Abstract
Despite the potential benefit of primaquine in reducing Plasmodium falciparum transmission and radical cure of Plasmodium vivax and Plasmodium ovale infections, concerns over risk of hemolytic toxicity in individuals with glucose-6-phosphate dehydrogenase deficiency (G6PDd) have hampered its deployment. A cross-sectional survey was conducted in 2014 to assess the G6PDd prevalence among 631 children between 6 and 59 months of age in southwestern Uganda, an area where primaquine may be a promising control measure. G6PDd prevalence was determined using three detection methods: a quantitative G6PD enzyme activity assay (Trinity Biotech® G-6-PDH kit), a qualitative point-of-care test (CareStart™ G6PD rapid diagnostic test [RDT]), and molecular detection of the G6PD A− G202A allele. Qualitative tests were compared with the gold standard quantitative assay. G6PDd prevalence was higher by RDT (8.6%) than by quantitative assay (6.8%), using a < 60% activity threshold. The RDT performed optimally at a < 60% threshold and demonstrated high sensitivity (≥ 90%) and negative predictive values (100%) across three activity thresholds (below 60%, 30%, and 40%). G202A allele frequency was 6.4%, 7.9%, and 6.8% among females, males, and overall, respectively. Notably, over half of the G202A homo-/hemizygous children expressed ≥ 60% enzyme activity. Overall, the CareStart™ G6PD RDT appears to be a viable screening test to accurately identify individuals with enzyme activities below 60%. The low prevalence of G6PDd across all three diagnostic modalities and absence of severe deficiency in our study suggests that there is little barrier to the use of single-dose primaquine in this region.
Introduction
As malaria transmission continues to decline in southwestern Uganda, aggressive strategies toward further reducing its burden must be considered. Because of its unique transmission-blocking potential and efficacy against hypnozoites, the scale-up of primaquine as a treatment strategy has been on the agenda of many malaria-endemic countries.1 Currently, the World Health Organization (WHO) recommends the use of primaquine in combination with artemisinin combination therapies (ACTs), as a transmission-blocking agent for patients with uncomplicated Plasmodium falciparum malaria living in low-transmission settings.1 Primaquine is also the only commercially available drug to prevent relapses of Plasmodium ovale and Plasmodium vivax infections by killing dormant hypnozoites in the liver.1,2
Uptake of primaquine in sub-Saharan Africa has been slow primarily due to safety concerns, namely the potential for severe hemolysis in those with glucose-6-phosphate dehydrogenase (G6PD) deficiency. G6PD deficiency is an X-linked trait and the most common enzymopathy in the world, with over 180 known alleles associated with varying phenotypic severity.3–5 The complexity of this disorder presents a diagnostic challenge, making it difficult to define the degree of deficiency in an individual, particularly in heterozygous females who exhibit variable X-chromosome inactivation. In addition, the correlation between the degree of deficiency and one's risk of clinically significant hemolysis remains unclear.6 Based on available data, the current recommended dose for preventing P. vivax and P. ovale clinical relapses is 0.25–0.5 mg/kg for 14 days for G6PD non-deficient individuals and 0.25 mg/kg as a single low dose in conjunction with an ACT for transmission-blocking activity without the need for G6PD testing.7
The G6PD A− variant, characterized by the presence of the G202A mutation, is the most prevalent variant of G6PD deficiency in sub-Saharan Africa.5 While it is generally associated with a milder degree of deficiency (residual red blood cell G6PD activity ∼5–13%8) as compared with the G6PD Mediterranean variant, and thus a lower risk of hemolysis, severe hemolytic events have been known to occur if the oxidative challenge is strong enough.4,9 The prevalence of this variant in southwestern Uganda, an area where all four major malaria species circulate, has not been established.10 More importantly, the comparative performance of varied G6PD deficiency diagnostic modalities in field-based settings requires further investigation to determine the most viable method to exclude at-risk G6PD-deficient persons before a 14-day course of primaquine.
Materials and Methods
Study population and design.
A cross-sectional survey was conducted between August and October 2014 in Mbarara, Bushenyi, and Isingiro districts, situated in southwestern Uganda. A stratified, two-stage cluster sampling design was used to select a total of 631 children between 6 and 59 months of age as previously described.10 Briefly, 60 villages were randomly selected (20 per district) using probability proportionate to population size sampling and stratified by their urban and rural status. The number of households sampled per village was determined by the total number of participants required from each district, which was weighted by population size. Households were then randomly selected from each village using the WHO's Expanded Program on Immunization (EPI) methodology.11 Only one child was randomly selected per household. Children were included in the survey if they were between 6 and 59 months of age (inclusive) and the parent or guardian provided informed consent.
A previous study reported G6PD deficiency in eastern Uganda to be ∼10% by quantitative assay.12 A sample size of 637 children was sufficient to detect a difference in G6PD deficiency from 10% with 80% power and ±4% precision. The sample size was increased to account for a design effect of 1.5 and a 10% nonresponse rate to generate the minimum sample size of 637 children.
