Abstract
In 2006, artemether–lumefantrine (AL) became the first-line treatment of uncomplicated malaria in Senegal, Mali, and the Gambia. To monitor its efficacy, between August 2011 and November 2014, children with uncomplicated Plasmodium falciparum malaria were treated with AL and followed up for 42 days. A total of 463 subjects were enrolled in three sites (246 in Senegal, 97 in Mali, and 120 in Gambia). No early treatment failure was observed and malaria infection cleared in all patients by day 3. Polymerase chain reaction (PCR)-adjusted adequate clinical and parasitological response (ACPR) was 100% in Mali, and the Gambia, and 98.8% in Senegal. However, without PCR adjustment, ACPR was 89.4% overall; 91.5% in Mali, 98.8% in Senegal, and 64.3% in the Gambia (the lower value in the Gambia attributed to poor compliance of the full antimalarial course). However, pfmdr1 mutations were prevalent in Senegal and a decrease in parasite sensitivity to artesunate and lumefantrine (as measured by ex vivo drug assay) was observed at all sites. Recrudescent parasites did not show Kelch 13 (K13) mutations and AL remains highly efficacious in these west African sites.
Introduction
Plasmodium falciparum malaria is a major public health problem, particularly in sub-Saharan Africa. Its control is threatened by the possible emergence of resistance to commonly used treatments. Resistance to antimalarials by P. falciparum has been an ongoing global public health concern since chloroquine resistance emerged in the 1960s.1 Several factors such as suboptimal treatment dosage or counterfeit/substandard medications increase the selective pressure on the local parasite populations and thus favor the spread of drug-resistant parasites. The approach of treating malaria patients with a combination of drugs is thought to decrease the risk of selecting resistant parasites.2 The artemisinin component has a short half-life (< 8 hours) and rapidly reduce parasite biomass, whereas the partner drug can clear any remaining parasites. For this reason, malaria-endemic countries, including those in sub-Saharan Africa, have adopted artemisinin-based combination therapies (ACT) as first-line treatments for uncomplicated malaria.1,3–5 One of these is artemether–lumefantrine (AL) that has been proven to be highly efficacious and well tolerated.6–8 Senegal, Mali, and the Gambia adopted it as first-line malaria treatment in 2006.9
Considering the emergence of artemisinin resistance along the Thai–Cambodian border,10 treatment efficacy should be monitored at regular intervals.11 This involves directly measuring parasite drug responses, or indirectly measuring the prevalence of specific mutations in several parasite genetic loci associated to lower treatment efficacy.12 Monitoring their prevalence over time can reveal trends that inform on the possible therapeutic life of a given treatment.11,13
Materials and Methods
This study was carried out between September 2011 and November 2014 in four sites, two in Senegal (Section de Lutte AntiParasitaire (SLAP) clinic in Thiès and in Dakar) and one in each of the other two countries, Dioro in Mali and Gambissara in The Gambia. The two sites in Senegal are urban, with low and seasonal (September–November) P. falciparum malaria transmission. The entomological inoculation rate (EIR) is estimated at < 5.14,15 Dioro is a rural community at the edge of the Sahel with hyper-endemic, year-round malaria caused by both the seasonal rainfall (June–July to October–November) and the irrigation scheme from the Niger River for the rice fields.16 Gambissara is a large village in the upper river region (URR) of the Gambia where malaria transmission is moderate and seasonal (July–December).17,18
Suspected malaria patients were explained the study objectives and procedures before being asked to sign an informed consent form. For patients < 18 years old, parents/guardian were asked to sign the informed consent. Inclusion criteria were as follows: P. falciparum mono-infection with a density between 2,000 and 200,000/μL, age between 2 and 15 years in Gambissara and Dioro, and 2–20 years in Thiès and Dakar. Exclusion criteria included the following: severe malaria, other acute or chronic potentially confounding diseases, concomitant infection, history of human immunodeficiency virus, and an inability to take oral medicine.
After consenting, a rapid diagnostic test (RDT) (Pf HRP2) was performed and a thick and thin blood film was collected for microscopy; hemoglobin was measured by Hemocue® Hb 201+ at days 0 and 28 and glycemia was also determined. A few drops of blood were collected on Whatman Flinders Technology Associates (FTA) filter paper cards or ethylenediaminetetraacetic acid (EDTA) tubes for later P. falciparum genotyping.19
AL (20 mg of artemether and 120 mg lumefantrine) was administered twice a day for 3 days according to patient weight: 5–14 kg, one tablet per dose; 15–24 kg, two tablets per dose; 25–34 kg, three tablets per dose. Morning doses were given under direct observation in the study clinic and the rest was given to the patient to be taken at home. A full dose was readministered if the patient vomited within 30 minutes; if patients vomited a second time within 30 minutes, they were referred for parenteral treatment and withdrawn from the study.
Patients were asked to return for follow-up visits, physical examinations, and finger prick (for thick and thin blood smear, and filter paper collection) on days 1, 2, 3, 7, 14, 21, 28, 35, and 42, as well as any day they felt unwell. Treatment failure was treated with quinine.
