Abstract
Primaquine is the only licensed antimalarial drug that is capable of clearing dormant Plasmodium vivax liver stage parasites. To date, there is no clear evidence of resistance of the liver stage parasite against this drug, because of the difficulty in ascertaining the cause of recurrent infection. We followed 52 Thai P. vivax patients for 9 months after directly observed treatment of 15 mg primaquine daily for 14 days. Blood samples taken at 2–4 weekly intervals were assessed by microscopy and polymerase chain reaction (PCR) for the presence of parasites. Only four of 52 (7.7%) volunteers had recurrent P. vivax infections, all at least 8 weeks after treatment. This demonstrates that primaquine retains a high efficacy in this population. Although a risk of new infections could not be ruled out, parasite genotyping at two polymorphic markers suggested a high probability of late relapsing infections in these volunteers. Continued monitoring of primaquine efficacy in this region is advisable.
Plasmodium vivax is widespread throughout southeast Asia, and is now considered the dominant parasite causing malaria within Thailand.1 Plasmodium vivax may not be as fatal as Plasmodium falciparum, but its wide geographical distribution and tendency for relapse makes it difficult to eliminate.2 Currently, primaquine is the only licensed drug that is capable of eliminating hypnozoites, the dormant liver stage parasites responsible for relapsing infections. Nevertheless, the drug is neither widely nor appropriately used in many regions with P. vivax malaria. There are a number of drawbacks to primaquine, in particular the risk of acute hemolysis in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency. In addition, certain genotypes of the cytochrome P450 2D6 alleles (CYP2D6) fail to sufficiently metabolize primaquine to its active form, and failure of treatment has been reported.3,4 The lengthy duration of treatment (14 days) has also led to adherence issues, and directly observed therapy (DOT) has resulted in far better rates of efficacy in preventing recurrent infections.5
Primaquine has been used for over 60 years, yet the evidence is unclear as to whether resistance has developed (at the liver stage).6–9 This is generally attributed to the confounding factors present in most studies: for example, nonadherence or inadequate dosing of primaquine, low metabolizers (which has only recently been identified, and hence has not been a major focus of early studies), chloroquine resistance, and, in studies conducted in endemic regions, the risk of new infections (reviewed in Ref. 6). This makes it difficult to eliminate other possibilities of posttreatment recurrences.
To investigate the efficacy of primaquine treatment in the Tak Province, western Thailand, we enrolled 57 symptomatic P. vivax patients (G6PD normal) from the Tha Song Yang malaria clinic or hospital on an ongoing basis, with all patients giving informed consent or assent. The study was approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Thailand (MUTM 2014-025-01 and 02), and was conducted from April 2014 to September 2015. The Tak Province has low endemic malaria transmission, with a community survey in 2012 identifying a parasite prevalence of 11.4%.1 Enrolled volunteers were treated with chloroquine (25 mg base/kg body weight, administered over 3 days) and primaquine (15 mg daily, for 14 days), according to standard Thai treatment. Primaquine was administered under DOT. Finger-prick blood samples were collected from volunteers before treatment and at 1 and 2 weeks after enrollment, then every 2 weeks for 6 months, and every 4 weeks until 9 months. Malaria parasites were detected by expert microscopy, genus-specific quantitative polymerase chain reaction (PCR) (qMAL),10 nested single-species PCR and single-species qPCR,10,11 as previously described. The limit of detection of the molecular methods was 1–3 copy numbers/μL.10 The nested PCRs were modified as detailed in the Supplemental Materials (SMs). Samples were defined as positive once the species was confirmed by two independent molecular methods (see Supplemental Figure 1).
