Abstract
Zika virus (ZIKV) is a mosquito-borne flavivirus with a significant public health impact highlighted by the ongoing epidemic in the Americas. We describe a 44-year-old male presenting to our tropical medicine center with complaints of fever, headache, joint pain, and rash after recent travel to Guyana. The patient subsequently developed gait imbalance and lower extremity weakness with clinical examination, cerebrospinal fluid studies, and magnetic resonance imaging of the spine consistent with a diagnosis of Guillain–Barré syndrome (GBS). ZIKV infection was confirmed via detection of ZIKV RNA in urine by polymerase chain reaction. The patient was treated with intravenous immunoglobulin and experienced near-complete neurologic recovery, reporting ongoing mild paresthesia up to 2 months later. This case highlights the diagnostic challenges posed by ZIKV and underscores the need for clinician awareness of the potential for neurological complications such as GBS with ZIKV infection.
Background
Zika virus (ZIKV) is a mosquito-borne flavivirus that was first discovered in the Zika forest of Uganda in 1947, and was first isolated from a human in 1952.1 In the decades following this discovery, serologic evidence of exposure to ZIKV and sporadic cases in humans were noted in several countries in Africa and Asia.2–6 A significant outbreak occurred on Yap Island in 2007, affecting nearly three-quarters of inhabitants and providing an important opportunity to describe the natural history of ZIKV infection.7 The most common symptoms in this outbreak were rash, fever, arthritis or arthralgia, and nonpurulent conjunctivitis. The absence of reported symptoms in many individuals with evidence of acute infection suggested a predominance of asymptomatic ZIKV infections (perhaps 80%).
Neurological complications of ZIKV infection, including Guillain–Barré syndrome (GBS), were not noted until a subsequent, large epidemic in French Polynesia in 2013–2014.8 GBS is a neurological syndrome characterized by acute areflexic paralysis and albuminocytologic dissociation (high cerebrospinal fluid [CSF] protein with normal cell count). The syndrome was first described in 1916.9 In the post-polio virus era, GBS is the most frequent cause of acute flaccid paralysis worldwide. The most common preceding infection is Campylobacter jejuni,10 with other possible infectious agents including cytomegalovirus, Epstein–Barr virus, varicella zoster virus, and Mycoplasma pneumoniae.11–13 GBS has also been associated with West Nile virus, Japanese encephalitis virus, chikungunya virus (CHIKV), and dengue virus (DENV).14–17
The current ZIKV outbreak in the Americas began in May 2015 with confirmed autochthonous transmission in Brazil.18 ZIKV has since spread rapidly throughout the region and, as of September 2016, endemic transmission has been reported in nearly every country or territory in the Americas and Caribbean including the United States.19 Additionally, the Centers for Disease Control and Prevention (CDC) have reported over 2,700 cases of travel-associated ZIKV infection in the United States as of September 2016.20 A concurrent increase in cases of microcephaly in Brazil led to speculation regarding ZIKV-associated birth defects,21 a concern which was further supported by finding ZIKV in the brain tissue of microcephalic fetuses.22 There is now a sufficient burden of evidence to indicate a causal relationship between microcephaly and ZIKV infection.23 The ZIKV epidemic in the Americas has been associated with increases in the occurrence of GBS, with seven cases of ZIKV-associated GBS reported in U.S. states.19 We report a visitor of friends or relatives (VFR) traveler to Guyana that developed clinical GBS after an episode of symptomatic ZIKV infection.
Case Report
A 44-year-old male, born in Guyana and presently residing in the United States, without significant past medical history, contacted our tropical medicine center by telephone. His main concerns were subjective fever, headache, joint pain, and rash for 3 days. The patient had recently traveled to an urban region along the South Atlantic Coast in Guyana to visit friends and relatives for 16 days in March 2016, returning 8 days before onset of the illness. The patient reported having a diffuse erythematous rash, joint pain in both hands and fingers, and headache. His reported exposures during travel included multiple mosquito bites, brushing teeth with tap water, and staying in a local home. He was taking naproxen, with some relief of joint pain and subjective fever. He did not seek pretravel medical counsel and was not taking malaria prophylaxis. He denied a history of yellow fever vaccination or infection. An appointment was made for him to be seen in our tropical medicine center the same day; however, the patient did not keep his appointment.
He presented to an urgent care the next day (day 4 of illness), with continued subjective fever, joint pain, headache, and rash. On presentation to urgent care, the patient was afebrile with normal vital signs. Physical examination showed effusion in finger joints with tenderness to palpation, “sandpaper rash” on the torso, extremities, and face, and nonpurulent conjunctivitis. Neurological examination was normal. Initial laboratory studies included complete blood count (CBC), basic metabolic panel (BMP), liver function panel, and urinalysis (UA), all of which were within normal limits. CHIKV and DENV IgM and IgG enzyme-linked immunosorbent assays (ELISAs) were sent and results remained pending for several days. Trioplex polymerase chain reaction (PCR) was also requested from the Minnesota Department of Health for DENV, CHIKV, and ZIKV testing from serum. The patient was discharged from urgent care with the presumptive diagnosis of chikungunya infection and was given a prescription for hydrocodone–acetaminophen.