Blood slides were stained with 10% Giemsa and read independently by two technicians. Parasite density was estimated by counting the number of asexual parasites against 200–500 white blood cells (WBCs) and assuming a WBC count of 8,000/μL.20
DNA extraction for subsequent molecular analysis was only carried out in Senegal and the Gambia. In Senegal, DNA was extracted from blood preserved on Whatman FTA filter paper cards or whole blood EDTA samples using either a QIAmp DNA or Blood Mini Kit (Qiagen, Valencia, CA), whereas in the Gambia, whole blood was stored in EDTA tubes for this analysis. Infections were genotyped according to published protocols.21,22 As a tool to track parasite diversity, we used a previously developed “molecular barcode,” composed of assays for 24 single nucleotide polymorphisms (SNPs) across the P. falciparum genome. The high resolution melting (HRM) genotyping method detects the presence of sequence variation in a fragment of amplified DNA using a dsDNA binding dye and Hi-Res Melting.22,23 Drug resistance–associated mutations were detected based on changes in DNA sequence, and are referred to in the text, by their corresponding amino acid changes.21 Differentiation between recrudescence and reinfection was assessed by comparing the 24-SNP molecular barcode between day 0 and day of treatment failure.
We performed Sanger sequencing of the K13 propeller gene using protocols established in Centers for Disease Control and Prevention (CDC) Atlanta Malaria Genomic laboratory.24 Samples with treatment failure confirmation from Senegal were analyzed for mutations in the K13 propeller domain using the Geneious Pro R8 software (Biomatters Inc., Newark, NJ). An automated SNP calling workflow developed in CDC Atlanta using Geneious Pro R8 was used for this analysis. SNPs were only called if both the forward and reverse strands had the mutation.
The primary endpoint was treatment efficacy at day 42, both PCR adjusted and unadjusted, assessed by clinical and parasitological outcomes using the World Health Organization (WHO) definitions for adequate clinical and parasitological response (ACPR), early treatment failure (ETF), late treatment failure (LTF), late parasitological failure (LPF).25 Secondary endpoints were parasite clearance time by day 3, time to reinfection, and hemoglobin levels on days 0 and 28. Resistance markers for AL (pfmdr1 and pfcrt) were measured in the Gambia and Senegal.
Study forms were double entered. Statistical analysis was performed using STATA 12 version software (Stata Corp., College Station, TX). The per-protocol (PP) analysis excluded children withdrawn from the study for any reason. Kaplan–Meier curves were estimated for both the 28- and 42-day follow-up; the log-rank test was used for comparing the curves. A two-sided P value < 0.05 was considered as statistically significant.
The study was reviewed and approved by the respective institutional review boards (IRBs), and was carried out according the current WHO Guidelines for Good Clinical Practices.
Results
Out of 1,771 screened patients (704 in Mali, 471 in Senegal, and 596 in the Gambia) (Table 1), 463 were included in the study (97 in Mali, 246 in Senegal, and 120 in the Gambia). Mean Hb at day 0 was 10.9 g/dL and similar between countries (Table 1).
Table 1.
Clinical and parasitological outcomes from children enrolled in Senegal, Mali, and the Gambia over the duration of the study
| Year | 2011 | 2012 | 2013 | 2014 | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Senegal | Mali | The Gambia | Senegal | Mali | The Gambia | Senegal | Mali | The Gambia | Senegal | Mali | The Gambia | Senegal | Mali | The Gambia |
| Number screened | 46 | 0 | 0 | 208 | 101 | 186 | 98 | 378 | 341 | 119 | 225 | 69 | 471 | 704 | 596 |
| Number enrolled | 26 | 0 | 0 | 120 | 16 | 39 | 50 | 25 | 55 | 50 | 56 | 26 | 246 | 97 | 120 |
| Median age (years) | 11 | 0 | 0 | 11 | 6 | 6 | 12 | 10 | 7 | 14 | 8 | 9 | 13 | 9 | 7 |
| Mean age ± SD (years) | 11.10 ± 2.60 | 0 | 0 | 11.00 ± 2.80 | 6.40 ± 3.1 | 7.52 ± 2.95 | 12.50 ± 4.30 | 9.52 ± 3.48 | 7.37 ± 2.87 | 12.00 ± 2.30 | 8.21 ± 3.39 | 8.69 ± 2.49 | 11.80 ± 3.30 | 8.04 ± 3.32 | 7.52 ± 2.95 |
| Median parasite count (/mL) | 57,825 | 0 | 0 | 38,925 | 30,563 | 28,160 | 45,938 | 31,800 | 52,244 | 37,658 | 32,095 | 29,480 | 44,134 | 31,486 | 34,440 |
| Mean count ± SD (/mL) | 89,595 ± 81,253 | 0 | 0 | 57,426 ± 52,626 | 40,314 ± 41,414 | 31,953 ± 44,072 | 67,367 ± 61,247 | 40,088 ± 35,107 | 62,824 ± 48,799 | 53,453 ± 76,067 | 42,537 ± 69,965 | 42,335 ± 44,632 | 67,423 ± 66,053 | 40,979 ± 48,828 | 48,716 ± 44,072 |
| Persistent vomiting of meds | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| No asexual parasites by day 7 | 26 | 0 | 0 | 120 | 16 | 39 | 50 | 25 | 54 | 50 | 56 | 26 | 246 | 97 | 119 |
| Serious adverse events | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Clinical symptom persist after day 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hb level on day 28 mean ± SD (g/dL) | 11.90 ± 1.30 | 0 | 0 | 12.10 ± 1.20 | NA | 10.91 ± 1.58 | 11.0 ± 2.20 | 11.50 ± 1.30 | 11.43 ± 1.45 | 12.0 ± 2.20 | 11.44 ± 1.08 | 11.70 ± 1.81 | 11.0 ± 1.70 | 11.47 ± 1.19 | 11.72 ± 1.59 |
| Follow-up through day 42 | 26 | 0 | 0 | 116 | 12 | 21 | 47 | 23 | 27 | 48 | 49 | 23 | 237 | 84 | 71 |
| Loss to follow-up | 0 | 0 | 0 | 3 | 2 | 6 | 2 | 0 | 8 | 1 | 1 | 1 | 6 | 3 | 15 |
| Recurrence uncorrected, n (%) | 0 (0) | 0 | 0 | 1 (0.9) | 2 (14.3) | 13 (39.4) | 1 (2.1) | 2 (8) | 20 (66.7) | 1 (2.0) | 4 (7.3) | 2 (8) | 3 (1.2) | 8 (8.5) | 35 (35.7) |
| Recurrence corrected, n (%) | 0(0) | 0 | 0 | 1 (0.9) | 0 (0) | 0 (0) | 1 (2.1) | 0 (0) | 0 (0) | 1 (2.0) | 0 (0) | 0 (0) | 3 (1.2) | 0 (0) | 0 (0) |
NA = not applicable.