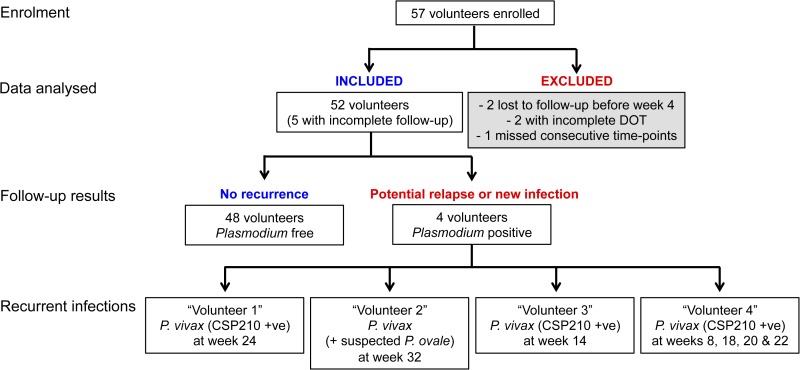
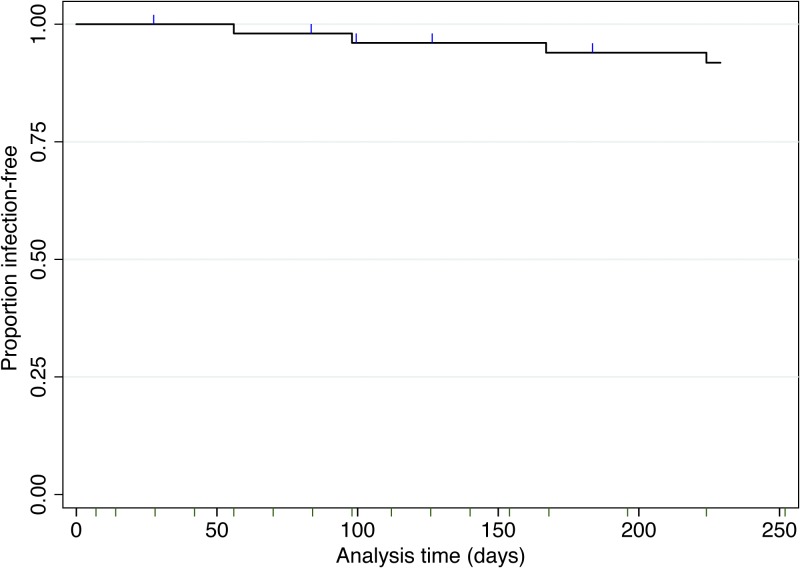
Data from 52 volunteers were analyzed for the presence of recurrent infections (five excluded as detailed in Figure 1 ). These volunteers ranged in age from 7 to 71 years (median 22), 63% were male, 58% had a self-reported history of past malarial infection(s), and all were of Thai or Karen ethnicity. Over 9 months of follow-up, only four volunteers had recurrent P. vivax infections, all of which occurred at least 8 weeks and up to 32 weeks after the initial infection (Table 1). Tropical strain P. vivax parasites, as found in Thailand, are expected to relapse within 3–4 weeks of primary infection.13 All recurrent infections were of lower parasite density than the initial infection at enrollment. In three of the four volunteers, the recurrent infections were short lasting (only detected at one visit), asymptomatic, and in one volunteer, only detected by PCR. The fourth volunteer had multiple recurrences. The first was a symptomatic P. vivax infection at week 8, which was treated, followed by an asymptomatic infection lasting from weeks 18 to 22 (only detectable by microscopy at weeks 20 and 22). In addition, one of the four volunteers was identified as coinfected with Plasmodium ovale at week 32 (Figure 1). Overall, 92.3% of volunteers remained Plasmodium infection free (Figure 2 ), demonstrating that primaquine remains highly efficacious in this region. As individual weight was not recorded in this study, we cannot determine whether the recurrent infections were related to a lower total primaquine dose.
Figure 1.
Recurrence of Plasmodium vivax infections during follow-up over 9 months. Of the 52 volunteers, five were lost to follow-up, in the survival analysis these volunteers were censored at their last visit.
Table 1.
Detailed information on recurrent infections
| Volunteer | Age (year) | Sex | Occupation | CYP2D6 | Time point | qMAL‡ | Microscopy§ | Disease¶ | MS2 | Msp1F3 | Classification** |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 28 | F | Agriculture | EM | Enrollment | 2.6 × 106 | Yes | Symptomatic | 196.77 | 312.45 | |
| Week 24 | 2.3 × 105 | Yes | Asymptomatic | 196.69 | 312.63 | Identical | |||||
| 2 | 62 | M | Agriculture | EM | Enrollment | 5 × 105 | Yes | Symptomatic | n/a∥ | n/a∥ | |
| Week 32 | 1.9 × 101 | No | Asymptomatic | n/a∥ | n/a∥ | Undetermined | |||||
| 3* | 14 | M | Student | EM | Enrollment | 5.1 × 106 | Yes | Symptomatic | 216.19 | 256.45 | |
| Week 14 | 9.8 × 105 | Yes | Asymptomatic | 216.19 | 256.45 | Identical | |||||
| 4* | 38 | M | Employee | IM | Enrollment | 1.4 × 106 | Yes | Symptomatic | 200.8 | 256.45 | |
| Week 8 | 3.6 × 105 | Yes | Symptomatic | 200.8 | 256.45 and 262.71 | Related | |||||
| Week 18† | 8.6 × 102 | No† | Asymptomatic | 255.19 | 262.71 | Different |
EM = extensive metabolizer; IM = intermediate metabolizer.