The next presentation to medical care was to an emergency department 4 days later (day 8 of illness). He reported continued “general body aches,” rash and headaches. He also reported cramping and weakness in the feet, hands, arms, and unsteady gait. Physical examination showed him to be afebrile with normal vital signs. Conjunctivitis was no longer present, and the rash was now confined to a small area on the left antecubital fossa. Neurological examination was normal. CBC, BMP, and UA were again within normal limits and the patient was counseled to continue supportive care and monitor for significant changes. The diagnosis of chikungunya infection was again presumed to be the most likely; however, diagnostic testing of CHIKV had not yet returned. The patient was discharged home with plan to follow up with primary care provider and neurology within 1 week.
On day 9 of illness, the patient presented to our tropical medicine center with worsening gait instability. The patient reported a “tingling” sensation in his hands and feet and a “heaviness” of the tongue. He stated “my legs cannot carry me” and walked into the travel clinic with the support of family members. On examination, he was found to have full strength in the upper extremities, but reduced strength to 4/5 in the lower extremities, and an unsteady gait. His reflexes were noted to be “minimal to absent” in the lower extremities. By this time, CHIKV serology had returned and was negative by IgM and IgG. DENV serology was found to be positive with IgG of 9.65 (upper limit of normal, 1.1) and IgM of 1.35 (upper limit of normal, 1.35) (studies performed at Focus Diagnostics, San Juan Capistrano, CA) (Table 1). The patient was admitted directly to the hospital, with a working diagnosis of probable ZIKV infection and associated GBS.
Table 1.
Timing of specimen collection for diagnostic testing
| Day of illness | Date | Diagnostic test (specimen) | Result |
|---|---|---|---|
| Day 0 | April 4, 2016 | – | – |
| Day 4 | April 7, 2016 | Trioplex PCR (serum) | Negative for ZIKV, DENV, CHIKV |
| CHIKV ELISA (serum) | IgM, IgG negative | ||
| DENV ELISA (serum) | IgG: 9.65 (ULN = 1.1)IgM: 1.35 (ULN = 1.35) | ||
| Day 9 | April 12, 2016 | ZIKV IgM ELISA (serum) | Positive* |
| ZIKV PRNT (serum) | Titer > 1:1,280 | ||
| DENV PRNT (serum) | Titer > 1:1,280 | ||
| Day 11 | April 14, 2016 | ZIKV IgM ELISA (CSF) | Positive* |
| Day 15 | April 18, 2016 | Trioplex PCR (urine) | Positive for ZIKV† |
CHIKV = chikungunya virus; CSF = cerebrospinal fluid; DENV = dengue virus; ELISA = enzyme-linked immunosorbent assay; PCR = polymerase chain reaction; PRNT = plaque-reduction neutralization test; ULN = upper limit of normal; ZIKV = Zika virus.
Only qualitative results were available for ZIKV IgM.
Trioplex PCR was run only for ZIKV, as urine testing is not currently performed for DENV and CHIKV.
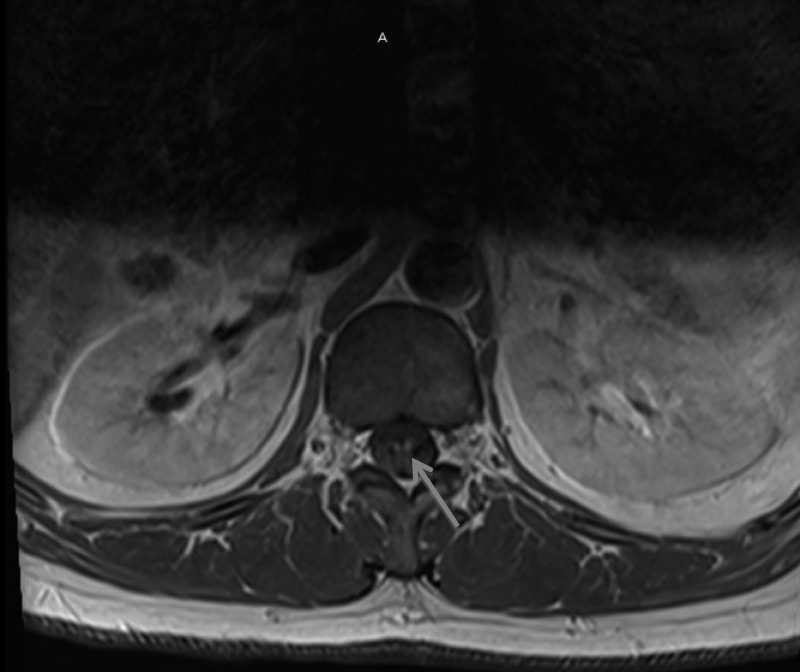
On admission, he had decreased sensation on the distal lower extremities to the knees bilaterally. Otherwise, the neurologic examination was similar to that in clinic. The patient had severe, sharp pains from the occiput radiating down his spine and from his knees to his hips bilaterally, concerning for neuropathic pain. Laboratory studies showed normal CBC, BMP, thyroid stimulating hormone, erythrocyte sedimentation rate, and C-reactive protein. Malaria rapid antigen and thin/thick blood smear examinations found no evidence of Plasmodium spp. ZIKV serologies were sent on admission and pending for many days. Given concern for GBS, he was started on intravenous immunoglobulin (IVIG) therapy at a dose of 400 mg/kg every 24 hours. The Supplemental Video demonstrates the gait instability which was observed during admission (Supplemental Video—Permission was obtained from patient to publish video in public domain). The video was taken on day 12 of illness, after initiation of IVIG. A lumbar puncture showed clear spinal fluid with 910 red blood cells and four white blood cells (WBC). The differential WBC showed 20% polymorphonuclear