Field procedures and sample collection.
Demographic information was collected from a parent/guardian before sample collection. Approximately 250 μL of blood was collected by finger prick into an ethylenediaminetetraacetic acid–coated microtainer (BD Diagnostics, Franklin Lakes, NJ). Qualitative G6PD deficiency testing was assessed using the CareStart™G6PD rapid diagnostic test (RDT) (cat no.: RGP-M05082; Access Bio Inc., Somerset, NJ) and performed according to manufacturers' instructions. In brief, the CareStart™ G6PD RDT is a qualitative colorimetric assay based on the reduction of colorless nitro blue tetrazolium dye to dark-colored formazan.13 Hemoglobin levels (HemoCue® Hb 301; HemoCue®, Brea, CA) and thick and thin smears for malaria microscopy were also performed in the field at the time of sample collection by trained laboratory technicians. The remaining volume of blood was stored at 4°C and transported to Médecins Sans Frontières Epicentre Mbarara Research Base within 12 hours of sample collection. Samples were stored at 4°C until the G6PD quantitative assay was performed.
Laboratory procedures.
Quantitative spectrophotometric G6PD assay (cat no.: 345-B, Trinity Biotech® G-6-PDH Kit; Trinity Biotech, County Wicklow, Ireland) was performed according to manufacturer's instructions within 24 hours of sample collection. Runs using a commercial set of known normal and null G6PD activity controls (cat nos.: G6888 and G5888; Trinity Biotech®) were performed before running samples on that day. Sample runs were considered valid if control values fell within the given range of the manufacturer.
Percent G6PD activity was calculated based on their relative proportion to the adjusted median value of G6PD enzyme activity among the male population, upon exclusion of severely G6PD-deficient males (i.e., less than 10% G6PD enzyme activity of the median value), as recommended by an expert panel.6 Prevalence of G6PD deficiency was defined according to the WHO classification of G6PD deficiency (i.e., < 60% of G6PD enzyme activity).14
The remaining blood sample (∼150 μL) was collected onto filter paper (Whatman® 903 Protein Saver Card; Sigma-Aldrich, St. Louis, MO) and stored at −80°C. Dried blood spots were shipped dry at room temperature to the Yale School of Public Health, New Haven, CT, for the molecular detection of Plasmodium species and the G6PD A− G202A mutation. DNA was extracted using QIAamp DNA Mini Kit (Qiagen, Valencia, CA). DNA was eluted in 100 μL from each dried blood spot. For microscopic detection of malaria, thin and thick smears were stained with 10% Giesma (pH 7.2) for 15 minutes. Readings were independently made by two trained microscopists and discordant results were resolved by a third reader.15
Detection of the G6PD A− G202A mutation.
Restriction fragment length polymorphism of polymerase chain reaction (PCR)–amplified DNA products were used to evaluate the presence of the G6PD A− G202A allele.12 PCR amplification was performed using 5X GoTaq Flexi Buffer (Promega, Madison, WI), 1.5 mM MgCl2, 5% dimethyl sulfoxide, 0.2 mM of each deoxynucleotide triphosphate, 0.5 μM forward primer (5′-CCACCACTGCCCCTGTGACCT-3′), 0.5 μM reverse primer (5′-GGCCCTGACACCACCCACCTT-3′), 1 U GoTaq® (Promega) polymerase, and 10 ng of genomic DNA. Thermocycling conditions were as follows: initial denaturation at 95°C for 2 minutes, followed by 35 cycles of 95°C for 30 seconds, 67°C for 1 minute, 72°C for 30 seconds, and a final extension at 72°C for 5 minutes. Four microliters of PCR product was incubated with 1 U of NlaIII (New England BioLabs, Ipswich, MA) at 37°C for 3 hours. Genotypes were identified using 2.0% agarose gel electrophoresis. The overall call rate was 100%. For quality control, nine samples were sequenced and concordance rate was 100%.
Statistical analysis.
Statistical analysis was performed using the open software R (version 3.2.2; R Project for Statistical Computing; http://www.r-project.org/) and STATA, version 14.0 (StataCorp, College Station, TX).16 All statistical tests accounted for the clustered sampling design using svy commands in STATA to account for clustering at the village level and stratification of urban and rural villages. Categorical variables were compared using the χ2 test or Fischer's exact test, when appropriate. t tests were performed when comparing continuous variables, if the outcome was normally distributed. Mann–Whitney's U test was used when the variables were nonparametric. Linear regression was used to evaluate the relationship between covariates and continuous variables. Prevalence ratio estimates were calculated using a Poisson regression model with robust variance. P values less than 0.05 were considered statistically significant.