Volunteers three and four were only enrolled until 6 months.
Volunteer was Plasmodium vivax positive at weeks 18, 20, and 22, but genotyping was only performed at week 18. Parasites were identified by microscopy on weeks 20 and 22 only.
qMAL copy numbers shown. Symptomatic malaria generally requires > 104 (Nguitragool, unpublished data).
Parasites detectable by expert microscopy, yes or no.
All volunteers were symptomatic on enrollment. At the recurrent infections, temperature, and self-reported illness were recorded.
Genotyping was not performed due to low quantitative polymerase chain reaction values and failure of circumsporozoite protein (CSP) amplification at the recurrent infection.
Paired P. vivax samples (from enrollment and recurrence) were classified using the following criteria when genotyped with msp1F3 and MS2: (1) different, none of the alleles were the same, (2) identical, the same genotype was detected, and (3) related, at least one of the same alleles was detected.12
Figure 2.
Survival analysis after 14 days of directly observed therapy primaquine treatment, N = 52. Volunteers were censored at the time they were lost to follow-up (N = 5, shown as blue ticks). Follow-up schedule is shown by minor ticks (dark green) on the x axis.
To determine whether recurrent infections were likely due to relapse or new infections, genotyping was performed using the polymorphic merozoite surface protein 1 F3 fragment (msp1F3)14 and the microsatellite MS215 in samples taken from three of the four volunteers. Genetically identical or highly related parasites would be indicative of relapsing infections,16 as long as reinfections are genetically distinct from the initial infections detected at presentation. Genotyping was not attempted in volunteer 2 due to the low parasite density (Table 1). PCRs were performed as previously described,14 with slight modifications as detailed in the SM. We found that two recurrent infections were identical and one related to the parasites detected at enrollment (Table 1). We have recently genotyped over 400 P. vivax samples using these two markers in the neighboring Kanchanaburi Province; 42 alleles were identified for MS2, and 16 for msp1F3 (Nguitragool, Karl, Mueller and Sattabongkot, unpublished data). The MS2 alleles identified in our current study were found in < 6% of samples in Kanchanaburi, while the msp1F3 alleles were found in approximately 50% of samples (with the exception of the 312 fragment, which was not identified in Kanchanaburi). Assuming comparable allele frequencies, the probability of identifying identical genotypes at enrollment and recurrence was < 3%. We have also genotyped a smaller number of samples in the Tak Province for MS2 only (N = 42) (Kittichai, Nguitragool, Cui and Sattabongkot, unpublished data). Seventeen different alleles were identified, and the MS2 alleles seen in our current study were again not prevalent (found in < 7% samples), suggesting that the probability that these three volunteers experienced relapses was high. However, it is important to note that genotyping cannot currently distinguish a relapse from a reinfection without uncertainty.
In addition, we genotyped 51/52 volunteers for CYP2D6 polymorphisms using the Luminex xTAG CYP2D6 Kit v3 (Austin, TX) as previously described,17 with further details in the SM. The majority of volunteers were predicted to be of the extensive metabolizer phenotype (33/51, 65%). There was only one poor metabolizer (*5/*5, who remained infection free), and 12 intermediate metabolizers. Of the four volunteers with recurrent infections, three were extensive metabolizers while the remaining was an intermediate metabolizer (Table 1).
Our results indicate that primaquine has maintained high levels of efficacy in this endemic region of western Thailand, at least when administered under DOT. We used a total dose of 210 mg over 14 days (equating to approximately 3.50–5.25 mg/kg based on assumed weight between 40 and 60 kg), following the standard Thai regimen. This is the World Health Organization's recommendation for all transmission settings, and is presumed to be effective in Thailand where previous research has indicated that less than 2.75 mg/kg is associated with increased risk of recurrent P. vivax infections.5 Our current findings support current Thai treatment guidelines. However, DOT cannot be routinely administered to all patients, and hence adherence is likely the major factor contributing to the relapsing infections following primaquine treatment in this region. We also observed no detectable recrudescence of parasites within 28 days of treatment, indicating that chloroquine is still effective in this population, despite observations of resistance in another region on the Thai–Myanmar border.18
Predicted poor metabolizers of primaquine based on CYP2D6 polymorphisms are rare in this mixed Thai–Karen population. Predicted intermediate metabolizers are however relatively frequent; administration of primaquine under DOT appears to limit the risk of recurrent infections in these volunteers. However, one of the intermediate metabolizers did have recurrent infections and hence we cannot rule out the contribution of this phenotype.
