Abstract
We evaluated the efficacy of chloroquine and primaquine on uncomplicated Plasmodium vivax malaria in Cruzeiro do Sul, Brazil, in 2014. Patients ≥ 5 years of age with either fever or history of fever, and laboratory-confirmed P. vivax monoinfection received chloroquine (total dose = 25 mg/kg) and primaquine (total dose = 3.5 mg/kg), and were followed up for 168 days (24 weeks). We used microsatellite genotyping to differentiate recurrent infections caused by heterologous parasites from those caused by homologous ones. No new P. vivax episode occurred by Day 28 among 119 enrolled patients, leading to Day 28, with adequate clinical and parasitological response (ACPR) of 100% (95% confidence interval [CI] = 96.7–100%). Twenty-eight P. vivax episodes occurred by Day 168, with uncorrected ACPR of 69.9% (95% CI = 59.5–79.0%). Fifteen of these episodes were caused by either homologous haplotypes or haplotypes that could not be determined. Excluding the 13 recurrent episodes caused by heterologous parasites, Day 168 microsatellite-corrected ACPR was estimated at 81.2% (95% CI = 71.0–89.1%). Chloroquine and primaquine remain efficacious to treat acute uncomplicated P. vivax infection, but moderate recurrence rates were observed within 24 weeks of follow-up.
Introduction
Malaria remains an important cause of morbidity and mortality worldwide. The World Health Organization (WHO) has estimated that 214 million cases of malaria occurred globally in 2015 with 438,000 deaths.1 Although most cases occur in sub-Saharan Africa, malaria is also a concern in the Americas, and almost half of cases in the Americas occur in Brazil.1 In 2014, 143,551 malaria cases were reported in the Brazilian Amazon; 83.6% of those were Plasmodium vivax monoinfections, 15.9% were Plasmodium falciparum monoinfections, and 0.5% were mixed infections of these two species.2 Countries in the Americas follow WHO recommendations to control and prevent malaria; malaria treatment with efficacious antimalarials is a cornerstone of these recommendations.3 Although different methods, such as molecular and in vitro sensitivity assays, have been proposed to assist in evaluating malaria treatments, in vivo efficacy trials remain the only tool globally recommended for this purpose.4
The WHO and the Pan American Health Organization (PAHO) recommend routine monitoring of antimalarial treatment efficacy using standardized in vivo trials, with subsequent change in treatment policy when the efficacy of a regimen drops below 90%.4 The goal of such trials is to evaluate the efficacy of first- and second-line antimalarial regimens under directly observed therapy using quality-assured drugs based on standardized clinical and parasitological follow-up for 4 weeks or longer. In the Americas, the Amazon Network for the Surveillance of Antimalarial Drug Resistance (Spanish acronym, RAVREDA) and the Amazon Malaria Initiative (AMI) support therapeutic efficacy studies since 2001. The information generated by these efforts was crucial to motivate changes in P. falciparum treatment policies in the region.5
In Brazil, the recommended P. vivax treatment comprises a combination of chloroquine, given over 3 days (total dose = 25 mg/kg), and primaquine, given over 7 or 14 days (total dose = 3.5 mg/kg).6 Chloroquine resistance in P. vivax malaria was first reported in Papua New Guinea in 1989 among Australian soldiers and has either spread or independently emerged in other regions of the world.7,8 The possibility of chloroquine resistance is worrisome and has already led to the use of artemisinin-based combination therapy as first-line treatment of uncomplicated P. vivax malaria in Cambodia, Vanuatu, and Laos, for example.1 Reports of chloroquine resistance have also been made in the Americas, in countries such as Colombia, Peru, and Brazil.9–11 As part of the efforts to monitor antimalarials efficacy, we evaluated the efficacy of the combination of chloroquine and primaquine for the treatment of uncomplicated P. vivax malaria in Cruzeiro do Sul, Brazil.
Methods
Study site.
This study was conducted at the malaria diagnosis and treatment post at the Hospital Regional do Jurua in Cruzeiro do Sul, Acre State, Brazil, from February to December 2014. Cruzeiro do Sul, located in the western region of the Brazilian Amazon, is the second biggest city in Acre State with approximately 85,000 residents and is one of the sentinel sites for antimalarial efficacy monitoring in Brazil.2 In accordance with Brazilian national policies, malaria diagnosis and treatment are offered free of charge, and patients present directly to malaria diagnostic posts at public health-care facilities for clinical evaluation, laboratory testing, and treatment. The malaria post at Hospital Regional do Jurua is the busiest in Cruzeiro do Sul, with 19,331 patients presenting for malaria care in 2014, and was responsible for 18.5% of all microscopy-confirmed malaria cases in Cruzeiro do Sul also in 2014.
Study procedures.
Our study followed WHO recommendations with modifications from PAHO for in vivo antimalarial drug efficacy testing in areas of low transmission.4,12 Before study initiation, study staff underwent a 1-week intensive training covering patient enrollment and follow-up procedures, as well as microscopy standardization. In addition, two supervisory visits by national and international partners involved in the study took place during patient follow-up.
We enrolled patients ≥ 5 years of age with documented fever (axillary temperature > 37.5°C) or history of fever in the previous 48 hours; P. vivax monoinfection; and parasite density between 250 and 100,000 asexual parasites/μL. Those with clinical signs or symptoms of severe malaria were excluded.4 At enrollment (Day 0), after obtaining written informed consent, we collected demographic and clinical information using standard forms and then drew blood from a venous puncture for malaria confirmation, glucose 6-phosphate dehydrogenase (G6PD) activity determination, hemoglobin level, and pregnancy test (for women 10–49 years of age). In addition, we prepared and stored blood spots on filter paper for genotyping in case of recurrent parasitemia.