Among those enrolled, 404 (87.2%) were included in the day 42 analysis; 84 for Mali (86.5%), 237 for Senegal (96.3%), and 83 (69.2%) for the Gambia (Table 1). There was no persistent vomiting of medications or persisting symptoms after day 3 in any of the sites, with no other serious adverse events detected.
The only other reason for patient withdrawal from the analysis was lost to follow-up, which concerned 31 (6.7%) patients (Table 1). Mean hemoglobin level at day 28 improved from 10.9 ± 1.7 at day 0 to 11.7 ± 1.3, with the same trend seen in all three countries. Specific values per country were 10.00 ± 1.70 g/dL at day 28 versus 10.80 ± 1.71 g/dL at day 0 in Senegal (P = 0.003); 11.47 ± 11.19 g/dL at day 28 versus 10.86 ± 2.20 g/dL at day 0 in Mali (P = 0.004); and 11.72 ± 1.59 g/dL at day 28 versus 11.32 ± 1.50 g/dL at day 0 in the Gambia (P = 0.007).
In PP analysis, there were 8/94 (8.5%) treatment failures, all LPF, in Mali (one at day 21, one at day 28, three at day 35, and three at day 42); three LTF (3/240) in Senegal (one per day at days 14, 21, and 35); and 35 LTF in the Gambia (14 at day 28, eight at day 35, and eight at day 42).
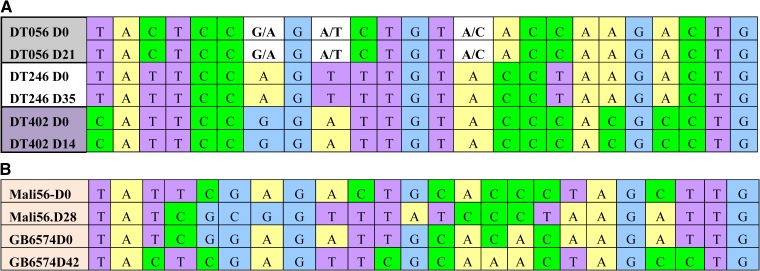
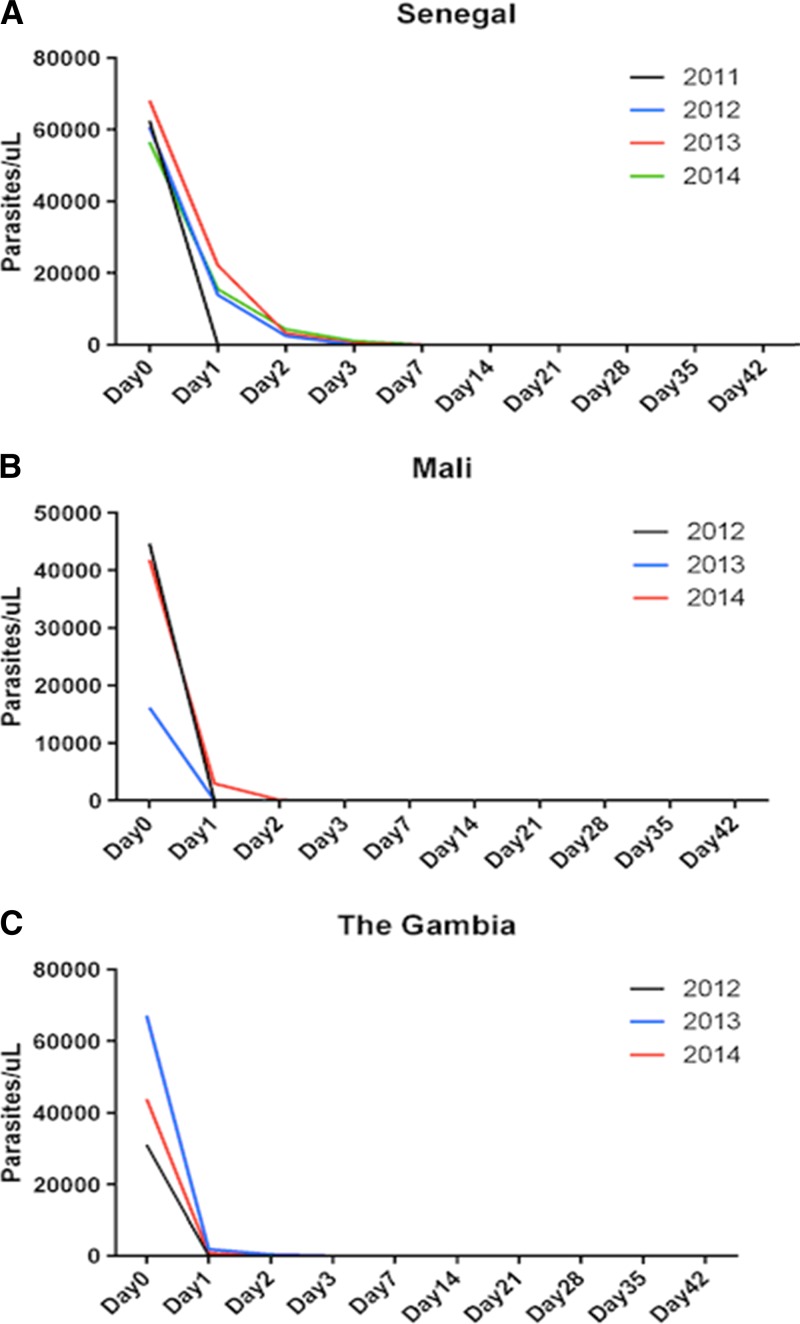
Nevertheless, PCR-corrected ACPR for all sites combined was 99.3% (3/N), with no recrudescence observed in Mali and the Gambia and three detected in Senegal, as confirmed by the 24 SNP molecular barcode analysis (Figure 1 ). All patients cleared their infection by day 3. Nevertheless, during the study period, parasite clearance was slightly delayed in Senegal (Figure 2A ) and Mali (Figure 2B), but not in the Gambia (Figure 2C).
