 and in F4 was 6.0, respectively. Surprisingly, in case of HCV/S. mansoni coinfection, the mean value for CMR was highly elevated to 12.2 in F2-F4 and 14.1 in F4, respectively, with a significant difference (P < 0.05, Mann–Whitney U test). The cutoff point of 60 μg/mL was chosen based on receiver-operating characteristic curve as the best balanced cutoff value for optimal identification of significant fibrosis (≥ F2).
Influence of elevated CMR on the progression rates of hepatic fibrosis.
The aforementioned mean values of CMR that was obtained in different hepatic fibrosis stages were then used as cutoff points for their corresponding hepatic fibrosis stages to estimate the impact of elevated collagen III/MMP-1 ratio on the progression rates of liver fibrosis. As a consequence, the risk for developing F2-F4 and F4 was found to increase upon S. mansoni coinfection when compared with HCV-monoinfected patients as depicted in Figure 3C. The risk for developing F2-F4 and F4, in patients with S. mansoni coinfection, was increased several fold yielding an estimated odds ratio of 11.1 and 5.2, respectively. As depicted in Figure 3C, CHC patients with S. mansoni coinfection have a 6-fold and 2-fold increased risk of developing F2-F4 and F4, respectively, over HCV-monoinfected patients.
Correlation of different variables with liver fibrosis progression.
The results showed that HCV-NS4, collagen III, MMP-1, and CMR were significantly correlated with fibrosis progression in HCV-monoinfected patients with lower correlation coefficients than those produced in HCV/S. mansoni–coinfected patients (Table 2). Besides, S. mansoni antigen was found to be directly proportional to fibrosis progression with a significant correlation coefficient (r = 0.63, P < 0.0001).
Table 2.
Spearman's rank correlation coefficient of different variables with liver fibrosis progression from minimal fibrosis to cirrhosis in patients with HCV monoinfection and HCV/Schistosoma mansoni coinfection
| Variables | HCV monoinfection | HCV/S. mansoni coinfection | ||
|---|---|---|---|---|
| Correlation | P value* | Correlation | P value* | |
| HCV-NS4 (μg/mL) | 0.41 | < 0.0001 | 0.45 | < 0.0001 |
| S. mansoni Ag (μg/mL) | – | – | 0.63 | < 0.0001 |
| Collagen III (μg/mL) | 0.34 | < 0.0001 | 0.41 | < 0.0001 |
| MMP-1 (μg/mL) | −0.20 | 0.042 | −0.30 | < 0.0001 |
| CMR | 0.30 | 0.003 | 0.47 | < 0.0001 |
CMR = collagen III/matrix metalloproteinase-1 ratio; HCV = hepatitis C virus; MMP-1 = matrix metalloproteinase-1; NS4 = nonstructural protein-4.
P > 0.05 is considered nonsignificant, P < 0.05 is considered significant, and P < 0.0001 is considered extremely significant.
Discussion
In this work, we set out to examine whether there is an impact for S. mansoni coinfection with HCV on the level of HCV proteins in different METAVIR fibrosis stages. HCV-NS4 was identified using Western blot at 27 kDa in sera of CHC patients. In general and regardless of the presence or absence of S. mansoni, our patients with cirrhosis (F4) were found to have higher HCV-NS4 concentration than those who developed minimal fibrosis (F1) and moderate/sever fibrosis (F2-F3). This may be explained by the fact that patients with cirrhosis show an acquired immune deficiency because of dyshomeostasis and malnutrition. In addition, all host defense systems, antigen-specific as well as nonspecific functions are compromised in cirrhotic patients.22 The risen concentration of HCV-NS4 observed in F4 may also indicate that patients with higher viral protein concentration are more likely to be susceptible to develop end-stage liver disease. This may be explained by the fact that HCV proteins seem to modulate apoptosis and steatosis, ultimately leading to the activation of hepatic stellate cells (HSCs) which subsequently secrete large amounts of ECM. Moreover, the immune system, during chronic infection, may stimulate hepatocyte damage and fibrosis through direct cellular toxicity and the release of inflammatory cytokines.23 Consistent to our findings, Bataller and others24 demonstrated that HCV core and nonstructural proteins directly stimulate the inflammatory and fibrogenic actions of HSCs. In addition, Shin and others25 reported that HCV core proteins may contribute to the hepatic fibrogenesis via upregulation of transforming growth factor-β1 (TGF-β1). This may also apply to HCV-NS4 protein.