CareStart™ G6PD RDT was compared with the gold standard quantitative assay, Trinity Biotech® G-6-PDH kit, to assess its diagnostic performance. Sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) were calculated to assess RDT performance. Three clinically meaningful thresholds, < 60%, < 40%, and < 30% enzyme activity, were used to define deficiency. According to the WHO, less than 60% enzymatic activity denotes severe to moderate G6PD deficiency.14 Thirty and forty percent were chosen as cut-offs to demonstrate RDT performance at a threshold equivalent to the level of detection of G6PD deficiency of the fluorescent spot test and an acceptable level above which would be safe for primaquine use.6,8,17 An individual was considered a true positive case if they were classified as deficient by RDT and expressed a % G6PD activity below the threshold being tested. True negatives were defined as individuals who expressed % G6PD activity at or above the cut-off and tested G6PD normal by RDT. Individuals were considered false negative if they were considered G6PD normal by RDT and their expression of % G6PD activity was below the tested threshold. Conversely, false positives were defined as cases that were G6PD deficient by RDT, but their % G6PD activity was above the evaluated threshold.
Ethics statement.
Written informed consent was obtained by the participant's parent or guardian before questionnaires and sample collection. Children who had a positive malaria diagnosis were referred to the nearest health facility for treatment and G6PD-deficient children were notified of their status. Study approval was granted by the institutional review boards of Mbarara University of Science and Technology and Yale University and the Uganda National Council of Science and Technology.
Results
Demographic characteristics of study population.
A total of 631 children were included in the analysis (Table 1). Fifty-one percent of children were male and the mean age ± standard deviation (SD) of participants was 2.4 ± 1.3 years. The majority of children in our study population were of the Banyankole ethnic group (84.2%). Mean hemoglobin count ± SD was 11.9 ± 1.3 g/dL, and no child in our study was experiencing severe anemia (< 5 g/dL) at the time of the survey. Malaria prevalence by microscopy was 3.5% (95% confidence interval [CI]: 1.9 to 5.1) across all three districts.10
Table 1.
Demographic characteristics of children surveyed*
| Characteristics* | Male (N = 322) | Female (N = 309) | Total (N = 631) |
|---|---|---|---|
| Age (years), mean ± SD | 2.4 ± 1.2 | 2.4 ± 1.3 | 2.4 ± 1.3 |
| Hb count (g/dL) | 11.9 ± 1.3 | 12.0 ± 1.2 | 11.9 ± 1.3 |
| Ethnicity | |||
| Banyankole | 275 (85.4) | 256 (82.9) | 531 (84.2) |
| Bakiga | 29 (9.1) | 34 (11.0) | 63 (10.0) |
| Baganda | 11 (3.4) | 11 (3.6) | 22 (3.5) |
| Other | 7 (2.2) | 8 (2.6) | 15 (2.4) |
| Microscopy-confirmed malaria infection | 10 (3.1) | 12 (3.9) | 22 (3.5) |
| G6PD deficient by CareStart™ G6PD RDT† | 35 (10.9) | 19 (6.2) | 54 (8.6) |
| WHO classification of deficiency | |||
| Moderate (10% to < 30%) | 5 (1.6) | 5 (1.6) | 10 (1.6) |
| Mild (30% to < 60%) | 20 (6.2) | 13 (4.2) | 33 (5.2) |
| Normal (≥ 60%) | 297 (92.2) | 291 (94.2) | 588 (93.2) |
| G6PD 202A genotype (N = 625)‡ | |||
| Wild-type female | – | 272 (88.6) | 272 (43.5) |
| Heterozygous female | – | 31 (10.1) | 31 (5.0) |
| Homozygous mutant female | – | 4 (1.3) | 4 (0.6) |
| Wild-type male | 293 (92.1) | – | 293 (46.9) |
| Hemizygous male | 25 (7.9) | – | 25 (4.0) |
G6PD = glucose-6-phosphate dehydrogenase; Hb = hemoglobin; SD = standard deviation; WHO = World Health Organization.
Values are mean ± SD for continuous variables and n (column %) for categorical variables.
Deficiency defined as no background color in result window.
Dried blood spots were available for only 625 participants.
Prevalence of G6PD deficiency by quantitative assay.
G6PD enzyme activity values ranged from 1.2 to 12.2 U/g hemoglobin (Hb). Percent G6PD activity was determined from the adjusted male median observed at 7.9 U/g Hb. Prevalence of G6PD deficiency is presented in Table 1. No participant in our study exhibited severe G6PD deficiency (< 10% enzyme activity or < 0.8 U/g Hb), 1.6% (95% CI: 0.6 to 2.6; N = 10 children) exhibited G6PD activity < 30% (< 2.4 U/g Hb), and 6.8% (95% CI: 4.8 to 8.9) of children exhibited mild-to-moderate G6PD deficiency (10% to < 60% enzyme activity or 0.8 to < 4.7 U/g Hb). Deficiency, defined as < 60% G6PD activity, did not significantly differ by gender (7.8% males versus 5.8% females; P = 0.39) or by ethnic group (P = 0.28). However, mean G6PD activity was higher in Bagandan children compared with Banyankoles (8.6 versus 7.7 U/g Hb, respectively; P = 0.01). By district, Bushenyi had a significantly higher prevalence of G6PD-deficient children compared with Mbarara District (10.8% versus 3.7%) (unadjusted prevalence ratio = 2.9; 95% CI: 1.3 to 6.4; P = 0.01). Prevalence of G6PD-deficiency was higher in Isingiro (7.3%) compared with Mbarara, but this finding did not reach statistical significance (unadjusted prevalence ratio = 1.9; 95% CI: 0.9 to 4.3; P = 0.09). Malaria infection and hemoglobin count were not statistically associated with G6PD enzyme activity (P > 0.31).