Figure 1.
Single nucleotide polymorphism (SNP) barcode of parasite isolates from day 0 and day of treatment failure. (A) Barcodes of the three isolates from Senegal, in which the 24 SNPs were identical at day 0 and day of failure, indicating recrudescence. (B) Example of barcodes of two isolates from Mali and The Gambia, in which the pattern was different between day 0 and day of failure, indicating reinfection with a different strain of parasite.
Figure 2.
Parasite clearance time. (A) In Senegal, except in 2011, where parasite clearances were obtained by day 1, in 2012, 2013, and 2014, parasite clearances were generally delayed at day 3. (B) In Mali, during the first years of this study, parasite clearances were obtained by day 1 in 2012 and 2013, whereas parasite clearance was delayed by day 2 in 2014. (C) In Gambia, almost all parasite clearances were obtained by day 1 in 2012, 2013, and 2014.
HRM assays to determine the prevalence of the mutations on pfcrt and pfmdr1 genes were performed on 246 genomic DNA from Senegal and 154 from the Gambia (120 D0 and 34 reinfections). The prevalence of the pfcrt K76T mutation decreased during the study period in Thies, Senegal (38.5% in 2011, 26.7% in 2012, 18.5% in 2013) (P = 0.001), whereas in the Gambia, it tended to increase (30% in 2012 to 50.0% in 2014) (P = 0.08; Table 2). In Senegal, the pfmdr1 mutation at codon 86 showed a similar trend, with prevalence declined from 11.5% in 2011 to 2% in 2013 in Thies and 9.4% in Dakar. No mutation in codons 1042 and 1246 was observed (Table 2). In the Gambia, as for the pfcrt K76T mutation, the prevalence of the N86Y mutation increased from 13.6% in 2012 to 25.9% in 2014 (P = 0.0001); however, the Y184F mutation decreased 51.7% in 2012 to 25% in 2014 (P = 0.002; Table 2).
Table 2.
Pfcrt and Pfmdr polymorphisms detected in parasite isolates from the Gambia and Senegal
| Country | Site | Year | Number tested | Pfcrt | Pfmdr | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K76T (%) | N1042D (%) | N86Y (%) | Y184F (%) | D1246Y (%) | ||||||||||||||
| WT | MUT | MIX | WT | MUT | MIX | WT | MUT | MIX | WT | MUT | MIX | WT | MUT | MIX | ||||
| Senegal | Thies | 2011 | 26 | 50 | 38.5 | 11.5 | 100 | 0 | 0 | 88.5 | 11.5 | 0 | 42.3 | 57.7 | 0 | 100 | 0 | 0 |
| Thies | 2012 | 120 | 65.8 | 26.7 | 6.7 | 100 | 0 | 9 | 99.2 | 0.8 | 0 | 15 | 77.5 | 7,5 | 100 | 0 | 0 | |
| Thies | 2013 | 50 | 82 | 18.5 | 0 | 100 | 0 | 0 | 98.1 | 2 | 0 | 30 | 64.0 | 6 | 100 | 0 | 0 | |
| Dakar | 2014 | 50 | 92.5 | 0 | 7.5 | 100 | 0 | 0 | 90.6 | 9.4 | 0 | 30.1 | 67.9 | 1.9 | 100 | 0 | 0 | |
| The Gambia | Gambissara | 2012 | 51 | 57.5 | 30 | 12.5 | 83.4 | 7.1 | 9.6 | 81.8 | 13.6 | 4.6 | 48.3 | 51.7 | 0 | – | – | – |
| Gambissara | 2013 | 75 | 65.8 | 31.6 | 2.6 | 87.3 | 9.9 | 2.8 | 78.9 | 11.2 | 9.9 | 27.1 | 52.9 | 0 | – | – | – | |
| Gambissara | 2014 | 28 | 37.5 | 50 | 12.5 | 73 | 19.3 | 7.7 | 70.4 | 25.9 | 3.7 | 75 | 25 | 0 | – | – | – | |
MIX = mixed; MUT = mutant; WT = wild type.