HCV-NS4 concentration is then determined in CHC patients with and without schistosomiasis coinfection. Interestingly, patients with HCV/S. mansoni coinfection showed significantly (P < 0.05) higher concentration of HCV-NS4 compared with HCV-monoinfected patients in different hepatic fibrosis stages. The latter result may be explained by the fact that schistosomiasis triggers Th-2 cytokine response which suppresses Th-1 cytokine release, thereby hindering cellular and antiviral immunity, and promotes Th-2 host responses and fibrogenesis.6 In another way, it could be said that the impaired immune response brought by S. mansoni would allow the propagation of HCV and subsequently increase HCV-NS4 concentration.
On the other hand, Western-blot analysis revealed that specific monoclonal antibody reacted against S. mansoni antigen at an apparent molecular weight of 63 kDa in sera of CHC patients. Schistosoma mansoni antigen was then quantified using ELISA providing values that were found to increase with liver fibrosis progression with a significant difference. The increase in the concentration of S. mansoni antigen observed in advanced fibrosis stages may be explained by the compromised host defense systems and antigen-specific as well as nonspecific functions in this group of patients. Another logical elucidation is that dual infection with HCV and S. mansoni may have a potential role in the severity of S. mansoni infection. The risen concentration of S. mansoni antigen observed in cirrhotic patients may also indicate that patients with higher S. mansoni antigen concentration are more likely to be susceptible for developing end-stage liver disease and the worm burden has a primary importance in pathogenesis.
Next, collagen III and MMP-1, which are directly involved in the deposition and removal of ECM, were quantified in CHC patients with and without S. mansoni for evaluating how far S. mansoni could affect these fibrosis markers. Herein, collagen III was found to increase in parallel with liver fibrosis progression. This result may be explained by the fact that liver injury leads to activation of HSCs and transformation to active myofibroblastic phenotype, and secrete a large amount of collagen with decreased collagenase activity.26
In contrast, our results demonstrated that MMP-1 was found to decrease with fibrosis progression. The latter result is similar to results obtained by Leroy and others.27 These findings may be explained in light of relatively large literature which suggested that MMP activity diminishes as liver fibrosis progresses due to overexpression of tissue inhibitor of metalloproteinases (TIMPs).28
Surprisingly, our coinfected patients showed higher concentration of collagen III and depressed concentration of MMP-1 compared with HCV-monoinfected patients in different fibrosis stages. The latter result could be explained in light of the study performed by Kamal and others29 who showed that statistically significant elevation of TGF-β are found in individuals who have HCV/S. mansoni coinfection. TGF-β is considered to be the strongest known inducer of fibrogenesis in the effector cells of hepatic fibrosis. In addition, it was reported that TGF-β is considered to be the central cytokine in the process of abnormal ECM production which is known to upregulate gene transcription for collagen chains in cell nucleus.30,31 Knittel and others32 demonstrated that diminished matrix degradation during chronic tissue injury might be due to the action of TGF-β through TIMP induction which, in turn, inhibits MMP activity.
The question thus arises, “Does the concentration of HCV protein have an impact in inducing collagen/MMP-1 ratio and subsequently in the progression to end-stage liver disease?” This question could be answered by noting the elevation of CMR by increasing the concentration of HCV-NS4. This may be explained by the fact that HCV proteins seem to stimulate HSC activation23 which in turn induces the secretion of large amount of collagen with decreased MMP-1 activity.26
One of the most important findings in this study is that the etiology of liver disease must be taken into account upon using different noninvasive biomarkers for fibrosis staging. This is because monoinfected and coinfected patients with the same fibrosis stages may have different concentrations of collagen III and MMP-1 subsequently increasing the rates of false positive or false negative in the biomarkers test results.
In this work, we have focused on sampling HCV-infected patients with and without S. mansoni infection, but the healthy volunteers who were already negative for both S. mansoni and HCV were not taken into account during sampling. This is because the main aim of this work is to estimate the extent to which S. mansoni could influence HCV-NS4 and to estimate whether there is an impact of HCV-NS4 in inducing ECM deposition and subsequently in the progression to end-stage liver disease. Therefore, further prospective studies involving a greater number of patients and incorporating uninfected patients are warranted to determine the mean values of collagen III, MMP-1, and CMR in normal livers and to compare their values with infected patients.