Prevalence of G6PD A− G202A allele.
Dried blood spots were available for 625 participants in the study. Upon genotyping for the G202A allele, we observed 31 (5%) heterozygous females, four (0.6%) homozygous females, and 25 (4%) hemizygous males. Allele frequency of the G202A mutation was 6.4% among females, 7.9% among males, and 6.8% overall. Prevalence of the G202A mutation was statistically different by ethnic groups (P = 0.04), but not across districts (P = 0.68). Allele frequency among Banyankole, Bakiga, and Bagandan children ranged from 5.2% to 6.7%, though was much higher (21.7%) among children not identified as belonging to these three ethnic groups.
Of the 31 heterozygous females, 84.9% (N = 26) exhibited normal G6PD levels at the < 60% threshold and 93.6% (N = 29) at the < 40% threshold. Among the G202A wild-type children, only 4.4%, 1.4%, and 0.9% exhibited less than 60%, 40%, and 30% G6PD enzyme activity, respectively. Interestingly, among the 29 hemi- and homozygous children, 55.2%, 69.0% and 89.7% of children exhibited more than 60%, 40%, and 30% G6PD enzyme activity, respectively. However, mean G6PD enzyme activity among G202A hemi- and homozygotes was approximately 25% lower compared with wild-type genotypes (β = −2.0 U/g Hb; 95% CI: −3.1 to −0.9; P = 0.001). Heterozygous females were predicted to have 0.8 U/g Hb lower (i.e., 9% decrease) mean G6PD activity than their wild-type counterparts, though this finding did not reach statistical significance (95% CI: −1.5 to −0.3; P = 0.06).
Prevalence of G6PD deficiency by RDT.
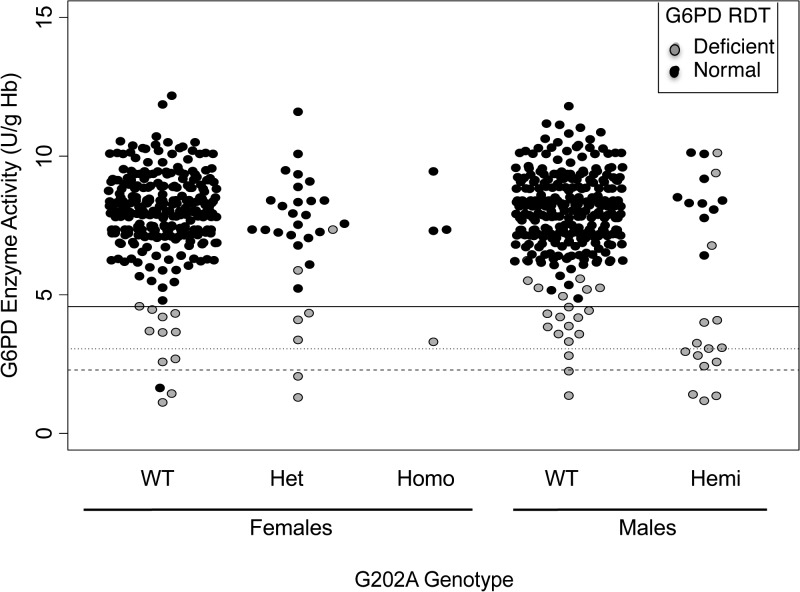
The prevalence of G6PD deficiency was 1.3-fold higher by CareStart™ G6PD RDT (8.6%; 95% CI: 6.6 to 10.0) than by quantitative assay (6.8%), using a threshold of < 60% activity. The distribution of G6PD enzyme activity by CareStart™ G6PD RDT is shown in Figure 1 . Median enzyme activity was significantly different between children identified as G6PD deficient by RDT compared with participants who tested normal (3.8 versus 8.1 U/g Hb, respectively; P < 0.001).
Figure 1.
Distribution of enzymatic activity by G202A genotype and CareStart™ G6PD rapid diagnostic test (RDT). Gray points represent individuals who tested deficient by CareStart™ G6PD RDT. Dotted line at 60% enzymatic activity represents the cut-off point between normal and mild G6PD deficiency as defined by the World Health Organization. Dotted line at 30% shows recent proposed cut-off to denote “deficient” males and females.8 Hemi = hemizygous; Het = heterozygous; Homo = homozygous; WT = wild-type.
Performance of the CareStart™ G6PD RDT.