We followed the national Brazilian antimalarial treatment guidelines.6 In brief, we initiated treatment with chloroquine (Farmanguinhos, Brazil, 150 mg per pill, lot no. 12080939) after enrollment procedures. We then instructed patients to return to the study clinic at the Hospital Regional do Jurua on Days 1 and 2 for clinical evaluation, two additional daily chloroquine doses to complete treatment (total chloroquine dose = 25 mg/kg), and microscopy testing (Day 2 only). If vomiting occurred within 30 minutes after a dose, a full dose was repeated. If vomiting reoccurred within 30 minutes after the repeat dose, patients were excluded from the study and referred for alternative treatment. All chloroquine doses were administered as directly observed therapy. Primaquine (Farmanguinhos, Brazil, lot nos. 13030259 [15 mg per pill] and 12101162 [5 mg per pill]) was initiated as soon as a normal G6PD level became available. G6PD determination was performed at a private laboratory in Cruzeiro do Sul using a fluorometric method. We administered primaquine also under directly observed therapy over 7 days (primaquine total dose = 3.5 mg/kg), except for patients weighing > 70 kg, in which cases, the total dose was calculated and was given over longer periods of time at a maximum daily dose of 30 mg/day.
We instructed patients to return to the study clinic, or made arrangements for home visits, on Days 3, 7, 14, 21, 28, and every 4 weeks until Day 168 (24 weeks) for clinical evaluation and laboratory follow-up. Axillary temperature was measured and blood smears were prepared on each visit. We considered patients who were not located within 1 day of the expected visit to have been lost to follow up and censored those from the study at that point. We instructed patients to contact the study team, telephone numbers were provided, if they presented fever, chills, headache, or any other symptom or concern related to malaria.
Sample size calculations were based on the expected proportion of P. vivax treatment failures with chloroquine and primaquine in the study population during the first 28 days of follow-up, our primary objective. Assuming a maximum of 5% treatment failures at Day 28 in a population of infinite size, precision of 5%, and a 95% confidence level, 73 patients reaching a valid endpoint (clinical and parasitological cure or failure) were needed. As an attempt to reach Day 168 with enough patients to infer efficacy within the same parameters, we increased the sample size by 60%, to at least 117 patients.
Blood smears.
Experienced microscopists from Instituto Evandro Chagas, a Brazilian national public health institute, trained study microscopists before study initiation and provided all reagents needed for slide staining. We prepared both thick and thin blood smears to identify species and estimate parasite density. Using diluted Giemsa stain (1:50, v/v), thick smears were stained for 20 minutes, and thin smears for 30 minutes. We estimated parasite density by counting the number of asexual parasites using a hand tally counter against 200 white blood cells (WBCs) in the thick smear, based on an estimated WBC count of 6,000 WBC/μL. If the parasite count was < 100 parasites per 200 WBCs, counting continued until 500 WBCs had been counted. A total of 1,000 WBCs were counted before a blood smear was considered negative. Gametocytes were counted and the number of gametocytes per microliter was also estimated.
All blood smears were examined by two independent microscopists, who were blinded to each other's results. Blood smears with differences in species determination or discrepancies in asexual parasite density of > 50% between the two microscopists were reexamined by a third, independent microscopist at the Acre State regional malaria laboratory in Cruzeiro do Sul. We calculated asexual parasite density based on the geometric mean of the two concordant results.
Microsatellite characterization.
As an effort to attempt to differentiate homologous and heterologous parasites causing recurrent infections, we collected blood onto filter paper on Day 0 to be processed in parallel in case of recurrent parasitemia on or after Day 7. We used seven neutral microsatellite markers previously described for this purpose: MS2 (chromosome 6), MS6 (chromosome 11), MS2.21 (chromosome 2), MS3.502 (chromosome 3), MS11.162 (chromosome 11), MS12.335 (chromosome 12), and MS038 (chromosome 6).13–15 We extracted DNA of the paired samples using commercial kits (Qiagen, Valencia, CA), and conducted polymerase chain reaction (PCR) to amplify these seven microsatellites. We analyzed fluorescent-labeled PCR products; alleles with a length difference of at least two base pairs were considered different. Paired samples with at least one microsatellite difference among the seven evaluated were considered heterologous haplotypes.
We also estimated the haplotypes circulating in Cruzeiro do Sul and their frequency by including all Day 0 samples. This information enabled calculation of probability of a homologous haplotype reinfection and not a relapse or a recrudescence caused by a homologous haplotype. The genetic diversity at each locus was measured by calculating the expected heterozygosity and number of alleles per locus using all Day 0 samples by means of Arlequin v3.5 (Swiss Institute of Bioinformatics, Bern, Switzerland).16 The probability of a recurrent episode with a particular haplotype occurring purely by chance P-match was calculated as previously described.17 In addition, we determined the multiplicity of infection (MOI), which describes the minimum number of coinfecting genotypes in a population, by measuring the average number of haplotypes in each sample using the seven unlinked microsatellite loci.
Outcome measures.
We evaluated treatment efficacy based on clinical and parasitological outcomes and study endpoints in accordance to WHO guidelines for in vivo efficacy monitoring.4 Cases classified as early treatment failure (ETF) included 1) development of danger signs or severe malaria with asexual parasitemia on Days 1, 2, or 3; 2) Day 2 asexual parasite density greater than that of Day 0; 3) positive asexual parasitemia on Day 3 with axillary temperature ≥ 37.5°C; or 4) Day 3 parasite density > 25% of Day 0. Late treatment failure (LTF) included late clinical failure (LCF) and late parasitologic failure (LPF). LCF was defined as the development of danger signs or severe malaria with asexual parasitemia, or axillary temperature ≥ 37.5°C with asexual parasitemia from Day 3 through Day 28 without meeting the criteria of ETF. LPF was the presence of asexual parasitemia between Day 7 and Day 28 and axillary temperature < 37.5°C without meeting criteria of ETF or LCF. An adequate clinical and parasitological response (ACPR) included patients who did not fulfill the criteria of ETF or LTF.
In addition to the WHO criteria listed above, the occurrence of asexual parasitemia between Day 28 and Day 168, with or without symptoms, was classified as extended follow-up failure. We used the microsatellite genotyping to differentiate recurrent episodes in infections caused by heterologous parasites, which could be reinfections or relapses, and those caused by homologous ones, assumed to be relapses. We conservatively considered recurrent infections where at least one of the paired isolates did not yield a complete set of microsatellite alleles to be caused by homologous haplotypes. We referred all patients with recurrent parasitemia for clinical evaluation and alternative treatment.