The recurrent infections classified as recrudescence in three Senegalese patients were wild type in the K13 propeller domain; none of the mutations related to artemisinin resistance, namely C580Y, R539T, Y493H, I543T, P553T, V568G, N458Y, was detected. Similarly, in the Gambia, no mutation in the K13 propeller domain region was observed in the 154 samples sequenced.
Discussion
AL remains efficacious in west African patients with uncomplicated P. falciparum malaria, with a cure rate close to 100% given that most recurrent infections were classified as new ones according to the results of the PCR SNP barcoding.
The identical PCR SNP barcodes observed in the three (∼1%) suspected treatment failures were from Senegal, which suggests that these are true treatment failures and may be attributed to key resistance mutations. For example, wild-type pfcrt has previously been reported to be associated with reduced susceptibility to lumefantrine, in line with the declining trend of the K76T mutation observed in Senegal. In addition, pfmdr mutations have been reported as associated with resistance to artemisinin partner drugs.26–32 We did not find any mutations in the K13 propeller domain region that correlates with resistance or delayed clearance time to artemisinin,10,33–35 it may be that these genetic loci are not responsible in the west African population and therefore further population genetic analysis is required to find those mutations associated with artemisinin resistance in west Africa. Alternatively, given the late occurrence of the treatment failures during the follow-up period, the possibility remains that these may be reinfections of parasites harboring the same genotype in the population.
The treatment failure rates observed in Senegal are also consistent with previous studies within Senegal.36 However, other studies from west Africa show slightly higher failure rates with values of 4% in Senegal,37 5% in Mali,38 > 4% in Burkina Faso,39 3% in Cote d'Ivoire,40 and 7% in Togo.41 Most of these results are within the confidence intervals and differences may be attributable to a number of factors, including but not limited to regional variations in the duration of deployment of AL, the period of deployment, the age distribution of participants, and prevalence levels as well as drug administration practices.
In the Gambia, the PCR corrected rate showed 100% efficacy; however, the high rate of apparent reinfection observed is in line with previous reports.42 This can be attributed to a number of factors, including the intense seasonal transmission that occurs during the short malaria season in the Gambia. In addition, poor compliance to treatment at days 2 and 3 may be a factor, given patients were observed during the three days of the AL treatment in Mali and Senegal, whereas in the Gambia, only the first dose was supervised. Most of these late parasitological failures were identified on days 28, 35, and 42, with all treated with quinine, the standard second-line drug for the treatment of P. falciparum malaria in Mali, Senegal, and the Gambia.
All participants cleared parasitemia in the Gambia by day 1 in 2011, and by day 2 in subsequent years. A similar profile was observed in Mali. This parasite clearance time obtained in Mali and the Gambia is considered to be below the threshold indicating potentially emerging resistance and is comparable to previous findings from Ethiopia43 and Burkina Faso39 and is considerably lower than the 21.9% parasitemic patients on day 3 reported from a trial conducted in western Cambodia as early as 2007.33 In Senegal, parasite clearance was obtained by day 3, except in 2011, where parasites were cleared by day 1. This late parasite clearance time in Senegal compared with Mali and the Gambia is consistent with the three recrudescence samples obtained from this country.
At the pfcrt K76T locus, we observed a decrease in the mutation over the 4 years of the study in Senegal from 38.5% in 2011 to 0% in 2014, consistent with previous findings from Senegal.44–46 Surprisingly, in the Gambia, there was an increase in the mutation from 30% in 2012 to 37.5% in 2014, indicating possible continued use of chloroquine outside of the government policy. These findings indicate that there is additional pressure on this locus, and may have implications for drug use in the Gambia.
Conclusion
AL still shows efficacy in west Africa. Antimalarial compounds in use in Senegal and the Gambia (artemisinin derivatives, lumefantrine, and amodiaquine), as well as chloroquine, are sensitive in vitro to P. falciparum. We observed a general decrease in pfcrt and pfmdr1 mutations in west Africa among the populations tested.
ACKNOWLEDGMENTS
We thank the populations and patients from Medina Fall, Gambissara, Dioro, and the field team. We also thank Daba Zoumarou and Julie Thwing for a deep reading of the manuscript and Eldin Talundzic, Eric Halsey, Udhayakumar Venkatachalam, and the Kumar's Lab at CDC/Atlanta for supporting Senegalese scientists to perform K13 sequencing of the Senegal isolates.
Footnotes
Financial support: The work was supported by the NIH/ICEMR, International Centre of Excellence for Malaria Research, west Africa (U19AI089696).