At the < 60% G6PD activity cut-off value, the sensitivity and specificity of the CareStart™ G6PD RDT were 98% (Table 2). PPVs and NPVs were 78% and 100%, respectively. Among the 12 false-positive children (i.e., participants who were classified as deficient by RDT, but expressed greater than 60% G6PD activity), G6PD activity ranged from 4.9 to 10.1 U/g Hb (i.e., 62–128% G6PD activity) (Figure 1). The range of enzyme activity of true positives (i.e., participants that were deficient by both CareStart™ G6PD RDT and by gold standard quantitative analysis) was 1.2–4.6 U/g Hb (i.e., 15–58% G6PD activity) (Figure 1). Sensitivity and specificity marginally decreased, and PPV values dramatically decreased at lower thresholds of < 30% and < 40%, as compared with values at a < 60% threshold. At all thresholds, NPV closely approximated 100%, with only one child with a G6PD enzyme activity of 21% (G6PD enzyme activity = 1.6 U/g Hb) falsely testing G6PD normal by CareStart™ G6PD RDT.
Table 2.
Diagnostic performance of CareStart™ G6PD RDT at different cut-off points of enzymatic activity
| Cut-off values of % G6PD activity |
CareStart™ G6PD RDT Result |
Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Deficient* | Normal | ||||||
| < 60%† | Deficient | 42 | 1 | 98 (88–100) | 98 (97–99) | 78 (64–88) | 100 (99–100) |
| Normal | 12 | 576 | |||||
| < 40%‡ | Deficient | 18 | 1 | 95 (74–100) | 94 (92–96) | 33 (21–48) | 100 (99–100) |
| Normal | 36 | 576 | |||||
| < 30%‡ | Deficient | 9 | 1 | 90 (56–100) | 93 (90–95) | 17 (8–29) | 100 (99–100) |
| Normal | 45 | 576 | |||||
CI = confidence interval; G6PD = glucose-6-phosphate dehydrogenase; NPV = negative predictive value; PPV = positive predictive value; RDT = rapid diagnostic test.
Deficiency defined as no background color in result window.
Defined by the World Health Organization classification of severe to mild deficiency (Class I–III).
Threshold detection level of semiquantitative Beutler fluorescence spot test (< 30–40% enzyme activity).
The CareStart™ G6PD RDT was also able to discriminate well across G202A genotypes, that is, G6PD deficiency by RDT was significantly different across G202A genotypes (P < 0.001). The prevalence ratio of RDT-deficient children among heterozygotes was 4.1 (95% CI: 1.9 to 8.8; P < 0.001) and 10.1 among hemi- and homozygotes (95% CI: 6.2 to 16.2; P < 0.001).
G6PD deficiency and malaria prevalence.
Asymptomatic parasitemia was detected in 3.5% (N = 22) children by microscopy (Table 1). No significant differences were observed between G6PD enzyme activity defined across all significant thresholds (i.e., < 60%, 40%, and 30%) and malaria microscopy-positive status (P > 0.26). Similar findings were seen upon the exclusion of females (P > 0.41). Only one child, a heterozygous female with an enzyme activity of 7.9 U/g Hb, was found to be asymptomatically infected by P. falciparum. Of note, among the 22 microscopy-positive children, nested species PCR detected six mixed/mono P. ovale infections and two mixed P. vivax infections, though none exhibited lower than 60% G6PD enzyme activity.10
Discussion
Primaquine's efficacy, as a P. falciparum gametocytocide and as a radical cure hypnozoiticide for P. ovale and P. vivax, makes it a valuable tool for malaria control in sub-Saharan Africa. Yet, its widespread use has been limited due to concerns over the risk of hemolytic toxicity in G6PD-deficient individuals, and the lack of a field-friendly method of detection to identify those at risk. Here we report the prevalence of G6PD deficiency using three different diagnostic modalities in southwestern Uganda, an area of low P. falciparum transmission, but where all four major malaria species are endemic.10 Our prevalence estimate by quantitative/phenotypic analysis is lower than the ranges reported in eastern Uganda (6.8% versus 10–20%), as well as the G202A allele frequency (6.8% versus 12–15%).12,18–20 This may be due to the differences in ethnic group composition between these regions, however, our study was not powered to detect such differences.