Statistical analysis.
We double entered patient data into an EpiInfo 3.5.4 (Centers for Disease Control and Prevention [CDC], Atlanta, GA) database and used SAS version 9.1 (SAS Institute, Cary, NC) for data cleaning and analysis. The primary efficacy endpoint was the Day 28 ACPR. In addition, we estimated Day 28 and Day 168 survival rates by Kaplan–Meier analysis. Kaplan–Meier survival rates were calculated among all participants with any follow-up duration; patients were censored when they reached a study endpoint, or were lost to follow up or withdrawn. We calculated 95% confidence intervals (CIs) around the efficacy rates for the two groups using exact methods for a binomial proportion. A P value < 0.05 was considered statistically significant throughout the evaluation.
Ethical considerations.
Informed written consent from patients ≥ 18 years of age and permission from caregivers of patients < 18 years of age were obtained before patient enrollment. In addition, we obtained verbal assent from all participants with ages between 8 and 18 years. This study was reviewed and approved by the institutional review board (IRB) of Instituto Evandro Chagas and Brazilian National IRB (Conselho Nacional de Etica em Pesquisa) (reference no. 482171) in Brazil, and also by the U.S. CDC IRB (reference no. 6371). This evaluation was registered in clinicaltrials.gov (reference no. NCT02043652).
Results
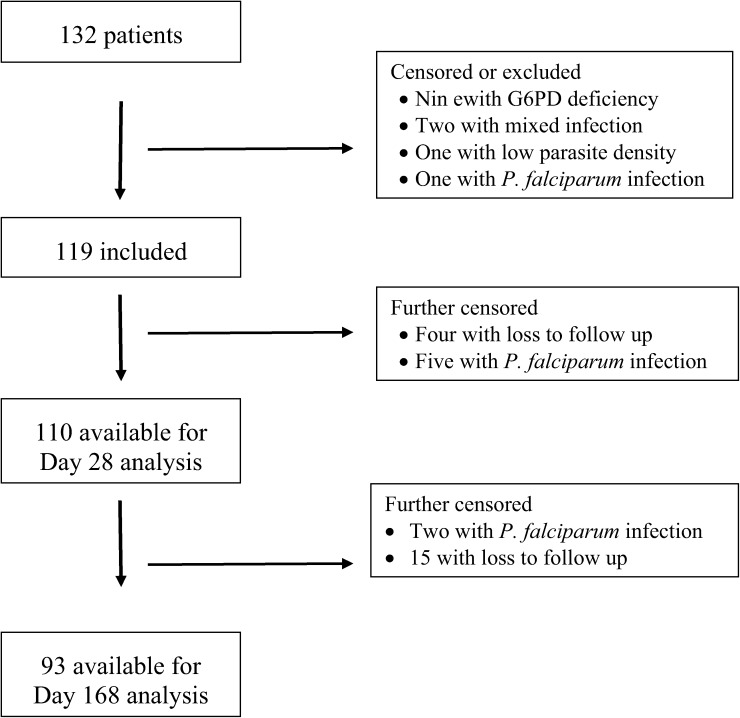
Patient enrollment lasted from February 17 to June 25, 2014. A total of 1,080 patients were diagnosed with vivax malaria during this period at Hospital Regional do Jurua. We offered participation in the study to 186 patients who presented during regular clinic hours; 132 were conditionally enrolled. Thirteen were later excluded due to G6PD deficiency (nine patients), mixed infection (two), low vivax parasite density (one), or P. falciparum infection (one) upon slide review. Therefore, we ultimately included a total of 119 patients in our study (Figure 1 ). Patient follow-up ended on December 17, 2014.
Figure 1.
Study profile of patients in the Plasmodium vivax in vivo efficacy trial, Cruzeiro do Sul (N = 132).
Patients' characteristics at enrollment are shown in Table 1. The median age was 23.4 years (range = 5–67.3 years); 65 (54.6%; 95% CI = 45.7–63.6) patients were male; and 31 (26.1%; 95% CI = 18.2–34.0) had documented fever at enrollment. Among the 109 patients who reported fever in the previous 48 hours, the median duration of fever was 2 days (range = 1–30 days). Other commonly reported symptoms included headache (111 patients), chills (64 patients), and vomiting (38 patients). The geometric mean of asexual parasite density at enrollment was 3,373 parasites/μL (range = 386–13,296 parasites/μL), whereas the arithmetic mean of gametocyte density was 133 parasites/μL (range = 0–671 parasites/μL). No patient had asexual parasitemia on Day 3.
Table 1.
Characteristics of enrolled patients, Cruzeiro do Sul, 2014 (N = 119)
| Variable | Value |
|---|---|
| Median age, years (range) | 23.4 (5–67.3) |
| Male, n (%) | 65 (54.6) |
| Median weight, kg (range) | 61.4 (16.0–100.3) |
| Fever on day of enrollment, n (%) | 31 (26.1) |
| Median duration of fever, days (range)* | 2 (1–30) |
| Median hemoglobin, g/dL (range) | 13.5 (9.7–17.1) |
| Asexual parasite density (geometric mean in parasites/μL) (range) | 3,373 (386–13,296) |
| Gametocyte density (arithmetic mean in parasites/μL) (range) | 133 (0–671) |
Only for those patients who reported fever.
Among the 119 patients, only three (2.5%) experienced vomiting after the first attempt for the first dose of chloroquine, but all three tolerated the repeat dose. The median total dose of chloroquine was 23.7 mg/kg (range = 15.0–35.7). Since the initiation of primaquine depended on the results of G6PD testing, primaquine was started on different days of treatment of each patient but within 24 hours of G6PD testing results becoming available. Most patients (25 patients or 22.3%) started primaquine on Day 16, but the start date varied from Day 9 to Day 22, and primaquine was given for as long as 11 days in patients weighting > 70 kg. No serious adverse event with either chloroquine or primaquine occurred. Median hemoglobin levels were 14.3 g/dL, 13.0 g/dL, and 13.3 g/dL on Days 0, 14, and 28, respectively.