Authors' addresses: Baba Dieye, Yaye D. Ndiaye, Jules F. Gomis, Mouhamadou Ndiaye, Aminata Mbaye, Ngayo Sy, Babacar Mbengue, Awa B. Deme, Ambroise D. Ahouidi, Tandakha Dieye, Jean L. Ndiaye, and Daouda Ndiaye, Université Cheikh Anta Diop, BP 5005, Dakar, Fann, Sénégal, E-mails: dieyebaba2004@yahoo.fr, ydndiaye@gmail.com, jules.gomis@gmail.com, mouhamadou.ndiaye@ucad.edu.sn, natou5002@yahoo.fr, ngayosy50@hotmail.com, b.mbengue@yahoo.fr, deme.awa@gmail.com, aahouidi@gmail.com, tandakha.dieye@ucad.edu.sn, jeanloab.ndiaye@ucad.edu.sn, and dndiaye@hsph.harvard.edu. Muna Affara, Fatou Joof, Ahmad Abdullahi, Abubakar Ismaela, Alfred Amambua Ngwa, Umberto D'Alessandro, and Davis Nwakanma, Medical Research Council Unit, Atlantic Boulevard, Fajara, Banjul, The Gambia, E-mails: maffara@mrc.gm, fajoof@mrc.gm, aahmad@mrc.gm, iabubakar@mrc.gm, angwa@mrc.gm, udalessandro@mrc.gm, and dnwakanma@mrc.gm. Lassana Sangre, Mouhamadou Diakite, Seydou Doumbia, Ayouba Diarra, Mamadou Coulibaly, and Ousmane Koita, Université de Bamako, BP 1805, Bamako, Mali, E-mails: lansana.sangare@gmail.com, mdiakite@icermali.org, sdoumbi@gmail.com, adiarra@icemrwaf.org, doudou@icermali.org, and okoita@icermali.org. Clint Welty, Jeffrey Shaffer, and Donald J. Krogstad, Tulane University, New Orleans, LA, E-mails: cwelty@icemrwaf.org, jshaffer@tulane.edu, and donkrogstad@gmail.com. Rachel Daniels, Sarah K. Volkman, and Dyann F. Wirth, Harvard T. H. Chan School of Public Health, Boston, MA, E-mails: rdaniels@broadinstitute.org, svolkman@hsph.harvard.edu, and dfwirth@hsph.harvard.edu.
References
- 1.Payne D. Spread of chloroquine resistance in Plasmodium falciparum. Parasitol Today. 1987;3:241–246. doi: 10.1016/0169-4758(87)90147-5. [DOI] [PubMed] [Google Scholar]
- 2.White NJ. Antimalarial drug resistance. J Clin Invest. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mens PF, Sawa P, van Amsterdam SM, Versteeg I, Omar SA, Schallig HD, Kager PA. A randomized trial to monitor the efficacy and effectiveness by QT-NASBA of artemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment and transmission control of uncomplicated Plasmodium falciparum malaria in western Kenya. Malar J. 2008;7:237. doi: 10.1186/1475-2875-7-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO . World Malaria Report 2008. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 5.Dorsey G, Staedke S, Clark TD, Njama-Meya D, Nzarubara B, Maiteki-Sebuguzi C, Dokomajilar C, Kamya MR, Rosenthal PJ. Combination therapy for uncomplicated falciparum malaria in Ugandan children: a randomized trial. JAMA. 2007;297:2210–2219. doi: 10.1001/jama.297.20.2210. [DOI] [PubMed] [Google Scholar]
- 6.Adjuik M, Babiker A, Garner P, Olliaro P, Taylor W, White N. International Artemisinin Study G Artesunate combinations for treatment of malaria: meta-analysis. Lancet. 2004;363:9–17. doi: 10.1016/s0140-6736(03)15162-8. [DOI] [PubMed] [Google Scholar]
- 7.Ashley EA, Stepniewska K, Lindegardh N, Annerberg A, Kham A, Brockman A, Singhasivanon P, White NJ, Nosten F. How much fat is necessary to optimize lumefantrine oral bioavailability? Trop Med Int Health. 2007;12:195–200. doi: 10.1111/j.1365-3156.2006.01784.x. [DOI] [PubMed] [Google Scholar]
- 8.Bousema JT, Schneider P, Gouagna LC, Drakeley CJ, Tostmann A, Houben R, Githure JI, Ord R, Sutherland CJ, Omar SA, Sauerwein RW. Moderate effect of artemisinin-based combination therapy on transmission of Plasmodium falciparum. J Infect Dis. 2006;193:1151–1159. doi: 10.1086/503051. [DOI] [PubMed] [Google Scholar]
- 9.WHO . The World Malaria Report. Geneva, Switzerland: World Health Organization; 2005. [Google Scholar]
- 10.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vestergaard LS, Ringwald P. 2007. Responding to the challenge of antimalarial drug resistance by routine monitoring to update national malaria treatment policies\ Am J Trop Med Hyg 77 153 159 [PubMed] [Google Scholar]
- 12.Wilson PE, Alker AP, Meshnick SR. Real-time PCR methods for monitoring antimalarial drug resistance. Trends Parasitol. 2005;21:278–283. doi: 10.1016/j.pt.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Sibley CH, Ringwald P. A database of antimalarial drug resistance. Malar J. 2006;5:48. doi: 10.1186/1475-2875-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas SM, Ndir O, Dieng T, Mboup S, Wypij D, Maguire JH, Wirth DF. In vitro chloroquine susceptibility and PCR analysis of pfcrt and pfmdr1 polymorphisms in Plasmodium falciparum isolates from Senegal. Am J Trop Med Hyg. 2002;66:474–480. doi: 10.4269/ajtmh.2002.66.474. [DOI] [PubMed] [Google Scholar]
- 15.Trape JF, Lefebvre-Zante E, Legros F, Ndiaye G, Bouganali H, Druilhe P, Salem G. Vector density gradients and the epidemiology of urban malaria in Dakar, Senegal. Am J Trop Med Hyg. 1992;47:181–189. doi: 10.4269/ajtmh.1992.47.181. [DOI] [PubMed] [Google Scholar]