Echoing reports in the literature, despite the similar overall prevalence of “deficiency” by G202A genotype and enzymatic phenotypic assay, we found significant discordance between these assays.12,21 While this lack of correlation is typical for heterozygous females who exhibit variable expression of G6PD activity, the degree of discordance is difficult to explain the large number of phenotypically normal, but genotypically hemi- and homozygous children. There may be a few reasons that explain this unusual finding. First, the child may have experienced a very recent hemolytic episode, thereby increase the number of reticulocytes and falsely elevating G6PD activity. However, we found that mean hemoglobin levels did not differ between the 29 children who were either hemi- or homozygous for the G202A mutation and expressed normal enzyme activity (N = 16) as compared with the 13 hemi- or homozygous for the G202A mutation and expressed < 60% enzyme activity (mean ± SD: 12.1 ± 1.1 versus 12.0 ± 1.1 g/dL, respectively; P = 0.912). Another potential reason for this finding may be due to the variability in biochemical and clinical penetrance of the G6PD A− G202A allele.22 Finally, the presence of certain genetic modifiers may have ameliorated the effect of the G202A mutation on enzyme activity, though limited data exist on this possibility.22
Not surprisingly, we found that a small percentage of G202A wild-type children exhibited low G6PD activity. It is likely that other G6PD A− single nucleotide polymorphisms (SNPs), such as A376G, A542T, G680T, and T986C, or perhaps non-G6PD A− alleles, may have been responsible for the lower enzyme activity in these individuals. Regardless of the etiology of the discrepancy between G202A genotype and enzymatic phenotype, it appears that the use of single SNP typing for G202A is unlikely to accurately identify all G6PD-deficient individuals in many east and west African settings.12,23
Overall, our results are comparable to the well-documented field studies that evaluated the diagnostic performance of the CareStart™ G6PD RDT.13,24–27 The diagnostic performance of the CareStart™ G6PD RDT in our setting was high across all the tested thresholds. Performance was optimal at a threshold of < 60% G6PD enzyme activity, demonstrating 98% sensitivity and specificity. We observed a low PPV of 78% at this threshold, which is likely reflective of the low G6PD deficiency prevalence in our setting. In the case of all three thresholds, the CareStart™ G6PD RDT exhibited a high NPV (100%), which has important clinical value when it comes to G6PD screening before primaquine therapy.
The CareStart™ G6PD RDT performed well at identifying “intermediate” ranges of G6PD deficiency (30–80%),8 typically seen in heterozygous females, and a range at which drug-induced hemolytic toxicity can still be observed.9,25 However, due to its qualitative nature, it is unable to differentiate those with severe deficiency (< 10%) from those with mild-to-moderate deficiency (10% to < 60%), the latter group of which may be eligible to receive alternative primaquine dosing for radical curative treatment under recent guidelines (0.75 mg/kg primaquine once weekly for 8 weeks).4,7
Overall, the CareStart™ G6PD RDT appears to be a promising RDT for use in southwestern Uganda,13,24–27 an area where our group has demonstrated continued strides in reducing the burden of P. falciparum (prevalence of asymptomatic P. falciparum parasitemia of 1.9%).10,15,28,29 In addition, the very low prevalence of under 30% enzyme activity (2%) in our study, combined with the likely safety of single-dose primaquine even in those with G6PD deficiency,1,4,30 suggests that there is little barrier to the use to single-dose primaquine in this region. The use of single-dose primaquine as a P. falciparum gametocytocide may serve to reduce the transmission of P. falciparum in such settings, which may aid in targeting the asymptomatic reservoir.1,30
In contrast, southwestern Uganda is also a region where it is clear that both P. vivax and P. ovale infections exist, albeit at low frequencies.10 Adequate treatment of these infections is critical to prevent relapse and reduce morbidity from severe anemia, and malnutrition.4,31 When used in this setting, however, the risk:benefit ratio of primaquine differs, as the 14-day course provides a much stronger oxidative challenge.2,4 It is therefore essential to identify all those at-risk for hemolysis before treatment. Although quantitative testing is best able to identify at-risk individuals, it is labor intensive, cost prohibitive, and requires electricity and reagents that are sensitive to light and heat.32 In our study, the CareStart™ G6PD RDT performed well for the purpose of accurately identifying G6PD-deficient individuals with an enzyme activity below 60%, which suggests that the RDT may be a good screening method before radical cure treatment in southwestern Uganda. However, alternative primaquine dosing for individuals with mild-to-moderate G6PD deficiency may not be possible due to the high discriminatory cut-off value of this RDT.
ACKNOWLEDGMENTS
We would like to thank the Médecins sans Frontières (MSF) Epicentre Mbarara research team for their laboratory support, without whom this research would not have been possible. We would also like to thank the population of Mbarara, Bushenyi, and Isingiro districts for their willingness to participate in the study. We would also like to thank Professor Andrew DeWan, from the Yale School of Public Health, for his assistance with genetic analyses.
Footnotes
Financial support: This study was supported by the Yale Downs Fellowship, Uganda Research Support Student Fund, and the Medical Education Partnership Initiative (MEPI-MESAU).
Authors' addresses: Michelle E. Roh, Martina Wade, and Sunil Parikh, Epidemiology of Microbial Diseases, Yale School of Public Health, New Haven, CT, E-mails: mroh@gmail.com, martina.wade@yale.edu, and sunil.parikh@yale.edu. Caesar Oyet and Gertrude N. Kiwanuka, Department of Biochemistry, Mbarara University of Science and Technology, Mbarara, Uganda, E-mails: caesaroyet@yahoo.com and gekiwa2001@yahoo.co.uk. Patrick Orikiriza, Department of Microbiology, Mbarara University of Science and Technology, Mbarara, Uganda, and Médecins sans Frontières Epicentre, Mbarara Research Centre, Mbarara, Uganda, E-mail: patrick.orikiriza@epicentre.msf.org. Juliet Mwanga-Amumpaire, Department of Pediatrics, Mbarara University of Science and Technology, Mbarara, Uganda, and Médecins sans Frontières Epicentre, Mbarara Research Centre, Mbarara, Uganda, E-mail: juliet.mwanga@epicentre.msf.org. Yap Boum II, Médecins sans Frontières Epicentre, Mbarara Research Centre, Mbarara, Uganda, and Department of Medicine, Mbarara University of Science and Technology, Mbarara, Uganda, E-mail: yap.boum@epicentre.msf.org.