Although the risk of new infections could not be excluded in our study, genotyping of two polymorphic markers indicated a high probability that in at least three of the volunteers the recurrent infections were most likely due to relapse. These relapses were asymptomatic and occurred relatively late compared with the common tropical phenotype of 3–4 weeks.13 However, treatment with primaquine in these volunteers may have led to a small number of hypnozoites remaining in the liver (either due to partial efficacy or a high initial hypnozoite burden); small numbers of parasites have been associated with late relapse periodicity.19 In addition, our data also indicate that untreated, asymptomatic P. vivax infections can be of very short duration (2–4 weeks). Together these observations highlight that anti-hypnozoite treatment efficacy studies need long-term follow-up with intensive and active monitoring for asymptomatic infections (ideally by PCR given the low densities)20; otherwise such studies may overestimate the efficacy of primaquine and novel anti-liver-stage drugs such as tafenoquine.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the volunteers enrolled in the study, in addition to Kaitisak Kanthawang, Janjira Jaihuek, and Ekkawat Konjunta from the Tha Song Yang Hospital; Kirakorn Kiattibutr, Nattawan Rachapaew, Nutcha Theerawarodom, and Rachaneeporn Jenwithisuk from MVRU; Connie Li Wai Suen for statistical support; Veerayuth Kittichai for providing genotyping data of MS2 from the Tha Song Yang region for comparison; and the National Research Council of Thailand for their support. We also thank all the members of the Mueller lab group for thoughtful feedback.
Footnotes
Financial support: This study was funded by the National Institute of Allergy and Infectious Diseases (NIH grant number 5R01 AI 104822) and the Foundation for Innovative New Diagnostics. Ivo Mueller is supported by an NHMRC Senior Research Fellowship (no. 1043345).
Authors' addresses: Rhea J. Longley and Ivo Mueller, Walter and Eliza Hall Institute of Medical Research, Victoria, Australia, E-mails: longley.r@wehi.edu.au and mueller@wehi.edu.au. Piyarat Sripoorote, Pornpimol Chobson, Teerawat Saeseu, Suparat Phuanukoonnon, and Jetsumon Sattabongkot, Mahidol Vivax Research Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, E-mails: pnt_94@hotmail.com, ppchobson@gmail.com, teerawatsaeseu@gmail.com, suparatp@hotmail.com, and jetsumon.pra@mahidol.ac.th. Chonlaphat Sukasem, Division of Pharmacogenomics and Personalized Medicine, Department of Pathology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand, E-mail: chonlaphat.suk@mahidol.ac.th. Wang Nguitragool, Department of Molecular Tropical Medicine and Genetics, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, E-mail: wang.nguitragool@gmail.com.
References
- 1.Baum E, Sattabongkot J, Sirichaisinthop J, Kiattibutr K, Davies DH, Jain A, Lo E, Lee MC, Randall AZ, Molina DM, Liang X, Cui L, Felgner PL, Yan G. Submicroscopic and asymptomatic Plasmodium falciparum and Plasmodium vivax infections are common in western Thailand—molecular and serological evidence. Malar J. 2015;14:95. doi: 10.1186/s12936-015-0611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cotter C, Sturrock HJ, Hsiang MS, Liu J, Phillips AA, Hwang J, Gueye CS, Fullman N, Gosling RD, Feachem RG. The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet. 2013;382:900–911. doi: 10.1016/S0140-6736(13)60310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingram RJ, Crenna-Darusallam C, Soebianto S, Noviyanti R, Baird JK. The clinical and public health problem of relapse despite primaquine therapy: case review of repeated relapses of Plasmodium vivax acquired in Papua New Guinea. Malar J. 2014;13:488. doi: 10.1186/1475-2875-13-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett JW, Pybus BS, Yadava A, Tosh D, Sousa JC, McCarthy WF, Deye G, Melendez V, Ockenhouse CF. Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. N Engl J Med. 2013;369:1381–1382. doi: 10.1056/NEJMc1301936. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi R, Lawpoolsri S, Imwong M, Kobayashi J, Kaewkungwal J, Pukrittayakamee S, Puangsa-art S, Thanyavanich N, Maneeboonyang W, Day NP, Singhasivanon P. Directly-observed therapy (DOT) for the radical 14-day primaquine treatment of Plasmodium vivax malaria on the Thai-Myanmar border. Malar J. 2010;9:308. doi: 10.1186/1475-2875-9-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39:1336–1345. doi: 10.1086/424663. [DOI] [PubMed] [Google Scholar]