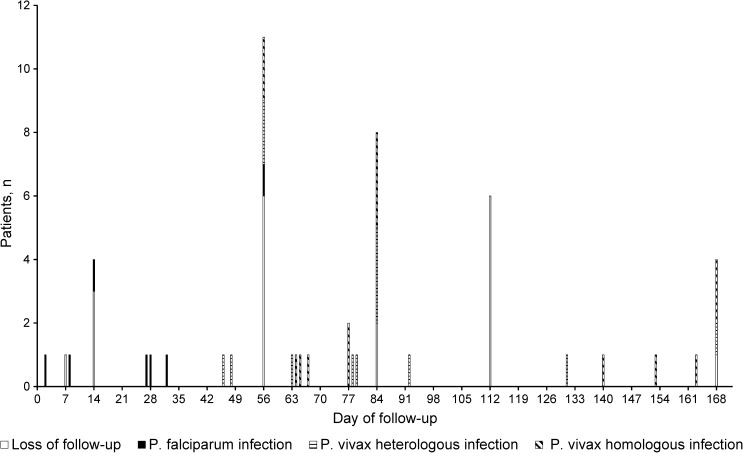
By Day 28, four patients (3.4%) were considered loss to follow-up and five (4.2%) presented with P. falciparum infection. No recurrent P. vivax episode was observed by Day 28; therefore; ACPR at Day 28 was estimated at 100% (95% CI = 96.7–100%). By Day 168, among 119 enrolled patients, 28 had recurrent P. vivax episode (all of them after Day 28), 19 were lost to follow up, and seven had P. falciparum infection. Figure 2 shows day of recurrent episodes, either by P. vivax or P. falciparum. Recurrent P. vivax episodes occurred as early as on Day 46 and as late as on Day 168, with no relationship with the timing of primaquine. Six (21.4%) of these 28 patients reported having fever in the days before recurrent episode was documented, whereas three (10.7%) had axillary temperature ≥ 37.5°C at the recurrent episode visit. Excluding patients lost to follow up and those with falciparum infection during the 168-day follow-up, Day 168 uncorrected ACPR could then be estimated at 69.9% (65 of 93 patients) (95% CI = 59.5–79.0%) and it was not associated with chloroquine dose per kilogram.
Figure 2.
Day of recurrent episode by species, Cruzeiro to Sul (N = 119).
Microsatellite typing of all Day 0 samples revealed the heterozygosity per locus ranged from 0.41 to 0.76 with number of alleles per locus ranging from four to nine, and an MOI for Day 0 samples of 1.06. Among the 28 cases of P. vivax recurrent episodes, a total of 56 samples, one sample from Day 0 and another from the day of recurrent episode for each patient, were available for microsatellite testing. Using the seven microsatellite markers, 20 samples did not yield valid amplifications by all seven microsatellites, or had more than one haplotype present. Among the remaining 36 samples, 19 distinct haplotypes, that is, those with at least one different microsatellite, were detected with prevalence varying from six occurrences (16.7%) to one (2.8%).
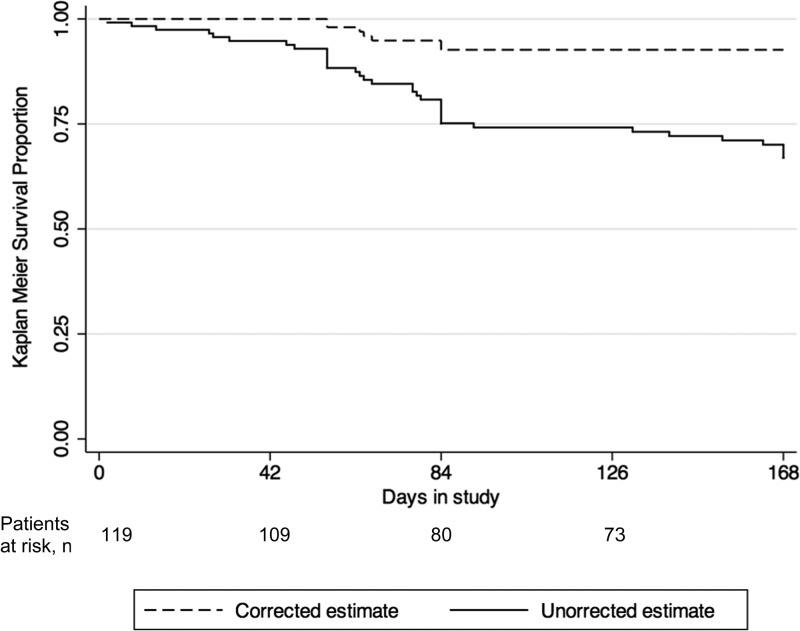
Comparing samples of Day 0 and day of recurrent episode from each of the 28 patients with recurrent P. vivax asexual parasitemia, 13 (46.4%) had an episode caused by a heterologous parasite during the 168-day follow-up. These could either be reinfections or relapses by heterologous haplotypes. The remaining 15 patients, had either recurrent episodes by homologous parasites (seven patients), or the haplotype could not be determined due to missed amplification in one of the microsatellites (eight patients). Of note, among the seven patients with recurrent episodes by homologous parasites, four were infected with haplotypes with P-match < 0.05, which means the chances of these infections being the result of reinfections is unlikely (Table 2).17 We considered these 15 cases as recurrent infections caused by homologous parasites during the extended follow-up. Not considering patients lost to follow up, P. falciparum infection, and P. vivax recurrent infections caused by heterologous parasites during the 168-day follow-up, Day 168 microsatellite-corrected ACPR was then estimated at 81.2% (65 of 80 patients) (95% CI = 71.0–89.1%). Figure 3 shows the Day 168 Kaplan–Meier survival curves showing both the uncorrected and microsatellite-corrected results.
Table 2.