References
- 1.World Health Organization . Policy Brief on Single-Dose Primaquine as a Gametocytocide in Plasmodium falciparum Malaria. Geneva, Switzerland: Global Malaria Programme, World Health Organization; 2015. [Google Scholar]
- 2.von Seidlein L, Auburn S, Espino F, Shanks D, Cheng Q, McCarthy J, Baird K, Moyes C, Howes R, Ménard D. Review of key knowledge gaps in glucose-6-phosphate dehydrogenase deficiency detection with regard to the safe clinical deployment of 8-aminoquinoline treatment regimens: a workshop report. Malar J. 2013;12:1. doi: 10.1186/1475-2875-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Niz M, Eziefula AC, Othieno L, Mbabazi E, Nabukeera D, Ssemmondo E, Gonahasa S, Tumwebaze P, DiLiberto D, Maiteki-Sebuguzi C. Tools for mass screening of G6PD deficiency: validation of the WST8/1-methoxy-PMS enzymatic assay in Uganda. Malar J. 2013;12:1. doi: 10.1186/1475-2875-12-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashley EA, Recht J, White NJ. Primaquine: the risks and the benefits. Malar J. 2014;13:1186. doi: 10.1186/1475-2875-13-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luzzatto L, Nannelli C, Notaro R. Glucose-6-phosphate dehydrogenase deficiency. Hematol Oncol Clin North Am. 2016;30:373–393. doi: 10.1016/j.hoc.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Domingo GJ, Satyagraha AW, Anvikar A, Baird K, Bancone G, Bansil P, Carter N, Cheng Q, Culpepper J, Eziefula C. G6PD testing in support of treatment and elimination of malaria: recommendations for evaluation of G6PD tests. Malar J. 2013;12:391. doi: 10.1186/1475-2875-12-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . Guidelines for the Treatment of Malaria. 3rd edition. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 8.Malaria Policy Advisory Committee Meeting . Point-of-Care G6PD Testing to Support Safe Use of Primaquine for the Treatment of Vivax Malaria. Geneva, Switzerland: WHO; 2015. WHO Evidence Review Group Meeting Report. [Google Scholar]
- 9.Pamba A, Richardson ND, Carter N, Duparc S, Premji Z, Tiono AB, Luzzatto L. Clinical spectrum and severity of hemolytic anemia in glucose 6-phosphate dehydrogenase-deficient children receiving dapsone. Blood. 2012;120:4123–4133. doi: 10.1182/blood-2012-03-416032. [DOI] [PubMed] [Google Scholar]
- 10.Roh ME, Oyet C, Orikiriza P, Wade M, Kiwanuka GN, Mwanga-Amumpaire J, Parikh S, Boum Y., 2nd Asymptomatic Plasmodium infections in children in low malaria transmission setting, southwestern Uganda. Emerg Infect Dis. 2016;22:1494–1498. doi: 10.3201/eid2208.160619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization . Training for Mid-Level Managers (MLM): The Epi Coverage Survey. Geneva, Switzerland: World Health Organization; 2008. Immunizations, Vaccines, and Biologicals. [Google Scholar]
- 12.Johnson MK, Clark TD, Njama-Meya D, Rosenthal PJ, Parikh S. Impact of the method of G6PD deficiency assessment on genetic association studies of malaria susceptibility. PLoS One. 2009;4:e7246. doi: 10.1371/journal.pone.0007246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S, Nguon C, Guillard B, Duong S, Chy S, Sum S, Nhem S, Bouchier C, Tichit M, Christophel E, Taylor WR, Baird JK, Menard D. Performance of the CareStart G6PD deficiency screening test, a point-of-care diagnostic for primaquine therapy screening. PLoS One. 2011;6:e28357. doi: 10.1371/journal.pone.0028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization Glucose-6-phosphate dehydrogenase deficiency. WHO working group. Bull World Health Organ. 1989;67:601–611. [PMC free article] [PubMed] [Google Scholar]
- 15.De Beaudrap P, Nabasumba C, Grandesso F, Turyakira E, Schramm B, Boum Y, Etard J-F. Heterogeneous decrease in malaria prevalence in children over a six-year period in south-western Uganda. Malar J. 2011;10:132. doi: 10.1186/1475-2875-10-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Core Team; 2013. [Google Scholar]
- 17.Baird JK, Dewi M, Subekti D, Elyazar I, Satyagraha AW. Noninferiority of glucose-6-phosphate dehydrogenase deficiency diagnosis by a point-of-care rapid test vs the laboratory fluorescent spot test demonstrated by copper inhibition in normal human red blood cells. Transl Res. 2015;165:677–688. doi: 10.1016/j.trsl.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okell LC, Bousema T, Griffin JT, Ouédraogo AL, Ghani AC, Drakeley CJ. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun. 2012;3:1237. doi: 10.1038/ncomms2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bwayo D, Kaddumukasa M, Ddungu H, Kironde F. Prevalence of glucose-6-phosphate dehydrogenase deficiency and its association with Plasmodium falciparum infection among children in Iganga district in Uganda. BMC Res Notes. 2014;7:372. doi: 10.1186/1756-0500-7-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malaria Atlas Project Population Estimates for G6PD Deficiency Table for Uganda. http://www.map.ox.ac.uk/browse-resources/g6pd/g6pdd-estimates-table/uga/ Available at. Accessed October 12, 2014.