- 16.Sogoba N, Doumbia S, Vounatsou P, Bagayoko MM, Dolo G, Traore SF, Maiga HM, Toure YT, Smith T. Malaria transmission dynamics in Niono, Mali: the effect of the irrigation systems. Acta Trop. 2007;101:232–240. doi: 10.1016/j.actatropica.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Bogh C, Lindsay SW, Clarke SE, Dean A, Jawara M, Pinder M, Thomas CJ. High spatial resolution mapping of malaria transmission risk in the Gambia, west Africa, using LANDSAT TM satellite imagery. Am J Trop Med Hyg. 2007;76:875–881. [PubMed] [Google Scholar]
- 18.Mwesigwa J, Okebe J, Affara M, Di Tanna GL, Nwakanma D, Janha O, Opondo K, Grietens KP, Achan J, D'Alessandro U. On-going malaria transmission in The Gambia despite high coverage of control interventions: a nationwide cross-sectional survey. Malar J. 2015;14:314. doi: 10.1186/s12936-015-0829-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howie SR. Blood sample volumes in child health research: review of safe limits. Bull World Health Organ. 2011;89:46–53. doi: 10.2471/BLT.10.080010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO . Malaria Microscopy Quality Assurance Manual—Version 1. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 21.Daniels R, Ndiaye D, Wall M, McKinney J, Sene PD, Sabeti PC, Volkman SK, Mboup S, Wirth DF. Rapid, field-deployable method for genotyping and discovery of single-nucleotide polymorphisms associated with drug resistance in Plasmodium falciparum. Antimicrob Agents Chemother. 2012;56:2976–2986. doi: 10.1128/AAC.05737-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daniels R, Chang HH, Sene PD, Park DC, Neafsey DE, Schaffner SF, Hamilton EJ, Lukens AK, Van Tyne D, Mboup S, Sabeti PC, Ndiaye D, Wirth DF, Hartl DL, Volkman SK. Genetic surveillance detects both clonal and epidemic transmission of malaria following enhanced intervention in Senegal. PLoS One. 2013;8:e60780. doi: 10.1371/journal.pone.0060780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniels R, Volkman SK, Milner DA, Mahesh N, Neafsey DE, Park DJ, Rosen D, Angelino E, Sabeti PC, Wirth DF, Wiegand RC. A general SNP-based molecular barcode for Plasmodium falciparum identification and tracking. Malar J. 2008;7:223. doi: 10.1186/1475-2875-7-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talundzic E, Okoth SA, Congpuong K, Plucinski MM, Morton L, Goldman IF, Kachur PS, Wongsrichanalai C, Satimai W, Barnwell JW, Udhayakumar V. Selection and spread of artemisinin-resistant alleles in Thailand prior to the global artemisinin resistance containment campaign. PLoS Pathog. 2015;11:e1004789. doi: 10.1371/journal.ppat.1004789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization . Methods for Surveillance of Antimalarial Drug Efficacy. Geneva, Switzerland: WHO; 2009. [Google Scholar]
- 26.Duraisingh MT, Jones P, Sambou I, von Seidlein L, Pinder M, Warhurst DC. The tyrosine-86 allele of the pfmdr1 gene of Plasmodium falciparum is associated with increased sensitivity to the anti-malarials mefloquine and artemisinin. Mol Biochem Parasitol. 2000;108:13–23. doi: 10.1016/s0166-6851(00)00201-2. [DOI] [PubMed] [Google Scholar]
- 27.Duraisingh MT, Roper C, Walliker D, Warhurst DC. Increased sensitivity to the antimalarials mefloquine and artemisinin is conferred by mutations in the pfmdr1 gene of Plasmodium falciparum. Mol Microbiol. 2000;36:955–961. doi: 10.1046/j.1365-2958.2000.01914.x. [DOI] [PubMed] [Google Scholar]
- 28.Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature. 2000;403:906–909. doi: 10.1038/35002615. [DOI] [PubMed] [Google Scholar]
- 29.Mwai L, Kiara SM, Abdirahman A, Pole L, Rippert A, Diriye A, Bull P, Marsh K, Borrmann S, Nzila A. In vitro activities of piperaquine, lumefantrine, and dihydroartemisinin in Kenyan Plasmodium falciparum isolates and polymorphisms in pfcrt and pfmdr1. Antimicrob Agents Chemother. 2009;53:5069–5073. doi: 10.1128/AAC.00638-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tumwebaze P, Conrad MD, Walakira A, LeClair N, Byaruhanga O, Nakazibwe C, Kozak B, Bloome J, Okiring J, Kakuru A, Bigira V, Kapisi J, Legac J, Gut J, Cooper RA, Kamya MR, Havlir DV, Dorsey G, Greenhouse B, Nsobya SL, Rosenthal PJ. Impact of antimalarial treatment and chemoprevention on the drug sensitivity of malaria parasites isolated from Ugandan children. Antimicrob Agents Chemother. 2015;59:3018–3030. doi: 10.1128/AAC.05141-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickard AL, Wongsrichanalai C, Purfield A, Kamwendo D, Emery K, Zalewski C, Kawamoto F, Miller RS, Meshnick SR. Resistance to antimalarials in southeast Asia and genetic polymorphisms in pfmdr1. Antimicrob Agents Chemother. 2003;47:2418–2423. doi: 10.1128/AAC.47.8.2418-2423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veiga MI, Ferreira PE, Jornhagen L, Malmberg M, Kone A, Schmidt BA, Petzold M, Bjorkman A, Nosten F, Gil JP. Novel polymorphisms in Plasmodium falciparum ABC transporter genes are associated with major ACT antimalarial drug resistance. PLoS One. 2011;6:e20212. doi: 10.1371/journal.pone.0020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Artemisinin Resistance in Cambodia 1 Study C Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 34.Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, ler Moo C, Al-Saai S, Dondorp AM, Lwin KM, Singhasivanon P, Day NP, White NJ, Anderson TJ, Nosten F. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;379:1960–1966. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, Rahman MR, Hasan MM, Islam A, Miotto O, Amato R, MacInnis B, Stalker J, Kwiatkowski DP, Bozdech Z, Jeeyapant A, Cheah PY, Sakulthaew T, Chalk J, Intharabut B, Silamut K, Lee SJ, Vihokhern B, Kunasol C, Imwong M, Tarning J, Taylor WJ, Yeung S, Woodrow CJ, Flegg JA, Das D, Smith J, Venkatesan M, Plowe CV, Stepniewska K, Guerin PJ, Dondorp AM, Day NP, White NJ. Tracking Resistance to Artemisinin C Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faye B, Ndiaye JL, Ndiaye D, Dieng Y, Faye O, Gaye O. Efficacy and tolerability of four antimalarial combinations in the treatment of uncomplicated Plasmodium falciparum malaria in Senegal. Malar J. 2007;6:80. doi: 10.1186/1475-2875-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ndiaye JL, Faye B, Gueye A, Tine R, Ndiaye D, Tchania C, Ndiaye I, Barry A, Cisse B, Lameyre V, Gaye O. Repeated treatment of recurrent uncomplicated Plasmodium falciparum malaria in Senegal with fixed-dose artesunate plus amodiaquine versus fixed-dose artemether plus lumefantrine: a randomized, open-label trial. Malar J. 2011;10:237. doi: 10.1186/1475-2875-10-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sagara I, Fofana B, Gaudart J, Sidibe B, Togo A, Toure S, Sanogo K, Dembele D, Dicko A, Giorgi R, Doumbo OK, Djimde AA. Repeated artemisinin-based combination therapies in a malaria hyperendemic area of Mali: efficacy, safety, and public health impact. Am J Trop Med Hyg. 2012;87:50–56. doi: 10.4269/ajtmh.2012.11-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zongo I, Dorsey G, Rouamba N, Dokomajilar C, Sere Y, Rosenthal PJ, Ouedraogo JB. Randomized comparison of amodiaquine plus sulfadoxine-pyrimethamine, artemether-lumefantrine, and dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria in Burkina Faso. Clin Infect Dis. 2007;45:1453–1461. doi: 10.1086/522985. [DOI] [PubMed] [Google Scholar]
- 40.Toure OA, Kouame MG, Didier YJ, Berenger AA, Djerea K, Genevieve GO, Penali LK. Artesunate/mefloquine paediatric formulation vs. artemether/lumefantrine for the treatment of uncomplicated Plasmodium falciparum in Anonkoua koute, Cote d'Ivoire. Trop Med Int Health. 2011;16:290–297. doi: 10.1111/j.1365-3156.2010.02701.x. [DOI] [PubMed] [Google Scholar]
- 41.Dorkenoo MA, Barrette A, Agbo YM, Bogreau H, Kutoati S, Sodahlon YK, Morgah K. Surveillance of the efficacy of artemether-lumefantrine and artesunate-amodiaquine for the treatment of uncomplicated Plasmodium falciparum among children under five in Togo, 2005–2009. Malar J. 2012;11:338. doi: 10.1186/1475-2875-11-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunyo S, Sirugo G, Sesay S, Bisseye C, Njie F, Adiamoh M, Nwakanma D, Diatta M, Janha R, Sisay Joof F, Temple B, Snell P, Conway D, Walton R, Cheung YB, Milligan P. Randomized trial of safety and effectiveness of chlorproguanil-dapsone and lumefantrine-artemether for uncomplicated malaria in children in The Gambia. PLoS One. 2011;6:e17371. doi: 10.1371/journal.pone.0017371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Getnet G, Fola AA, Alemu A, Getie S, Fuehrer HP, Noedl H. Therapeutic efficacy of artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in Enfranze, north-west Ethiopia. Malar J. 2015;14:258. doi: 10.1186/s12936-015-0775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niang M, Marrama L, Ekala MT, Alioune G, Tall A, Ndiaye JL, Sarr D, Dangou JM, Lehesran JY, Bouchier C, Mercereau-Puijalon O, Jambou R. Accumulation of CVIET Pfcrt allele of Plasmodium falciparum in placenta of pregnant women living in an urban area of Dakar, Senegal. J Antimicrob Chemother. 2008;62:921–928. doi: 10.1093/jac/dkn299. [DOI] [PubMed] [Google Scholar]
- 45.Ndiaye M, Faye B, Tine R, Ndiaye JL, Lo A, Abiola A, Dieng Y, Ndiaye D, Hallett R, Alifrangis M, Gaye O. Assessment of the molecular marker of Plasmodium falciparum chloroquine resistance (Pfcrt) in Senegal after several years of chloroquine withdrawal. Am J Trop Med Hyg. 2012;87:640–645. doi: 10.4269/ajtmh.2012.11-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ly O, Gueye PE, Deme AB, Dieng T, Badiane AS, Ahouidi AD, Diallo M, Bei AK, Wirth DF, Mboup S, Sarr O. Evolution of the pfcrt T76 and pfmdr1 Y86 markers and chloroquine susceptibility 8 years after cessation of chloroquine use in Pikine, Senegal. Parasitol Res. 2012;111:1541–1546. doi: 10.1007/s00436-012-2994-7. [DOI] [PubMed] [Google Scholar]