P-match values of primary and recurrent infections with the same microsatellite profile, Cruzeiro do Sul (N = 7)
| Patient, day of collection | MS2 | MS6 | MS2.21 | MS3.502 | MS11.162 | MS12.335 | MS038 | P-match value |
|---|---|---|---|---|---|---|---|---|
| 006, Day 0 | 206 | 210 | 106 | 133 | 217 | 164 | 206 | 0.02 |
| 006, Day 57 | 206 | 210 | 106 | 133 | 217 | 164 | 206 | |
| 007, Day 0 | 210 | 210 | 106 | 133 | 217 | 164 | 198 | 0.19 |
| 007, Day 64 | 210 | 210 | 106 | 133 | 217 | 164 | 198 | |
| 009, Day 0 | 210 | 210 | 106 | 133 | 217 | 164 | 198 | 0.19 |
| 009, Day 56 | 210 | 210 | 106 | 133 | 217 | 164 | 198 | |
| 048, Day 0 | 210 | 239 | 102 | 133 | 209 | 174 | 190 | 0.01 |
| 048, Day 84 | 210 | 239 | 102 | 133 | 209 | 174 | 190 | |
| 054, Day 0 | 210 | 210 | 106 | 133 | 217 | 164 | 194 | 0.08 |
| 054, Day 67 | 210 | 210 | 106 | 133 | 217 | 164 | 194 | |
| 102, Day 0 | 210 | 210 | 102 | 133 | 185 | 174 | 194 | 0.01 |
| 102, Day 84 | 210 | 210 | 102 | 133 | 185 | 174 | 194 | |
| 112, Day 0 | 210 | 210 | 102 | 133 | 217 | 162 | 198 | 0.01 |
| 112, Day 65 | 210 | 210 | 102 | 133 | 217 | 162 | 198 |
Figure 3.
Uncorrected and microsatellite-corrected Kaplan–Meier survival curve up to Day 168, Cruzeiro do Sul (N = 119).
Discussion
We found ACPR at Day 28 for the treatment of uncomplicated P. vivax malaria with chloroquine and primaquine to be 100% in Cruzeiro do Sul. At Day 168, ACPR rates were 69.9% and 81.2% for uncorrected and microsatellite-corrected analyses, respectively. The uncorrected Day 168 ACPR is lower than what was found in other studies using primaquine (77.3%).18 Our findings show that P. vivax antimalarial regimen in Cruzeiro do Sul is highly efficacious for the acute phase of the infection, but is associated with moderate recurrent infection rates, mostly relapses, compared with a similar study in Peru.19 In addition, we noted very good tolerance and rapid parasite clearance with the current first-line treatment of uncomplicated P. vivax malaria in Brazil.
WHO and PAHO advise the evaluation of first- and second-line antimalarial regimens at regular intervals, every 3 years in low-endemic countries.4,5 The recommended duration of follow-up for P. vivax trials using chloroquine, in combination or not with primaquine, is often 28 days. We decided to extend our follow-up period to 168 days to evaluate recurrence rates. Malaria burden in the Americas is considerably lower than in sub-Saharan Africa; thus, sufficient patient enrollment can be difficult even in studies with follow-up duration of 28 or 42 days.20 To increase the chances of reaching desired sample size, we offered home follow-up visits for patients and also alerted neighboring health centers and health posts to increase patient enrollment. Other efforts in this evaluation included intensive staff training before study initiation to standardize practices and minimize study noncompliance. These efforts were also coordinated with Brazilian national reference institutions to train staff in slide preparation and reading. Finally, study staff was closely supervised with weekly phone calls and two site visits during patient enrollment and follow-up.
Plasmodium vivax infection can lead to hypnozoites, which are dormant liver stages responsible for relapses weeks and months after a mosquito-transmitted acute infection. For this reason, it is particularly challenging to correctly estimate P. vivax treatment efficacy.8 Hypnozoites are not sensitive to chloroquine and require radical treatment with primaquine. Therefore, recurrent P. vivax episodes can either be recrudescense, which is associated with reactivation of blood-stage parasites, or relapses, that is, recurrent episode originating from hypnozoites. Therapeutic efficacy trials for P. vivax can combine chloroquine and primaquine, as we did, or postpone the primaquine treatment in an effort to evaluate the efficacy of chloroquine alone.4 As such, our results cannot be attributed to chloroquine monotherapy but rather to the combination of chloroquine and primaquine. A review on P. vivax resistance suggests that recurrent episodes occurring before Day 16 of follow-up are almost certainly recrudescence and those between Day 17 and Day 28 could be either recrudescence or relapse, likely associated with chloroquine resistance in either case.21 A study in Brazil has shown therapeutic failure rate at Day 28 of 5.2% when chloroquine and primaquine were both administered at time of diagnosis, whereas another one, which used chloroquine alone, had higher failure rates (10.1%).22,23
We included the use of microsatellite genotyping as an attempt to differentiate heterologous infections, either reinfections or relapses caused by heterologous parasites, from homologous infections, either relapses or recrudescences. The use of molecular methods for this purpose in vivax trials is not formally recommended by WHO because previous studies have shown that relapses can arise from either homologous or heterologous parasites.24–26 We recognize this limitation and agree that definitive conclusions cannot be drawn from current genotyping methods used for P. vivax in vivo trials. Nevertheless, valuable P. vivax genetic information, such as the haplotype characterization as well as the minimum number of haplotypes per infection, can be inferred using microsatellite genotyping. A previous review of P. vivax efficacy trials showed that heterologous parasites were the cause of relapses in 63–78% of cases.24 This review assumed that all recurrent episodes during follow-up were relapses considering the low probability of reinfection due to the low malaria transmission where the different trials were conducted. Taking into account that the follow-up for some of the patients was limited, 28 days only, and that not all doses of chloroquine were observed, these review findings need to be considered with caution as recurrent episodes with different haplotypes on or before Day 28 could have been due to recrudescence of chloroquine-resistant parasites rather than relapse.24
In Cruzeiro do Sul, we found that roughly half (13 or 46.4%) of the recurrent episodes were caused by a haplotype different from the one causing the acute infection. This rate is lower than reported by some colleagues who proposed that genotype-specific immunity develops only against the predominant blood-stage parasites, with recurrent episodes often being caused by heterologous parasites.24 Moreover, this finding implies that, unlike Thailand, Myanmar, and India, this part of Brazil might have lower rates of simultaneous inoculations of different P. vivax genotypes, and multiple inoculations by different mosquitoes might be rare. When we calculated the mean MOI in Cruzeiro do Sul (MOI = 1.06), we found that it was lower than the ones reported in Myanmar and Thailand, which indicates lower genetic diversity of P. vivax parasites circulating in this area of Brazil.24