In conclusion, S. mansoni could increase HCV protein concentrations which in turn directly invigorate the inflammatory and fibrogenic actions of HSCs. Besides, not only the existence of HCV and S. mansoni coinfection affect natural history of liver fibrosis, but their concentrations also have a great impact on fibrosis progression. In addition, we could conclude that patients with different fibrosis stages who have HCV/S. mansoni coinfection showed higher collagen content and subsequently higher CMR values than those with HCV monoinfection. This would increase their susceptibility for progressing to subsequent fibrosis stage faster than those with HCV monoinfection. Thus, patients with HCV/S. mansoni coinfection should be treated with anti-Schistosoma therapy to decrease fibrosis-progression rate and not to retard response to HCV treatment. Moreover, patients with acute HCV should be considered for antiviral therapy to prevent progression to CHC.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank the staff of Biotechnology Research Center for their help during this study. This work has been carried out at Biotechnology Research Center, New Damietta, Egypt. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Footnotes
Authors' addresses: Abdelfattah M. Attallah, Mohamed S. Albannan, and Ahmed A. Attallah, Biotechnology Research Center, New Damietta, Egypt, E-mails: amattallah@hotmail.com, mohamedalbannan@yahoo.com, and ahmedattallah2009@hotmail.com. Sanaa O. Abdallah, Faculty of Science, Cairo University, Giza, Egypt, E-mail: sanaa.osman@gmail.com. Mohamed M. Omran, Faculty of Science, Helwan University, Cairo, Egypt, E-mail: drmmomran@yahoo.com. Khaled Farid, Faculty of Medicine, Mansoura University, Mansoura, Egypt, E-mail: khaled_som@mans.edu.eg.
References
- 1.European Association for Study of Liver EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392–420. doi: 10.1016/j.jhep.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Negro F. Curbing hepatitis C virus spread in Egypt. Lancet Glob Health. 2014;2:e495–e496. doi: 10.1016/S2214-109X(14)70292-X. [DOI] [PubMed] [Google Scholar]
- 3.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–115. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 4.Gryseels B. Schistosomiasis. Infect Dis Clin North Am. 2012;26:383–397. doi: 10.1016/j.idc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanghvi MM, Hotez PJ, Fenwick A. Neglected tropical diseases as a cause of chronic liver disease: the case of schistosomiasis and hepatitis C co-infections in Egypt. Liver Int. 2013;33:165–168. doi: 10.1111/liv.12052. [DOI] [PubMed] [Google Scholar]
- 7.Attallah AM, Yones E, Ismail H, El Masry SA, Tabll A, Elenein AA, El Ghawalby NA. Immunochemical characterization and diagnostic potential of a 63-kilodalton Schistosoma antigen. Am J Trop Med Hyg. 1999;60:493–497. doi: 10.4269/ajtmh.1999.60.493. [DOI] [PubMed] [Google Scholar]
- 8.Strickland GT. Liver disease in Egypt: hepatitis C superseded schistosomiasis as a result of iatrogenic and biological factors. Hepatol. 2006;43:915–922. doi: 10.1002/hep.21173. [DOI] [PubMed] [Google Scholar]
- 9.Van-Lume DS, Albuquerque Mde F, Souza AI, Domingues AL, Lopes EP, Morais CN, Montenegro SM. Association between schistosomiasis mansoni and hepatitis C: systematic review. Rev Saude Publica. 2013;47:414–424. doi: 10.1590/S0034-910.2013047004247. [DOI] [PubMed] [Google Scholar]
- 10.Brady MT, MacDonald AJ, Rowan AG, Mills KH. Hepatitis C virus non‐structural protein 4 suppresses Th1 responses by stimulating IL‐10 production from monocytes. Eur J Immunol. 2003;33:3448–3457. doi: 10.1002/eji.200324251. [DOI] [PubMed] [Google Scholar]