- 21.Maiga B, Dolo A, Campino S, Sepulveda N, Corran P, Rockett KA, Troye-Blomberg M, Doumbo OK, Clark TG. Glucose-6-phosphate dehydrogenase polymorphisms and susceptibility to mild malaria in Dogon and Fulani, Mali. Malar J. 2014;13:270. doi: 10.1186/1475-2875-13-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah SS, Macharia A, Makale J, Uyoga S, Kivinen K, Craik R, Hubbart C, Wellems TE, Rockett KA, Kwiatkowski DP, Williams TN. Genetic determinants of glucose-6-phosphate dehydrogenase activity in Kenya. BMC Med Genet. 2014;15:93. doi: 10.1186/s12881-014-0093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark TG, Fry AE, Auburn S, Campino S, Diakite M, Green A, Richardson A, Teo YY, Small K, Wilson J, Jallow M, Sisay-Joof F, Pinder M, Sabeti P, Kwiatkowski DP, Rockett KA. Allelic heterogeneity of G6PD deficiency in west Africa and severe malaria susceptibility. Eur J Hum Genet. 2009;17:1080–1085. doi: 10.1038/ejhg.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adu-Gyasi D, Asante KP, Newton S, Dosoo D, Amoako S, Adjei G, Amoako N, Ankrah L, Tchum SK, Mahama E. Evaluation of the diagnostic accuracy of CareStart G6PD deficiency rapid diagnostic test (RDT) in a malaria endemic area in Ghana, Africa. PLoS One. 2015;10:e0125796. doi: 10.1371/journal.pone.0125796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satyagraha AW, Sadhewa A, Elvira R, Elyazar I, Feriandika D, Antonjaya U, Oyong D, Subekti D, Rozi IE, Domingo GJ. Assessment of point-of-care diagnostics for G6PD deficiency in malaria endemic rural eastern Indonesia. PLoS Negl Trop Dis. 2016;10:e0004457. doi: 10.1371/journal.pntd.0004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Fricken ME, Weppelmann TA, Eaton WT, Masse R, de Rochars MVB, Okech BA. Performance of the CareStart glucose-6-phosphate dehydrogenase (G6PD) rapid diagnostic test in Gressier, Haiti. Am J Trop Med Hyg. 2014;91:77–80. doi: 10.4269/ajtmh.14-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roca-Feltrer A, Khim N, Kim S, Chy S, Canier L, Kerleguer A, Tor P, Chuor CM, Kheng S, Siv S. Field trial evaluation of the performances of point-of-care tests for screening G6PD deficiency in Cambodia. PLoS One. 2014;9:e116143. doi: 10.1371/journal.pone.0116143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uganda Bureau of Statistics (UBOS) and ICF International . Uganda Malaria Indicator Survey 2009. Kampala, Uganda and Rockville, MD: Uganda Malaria Surveillance Project Molecular Laboratory, National Malaria Control Programme, ICF Macro; 2010. [Google Scholar]
- 29.Uganda Bureau of Statistics (UBOS) and ICF International . Uganda Malaria Indicator Survey 2014–2015. Kampala, Uganda and Rockville, MD: Uganda Bureau of Statistics; 2015. [Google Scholar]
- 30.Bancone G, Chowwiwat N, Somsakchaicharoen R, Poodpanya L, Moo PK, Gornsawun G, Kajeechiwa L, Thwin MM, Rakthinthong S, Nosten S. Single low dose primaquine (0.25 mg/kg) does not cause clinically significant haemolysis in G6PD deficient subjects. PLoS One. 2016;11:e0151898. doi: 10.1371/journal.pone.0151898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77:79–87. [PMC free article] [PubMed] [Google Scholar]
- 32.Eziefula AC, Gosling R, Hwang J, Hsiang MS, Bousema T, von Seidlein L, Drakeley C. Rationale for short course primaquine in Africa to interrupt malaria transmission. Malar J. 2012;11:360. doi: 10.1186/1475-2875-11-360. [DOI] [PMC free article] [PubMed] [Google Scholar]