Considering the results of therapeutic response for the first 28 days, which is the WHO recommended endpoint for chloroquine trials, we found that chloroquine and primaquine treatment of uncomplicated P. vivax infection is quite efficacious (100%). This contrasts with results of a similar trial in Bolivia that documented ACPR of 89.6% at Day 28.27 The study in Bolivia and ours are comparable in design; however, the Bolivia trial did not include primaquine, and the combination of chloroquine and primaquine is more effective in treating chloroquine-sensitive and chloroquine-resistant P. vivax strains.28 On the other hand, our study shows a moderate rate of recurrent episodes occurring between Day 28 and the end of follow-up (Day 168). We observed 28 recurrent episodes during this long follow-up, 15 of those being of the same haplotype or haplotypes that could not be determined. These cases are most likely relapses due to homologous parasites, supporting the hypothesis that primaquine dose was insufficient to eliminate hypnozoites or its efficacy could have been impaired by other factors.29 The remaining 13 cases were of different microsatellite combination and reflect either reinfections or relapses by heterologous parasites, which have been reported in the literature.24
Although primaquine remains the only currently available drug for radical cure after P. vivax and Plasmodium ovale infections, different results in relapse prevention with primaquine have been reported.19,30 WHO recommends daily dose of 0.25 mg/kg/day (infections acquired in temperate regions) or 0.50 mg/kg/day (infections from tropical regions associated with frequent P. vivax relapses) over 14 days in both situations (total dose of 3.5 mg/kg or 7.0 mg/kg) after reports of frequent relapses in Oceania and east Asia.31–33 The concern around frequent relapses has led CDC to change its recommendation and increase the total primaquine dose to 7.0 mg/kg given over 14 days for radical cure of P. vivax infections acquired in all regions of the world.34 Primaquine can be associated with hemolytic anemia in patients with G6PD deficiency, and prior laboratory testing to confirm adequate G6PD activity is recommended in some countries, for example, United States.34 Alternatives to a 14-day therapy are highly desirable due to concerns around patient adherence. As such, single dose tafenoquine has been evaluated as an alternative for radical treatment.18 A study found that tafenoquine, which is not yet approved for market use, at single doses of 300 mg or 600 mg prevented relapses in 89.2% and 91.9% of cases, respectively, during a 6-month follow-up.18 Of note, G6PD testing is also recommended before tafenoquine use.
The AMI, a U.S. Agency for International Development–funded initiative, aims to assist in capacity building and generate evidence to support malaria control in the Americas. The low incidence of malaria in this region is an obstacle to complete the recommended in vivo protocols within a reasonable timeframe, 12 months.12,20 We, however, were able to institute changes in the original protocol, such as not limiting the catchment area of patient residence and hiring of extra study staff to perform home follow-up visits, to increase patient enrollment and decrease patient loss to follow-up. In addition, great attention and effort were devoted to train and build local capacity to conduct clinical trials and perform high-quality microscopy to minimize noncompliance to study standards.
Our evaluation has a few limitations. First, our findings are restricted to this region of Brazil and, until information from other parts of Brazil and the Amazon region can be collected, it should not be generalized. Second, we adopted a longer follow-up, 24 weeks, close to 6 months, as an attempt to detect relapses, and primaquine dosing was not initiated at the same time for all patients. We believe even longer duration of follow-up and strict primaquine dosing would be recommended to comprehensively estimate relapse rates in P. vivax studies. Longer studies, however, would increase the chance of reinfections, and due to the lack of adequate molecular markers, an accurate differentiation of relapses from reinfections is not yet possible. Finally, we do not have enough background information about the clonal variability of the P. vivax in this region for the seven microsatellite markers we used in our evaluation. It is possible that alleles in minor frequency exist in the population; therefore, the haplotype diversity we observed might have been underestimated. The use of more sensitive genotyping methods, such as deep sequencing, would allow for the identification of low-frequency alleles in small samples sizes of Plasmodium populations, such as those of in vivo trials.35 Nevertheless, in our study, we used seven of the most polymorphic microsatellite loci previously tested in our laboratories.
In summary, we provide evidence that chloroquine and primaquine continue to be very effective in treating the acute presentation of uncomplicated P. vivax malaria in the western Brazilian Amazon. It is, however, associated with frequent recurrent infections, most of them infections by homologous isolates or relapses, within 24 weeks after treatment, underscoring the need for studies with longer follow-ups to better understand treatment efficacy for P. vivax infections. In addition, consideration should be given to investigating possible causes for primaquine treatment failure, polymorphisms in cytochrome P-450 isoenzyme 2D6 (CYP2D6), and evaluating alternative regimens for relapse prevention in this population, for example, increasing the total primaquine dose.29 Despite the challenges in conducting efficacy trials in low-transmission areas such as the Americas, routine efficacy surveillance using WHO and PAHO guidelines should be conducted every 3 years.
ACKNOWLEDGMENTS
We would like to thank the Ministry of Health of Brazil and the Acre State Health Secretariat for their support to this evaluation, and all the patients and their caregivers who participated. We are indebted to Simone Daniel, Izanelda Magalhães, José Maria de Souza do Nascimento, José Mário Veloso Peres, Camila Damasceno, Oscar Lapouble, Luciana Flannery, and Naomi Lucchi for their assistance with implementation and sample processing.