- 11.Attallah AM, Omran MM, Nasif WA, Ghaly MF, El-Shanshoury AER, Abdalla MS, Sharada HM, Farid K, El-Shony W, Moussa ESM, El-Domany EB, Nour E, Eldosoky I. Diagnostic performances of hepatitis C virus-NS4 antigen in patients with different liver pathologies. Arch Med Res. 2012;43:555–562. doi: 10.1016/j.arcmed.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Chen CZ, Raghunath M. Focus on collagen: in vitro systems to study fibrogenesis and antifibrosis state of the art. Fibrogenesis Tissue Repair. 2009;2:7. doi: 10.1186/1755-1536-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arthur MJP. Degradation of matrix proteins in liver fibrosis. Pathol Res Pract. 1994;190:825–833. doi: 10.1016/S0344-0338(11)80985-4. [DOI] [PubMed] [Google Scholar]
- 14.Attallah AM, El-Far M, Abdel Malak CA, Omran MM, Farid K, Hussien MA, Albannan MS, Attallah AA, Elbendary MS, Elbesh DA, Elmenier NA, Abdallah MO. Fibro-check: a combination of direct and indirect markers for liver fibrosis staging in chronic hepatitis C patients. Ann Hepatol. 2015;14:225–233. [PubMed] [Google Scholar]
- 15.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.European Association for Study of Liver EASL recommendations on treatment of hepatitis C 2015. J Hepatol. 2015;63:199–236. doi: 10.1016/j.jhep.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatol. 2004;39:1147–1171. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 21.Booth JC, O'Grady J, Neuberger J. Clinical guidelines on the management of hepatitis C. Gut. 2001;49:I1–I21. doi: 10.1136/gut.49.suppl_1.I1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vilstrup H. Cirrhosis and bacterial infections. Rom J Gastroenterol. 2003;12:297–302. [PubMed] [Google Scholar]
- 23.Mengshol JA, Golden-Mason L, Rosen HR. Mechanisms of disease: HCV-induced liver injury. Nat Clin Pract Gastroenterol Hepatol. 2007;4:622–634. doi: 10.1038/ncpgasthep0961. [DOI] [PubMed] [Google Scholar]
- 24.Bataller R, Y-h Paik, Lindquist JN, Lemasters JJ, Brenner DA. Hepatitis C virus core and nonstructural proteins induce fibrogenic effects in hepatic stellate cells. Gastroenterol. 2004;126:529–540. doi: 10.1053/j.gastro.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 25.Shin JY, Hur W, Wang JS, Jang JW, Kim CW, Bae SH, Jang SK, Yang SH, Sung YC, Kwon OJ, Yoon SK. HCV core protein promotes liver fibrogenesis via up-regulation of CTGF with TGF-beta1. Exp Mol Med. 2005;37:138–145. doi: 10.1038/emm.2005.19. [DOI] [PubMed] [Google Scholar]
- 26.Arthur MJ, Mann DA, Iredale JP. Tissue inhibitors of metalloproteinases, hepatic stellate cells and liver fibrosis. J Gastroenterol Hepatol. 1998;13:S33–S38. doi: 10.1111/jgh.1998.13.s1.33. [DOI] [PubMed] [Google Scholar]
- 27.Leroy V, Monier F, Bottari S, Trocme C, Sturm N, Hilleret MN, Morel F, Zarski JP. Circulating matrix metalloproteinases 1, 2, 9 and their inhibitors TIMP-1 and TIMP-2 as serum markers of liver fibrosis in patients with chronic hepatitis C: comparison with PIIINP and hyaluronic acid. Am J Gastroenterol. 2004;99:271–279. doi: 10.1111/j.1572-0241.2004.04055.x. [DOI] [PubMed] [Google Scholar]
- 28.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamal SM, Turner B, He Q, Rasenack J, Bianchi L, Al Tawil A, Nooman A, Massoud M, Koziel MJ, Afdhal NH. Progression of fibrosis in hepatitis C with and without schistosomiasis: correlation with serum markers of fibrosis. Hepatol. 2006;43:771–779. doi: 10.1002/hep.21117. [DOI] [PubMed] [Google Scholar]
- 30.Inagaki Y, Okazaki I. Emerging insights into transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007;56:284–292. doi: 10.1136/gut.2005.088690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jarcuska P, Janicko M, Veseliny E, Jarcuska P, Skladaný L. Circulating markers of liver fibrosis progression. Clin Chim Acta. 2010;411:1009–1017. doi: 10.1016/j.cca.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Knittel T, Mehde M, Kobold D, Saile B, Dinter C, Ramadori G. Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non-parenchymal cells of rat liver: regulation by TNF-alpha and TGF-beta1. J Hepatol. 1999;30:48–60. doi: 10.1016/s0168-8278(99)80007-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.