- 7.Reddy P, Flaherty JP. Plasmodium vivax malaria relapses after primaquine prophylaxis. Emerg Infect Dis. 2006;12:1795–1796. doi: 10.3201/eid1211.060613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orjuela-Sanchez P, da Silva NS, da Silva-Nunes M, Ferreira MU. Recurrent parasitemias and population dynamics of Plasmodium vivax polymorphisms in rural Amazonia. Am J Trop Med Hyg. 2009;81:961–968. doi: 10.4269/ajtmh.2009.09-0337. [DOI] [PubMed] [Google Scholar]
- 9.Bright AT, Alenazi T, Shokoples S, Tarning J, Paganotti GM, White NJ, Houston S, Winzeler EA, Yanow SK. Genetic analysis of primaquine tolerance in a patient with relapsing vivax malaria. Emerg Infect Dis. 2013;19:802–805. doi: 10.3201/eid1905.121852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wampfler R, Mwingira F, Javati S, Robinson L, Betuela I, Siba P, Beck HP, Mueller I, Felger I. Strategies for detection of Plasmodium species gametocytes. PLoS One. 2013;8:e76316. doi: 10.1371/journal.pone.0076316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosanas-Urgell A, Mueller D, Betuela I, Barnadas C, Iga J, Zimmerman PA, del Portillo HA, Siba P, Mueller I, Felger I. Comparison of diagnostic methods for the detection and quantification of the four sympatric Plasmodium species in field samples from Papua New Guinea. Malar J. 2010;9:361. doi: 10.1186/1475-2875-9-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Restrepo E, Imwong M, Rojas W, Carmona-Fonseca J, Maestre A. High genetic polymorphism of relapsing P. vivax isolates in northwest Colombia. Acta Trop. 2011;119:23–29. doi: 10.1016/j.actatropica.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White NJ, Imwong M. Relapse. Adv Parasitol. 2012;80:113–150. doi: 10.1016/B978-0-12-397900-1.00002-5. [DOI] [PubMed] [Google Scholar]
- 14.Koepfli C, Ross A, Kiniboro B, Smith TA, Zimmerman PA, Siba P, Mueller I, Felger I. Multiplicity and diversity of Plasmodium vivax infections in a highly endemic region in Papua New Guinea. PLoS Negl Trop Dis. 2011;5:e1424. doi: 10.1371/journal.pntd.0001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koepfli C, Rodrigues PT, Antao T, Orjuela-Sanchez P, Van den Eede P, Gamboa D, van Hong N, Bendezu J, Erhart A, Barnadas C, Ratsimbasoa A, Menard D, Severini C, Menegon M, Nour BY, Karunaweera N, Mueller I, Ferreira MU, Felger I. Plasmodium vivax diversity and population structure across four continents. PLoS Negl Trop Dis. 2015;9:e0003872. doi: 10.1371/journal.pntd.0003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bright AT, Manary MJ, Tewhey R, Arango EM, Wang T, Schork NJ, Yanow SK, Winzeler EA. A high resolution case study of a patient with recurrent Plasmodium vivax infections shows that relapses were caused by meiotic siblings. PLoS Negl Trop Dis. 2014;8:e2882. doi: 10.1371/journal.pntd.0002882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanwong N, Ngamsamut N, Hongkaew Y, Nuntamool N, Puangpetch A, Chamnanphon M, Sinrachatanant A, Limsila P, Sukasem C. Detection of CYP2D6 polymorphism using Luminex xTAG technology in autism spectrum disorder: CYP2D6 activity score and its association with risperidone levels. Drug Metab Pharmacokinet. 2016;31:156–162. doi: 10.1016/j.dmpk.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Rijken MJ, Boel ME, Russell B, Imwong M, Leimanis ML, Phyo AP, Muehlenbachs A, Lindegardh N, McGready R, Renia L, Snounou G, Singhasivanon P, Nosten F. Chloroquine resistant vivax malaria in a pregnant woman on the western border of Thailand. Malar J. 2011;10:113. doi: 10.1186/1475-2875-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White NJ. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J. 2011;10:297. doi: 10.1186/1475-2875-10-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.John GK, Douglas NM, von Seidlein L, Nosten F, Baird JK, White NJ, Price RN. Primaquine radical cure of Plasmodium vivax: a critical review of the literature. Malar J. 2012;11:280. doi: 10.1186/1475-2875-11-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.