Disclaimer: The funding sources for this study had no role in study design, data collection, analysis, or interpretation. The opinions expressed herein are those of the authors and do not necessarily reflect the views of the USAID or the U.S. Centers for Disease Control and Prevention (CDC).
Footnotes
Financial support: Funding for this evaluation was partially provided by the U.S. Agency for International Development (USAID) through the Amazon Malaria Initiative (AMI). Stella M. Chenet was supported by the American Society of Microbiology/CDC Postdoctoral Fellowship.
Authors' addresses: Suiane Negreiros, Samela Farias, and Thayna Maria Holanda de Souza, Acre State Health Secretariat, Cruzeiro do Sul, Acre, Brazil, E-mails: omsvalle@hotmail.com, samela_86@hotmail.com, and thayna.souza21@gmail.com. Giselle Maria Rachid Viana and Marinete Marins Povoa, Instituto Evandro Chagas, Brazilian Ministry of Health, Ananindeua, Brazil, E-mails: giselleviana@iec.pa.gov.br and povoamm@gmail.com. Sheila Akinyi Okoth, Stella M. Chenet, and Alexandre Macedo de Oliveira, Malaria Branch, Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: jyo3@cdc.gov, ynw0@cdc.gov, and acq7@cdc.gov. Paola Marchesini and Ana Carolina Faria e Silva Santelli, National Malaria Control Program, Brazilian Ministry of Health, Brasilia, Brazil, E-mails: paola.marchesini@saude.gov.br and anacarolina.santelli@gmail.com. Venkatachalam Udhayakumar, Malaria Branch, Centers for Disease Control and Prevention, Chamblee, GA, E-mail: vxu0@cdc.gov.
References
- 1.World Health Organization World Malaria Report1. 2015. http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/ Available at. Accessed January 20, 2016.
- 2.Brazil MdS. Boletim Epidemiologico. 2015. http://www.sivep_malaria/relatorio/rel_resumo_nacional_mensal.asp?tx_opcao_agravo=MALARIA&opcao_agravo=B54%20&dt_inicial=01/01/2014&dt_final=31/12/2014&niv_relatorio=NACIONAL Available at. Accessed January 20, 2016.
- 3.World Health Organization . Global Technical Strategy for Malaria 2016–2030. Geneva, Switzerland: World Health Organization; 2015. http://www.who.int/malaria/publications/atoz/9789241564991/en/ Available at. Accessed January 20, 2016. [Google Scholar]
- 4.World Health Organization . Methods for Surveillance of Antimalarial Drug Efficacy. Geneva, Switzerland: World Health Organization; 2009. http://www.who.int/malaria/publications/atoz/9789241597531/en/ Available at. [Google Scholar]
- 5.Pan American Health Organization RAVREDA-AMI: Amazon Network for the Surveillance of Antimalarial Drug Resistance (RAVREDA)/Amazon Malaria Initiative (AMI) 2015. http://www.paho.org/english/ad/dpc/cd/ravreda-ami.htm Available at. Accessed August 22, 2015.
- 6.Departamento de Vigilância Epidemiológica, Brasil Ministério da Saúde . Guia Prático de Tratamento da Malária no Brazil. Brasilia, Brazil: Ministério da Saúde; 2010. p. 36. [Google Scholar]
- 7.Rieckmann KH, Davis DR, Hutton DC. Plasmodium vivax resistance to chloroquine? Lancet. 1989;2:1183–1184. doi: 10.1016/s0140-6736(89)91792-3. [DOI] [PubMed] [Google Scholar]
- 8.Price RN, von Seidlein L, Valecha N, Nosten F, Baird JK, White NJ. Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:982–991. doi: 10.1016/S1473-3099(14)70855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alecrim Md G, Alecrim W, Macedo V. Plasmodium vivax resistance to chloroquine (R2) and mefloquine (R3) in Brazilian Amazon region. Rev Soc Bras Med Trop. 1999;32:67–68. doi: 10.1590/s0037-86821999000100013. [DOI] [PubMed] [Google Scholar]
- 10.Soto J, Toledo J, Gutierrez P, Luzz M, Llinas N, Cedeno N, Dunne M, Berman J. Plasmodium vivax clinically resistant to chloroquine in Colombia. Am J Trop Med Hyg. 2001;65:90–93. doi: 10.4269/ajtmh.2001.65.90. [DOI] [PubMed] [Google Scholar]
- 11.Ruebush TK, 2nd, Zegarra J, Cairo J, Andersen EM, Green M, Pillai DR, Marquino W, Huilca M, Arevalo E, Garcia C, Solary L, Kain KC. Chloroquine-resistant Plasmodium vivax malaria in Peru. Am J Trop Med Hyg. 2003;69:548–552. [PubMed] [Google Scholar]
- 12.Pan American Health Organization Generic Protocols and Flow Diagram for In Vivo Antimalarial Drug-Efficacy Studies in the Americas. 2015. http://www.paho.org/english/ad/dpc/cd/mal-antimalarials.htm Available at. Accessed June, 2015.
- 13.Imwong M, Sudimack D, Pukrittayakamee S, Osorio L, Carlton JM, Day NP, White NJ, Anderson TJ. Microsatellite variation, repeat array length, and population history of Plasmodium vivax. Mol Biol Evol. 2006;23:1016–1018. doi: 10.1093/molbev/msj116. [DOI] [PubMed] [Google Scholar]
- 14.Imwong M, Boel ME, Pagornrat W, Pimanpanarak M, McGready R, Day NP, Nosten F, White NJ. The first Plasmodium vivax relapses of life are usually genetically homologous. J Infect Dis. 2012;205:680–683. doi: 10.1093/infdis/jir806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thanapongpichat S, McGready R, Luxemburger C, Day NP, White NJ, Nosten F, Snounou G, Imwong M. Microsatellite genotyping of Plasmodium vivax infections and their relapses in pregnant and non-pregnant patients on the Thai-Myanmar border. Malar J. 2013;12:275. doi: 10.1186/1475-2875-12-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Excoffier L, Lischer HE. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour. 2010;10:564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 17.McCollum AM, Soberon V, Salas CJ, Santolalla ML, Udhayakumar V, Escalante AA, Graf PC, Durand S, Cabezas C, Bacon DJ. Genetic variation and recurrent parasitaemia in Peruvian Plasmodium vivax populations. Malar J. 2014;13:67. doi: 10.1186/1475-2875-13-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llanos-Cuentas A, Lacerda MV, Rueangweerayut R, Krudsood S, Gupta SK, Kochar SK, Arthur P, Chuenchom N, Mohrle JJ, Duparc S, Ugwuegbulam C, Kleim JP, Carter N, Green JA, Kellam L. Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomised, phase 2b dose-selection study. Lancet. 2014;383:1049–1058. doi: 10.1016/S0140-6736(13)62568-4. [DOI] [PubMed] [Google Scholar]
- 19.Durand S, Cabezas C, Lescano AG, Galvez M, Gutierrez S, Arrospide N, Alvarez C, Santolalla ML, Bacon DJ, Graf PC. Efficacy of three different regimens of primaquine for the prevention of relapses of Plasmodium vivax malaria in the Amazon Basin of Peru. Am J Trop Med Hyg. 2014;91:18–26. doi: 10.4269/ajtmh.13-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruebush TK, Levin A, Gonzaga V, Neyra D, Marquino W. Evaluation of a simple operational approach for monitoring resistance to antimalarial drugs in Peru. Trop Med Int Health. 2003;8:910–916. doi: 10.1046/j.1365-3156.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- 21.Baird JK, Leksana B, Masbar S, Fryauff DJ, Sutanihardja MA, Suradi Wignall FS, Hoffman SL. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine levels. Am J Trop Med Hyg. 1997;56:621–626. doi: 10.4269/ajtmh.1997.56.621. [DOI] [PubMed] [Google Scholar]
- 22.de Santana Filho FS, Arcanjo AR, Chehuan YM, Costa MR, Martinez-Espinosa FE, Vieira JL, Barbosa M, Alecrim WD, Alecrim M. Chloroquine-resistant Plasmodium vivax, Brazilian Amazon. Emerg Infect Dis. 2007;13:1125–1126. doi: 10.3201/eid1307.061386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marques MM, Costa MR, Santana Filho FS, Vieira JL, Nascimento MT, Brasil LW, Nogueira F, Silveira H, Reyes-Lecca RC, Monteiro WM, Lacerda MV, Alecrim MG. Plasmodium vivax chloroquine resistance and anemia in the western Brazilian Amazon. Antimicrob Agents Chemother. 2014;58:342–347. doi: 10.1128/AAC.02279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imwong M, Snounou G, Pukrittayakamee S, Tanomsing N, Kim JR, Nandy A, Guthmann JP, Nosten F, Carlton J, Looareesuwan S, Nair S, Sudimack D, Day NP, Anderson TJ, White NJ. Relapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoites. J Infect Dis. 2007;195:927–933. doi: 10.1086/512241. [DOI] [PubMed] [Google Scholar]
- 25.Chen N, Auliff A, Rieckmann K, Gatton M, Cheng Q. Relapses of Plasmodium vivax infection result from clonal hypnozoites activated at predetermined intervals. J Infect Dis. 2007;195:934–941. doi: 10.1086/512242. [DOI] [PubMed] [Google Scholar]
- 26.Collins WE. Further understanding the nature of relapse of Plasmodium vivax infection. J Infect Dis. 2007;195:919–920. doi: 10.1086/512246. [DOI] [PubMed] [Google Scholar]
- 27.Anez A, Moscoso M, Laguna A, Garnica C, Melgar V, Cuba M, Gutierrez S, Ascaso C. Resistance of infection by Plasmodium vivax to chloroquine in Bolivia. Malar J. 2015;14:261. doi: 10.1186/s12936-015-0774-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baird JK, Wiady I, Fryauff DJ, Sutanihardja MA, Leksana B, Widjaya H, Kysdarmanto Subianto B. In vivo resistance to chloroquine by Plasmodium vivax and Plasmodium falciparum at Nabire, Irian Jaya, Indonesia. Am J Trop Med Hyg. 1997;56:627–631. doi: 10.4269/ajtmh.1997.56.627. [DOI] [PubMed] [Google Scholar]
- 29.Bennett JW, Pybus BS, Yadava A, Tosh D, Sousa JC, McCarthy WF, Deye G, Melendez V, Ockenhouse CF. Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. N Engl J Med. 2013;369:1381–1382. doi: 10.1056/NEJMc1301936. [DOI] [PubMed] [Google Scholar]
- 30.Hill DR, Baird JK, Parise ME, Lewis LS, Ryan ET, Magill AJ. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am J Trop Med Hyg. 2006;75:402–415. [PubMed] [Google Scholar]
- 31.World Health Organization . Guidelines for the Treatment of Malaria. Geneva, Switzerland: World Health Organization; 2015. http://www.who.int/malaria/publications/atoz/9789241549127/en/ Available at. Accessed January 20, 2016. [Google Scholar]
- 32.Collins WE, Jeffery GM. Primaquine resistance in Plasmodium vivax. Am J Trop Med Hyg. 1996;55:243–249. doi: 10.4269/ajtmh.1996.55.243. [DOI] [PubMed] [Google Scholar]
- 33.Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39:1336–1345. doi: 10.1086/424663. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention . Malaria Treatment (United States) Atlanta, GA: Centers for Disease Control and Prevention; 2015. http://www.cdc.gov/malaria/diagnosis_treatment/treatment.html Available at. Accessed January 20, 2016. [Google Scholar]
- 35.Lin JT, Hathaway NJ, Saunders DL, Lon C, Balasubramanian S, Kharabora O, Gosi P, Sriwichai S, Kartchner L, Chuor CM, Satharath P, Lanteri C, Bailey JA, Juliano JJ. Using amplicon deep sequencing to detect genetic signatures of Plasmodium vivax relapse. J Infect Dis. 2015;212:999–1008. doi: 10.1093/infdis/jiv142. [DOI] [PMC free article] [PubMed] [Google Scholar]