cells, 59% lymphocytes, and 21% monocytes. Protein was 118 mg/dL and glucose 70 mg/dL. The patient had magnetic resonance imaging (MRI) and angiography of the head without significant abnormality noted. MRI of the spine (Figure 1 ) showed findings of “subtle enhancement of cervical nerve roots” along with “areas of enhancement within the lumbar nerve roots/nerve roots of the cauda equina,” which were thought to be consistent with GBS. Electromyography showed sensorimotor peripheral neuropathy with involvement of the upper extremities greater than the lower extremities, consistent with a demyelinating disease. With this evaluation, the diagnosis of GBS was confirmed with the Brighton Collaborative Criteria for GBS, at Level 1 certainty.24
Figure 1.
Magnetic resonance imaging of lumbar spine showing enhancement of lumbar nerve roots and cauda equina (blue arrow), a finding often seen in Guillain–Barré syndrome.
Over the course of his hospitalization, he improved but complained of ongoing numbness in his feet, hands, and tongue as well as dysgeusia. His reflexes improved and strength in lower extremities normalized. His gait remained unsteady, but significantly improved from admission, requiring minimal assistance to walk several feet at the time of discharge (day 15 of illness). His urine specimen collected before discharge (day 18 of illness) ultimately resulted as showing evidence of ZIKV RNA using the Trioplex real-time RT-PCR assay (https://www.cdc.gov/zika/pdfs/fact-sheet-for-hcp-eua-trioplex-rt-pcr-zika.pdf), confirming the diagnosis of ZIKV infection. Weeks later, his serum (collected on day 9 of illness) and CSF (collected on day 13 of illness) both resulted as positive for ZIKV IgM, further confirming the diagnosis of ZIKV-associated GBS.
The patient was contacted by telephone 1 month after hospital discharge. He noted recovery of gross strength and balance, with ongoing paresthesias in the palms of the hands and soles of the feet. At last contact, 2 months after the onset of GBS, the patient reported ongoing and unchanged paresthesias.
Discussion
We report a case of ZIKV infection acquired in Guyana with characteristic fever, rash, conjunctivitis, and arthritis with subsequent development of clinical Guillain–Barré syndrome on day 8–9 of illness. This case serves to highlight the ongoing risk of ZIKV infection in multiple regions worldwide and adds to the literature regarding the association between ZIKV and GBS. Clinicians should have ZIKV infection on their differential diagnosis in travelers returning from epidemic areas with fever and/or rash and should be aware of potential complications such as birth defects and neurological disease. This patient was a VFR traveler, returning to his native country for a visit. VFR travelers are at higher risk for some diseases, such as malaria and typhoid fever,25,26 due to the fact that they may stay longer than other tourists, reside in local homes and eat local food, and may be less likely to take precautions such as using effective insect repellant or seeking pretravel counsel.
Diagnosis of acute ZIKV infection and possible ZIKV-related neurological disease is complicated by significant serologic cross-reactivity between the Flaviviridae, particularly DENV, yellow fever virus, and ZIKV.27 This is particularly problematic given that the Americas are currently experiencing simultaneous epidemics of ZIKV, DENV, and CHIKV. The patient denied a history of yellow fever vaccination or infection; however, this was not confirmed serologically. He had probably been previously exposed to DENV based upon his IgG ELISA results. Although acute coinfection with DENV and ZIKV cannot be ruled out on the basis of the diagnostic tests performed, the finding of ZIKV in the urine confirms the diagnosis of ZIKV-associated GBS.28,29
The fact that his serum PCR was negative for ZIKV on day 4 of illness but urine PCR was positive on day 18 is not surprising; viral persistence in urine has been documented in multiple prior studies.30,31 The viremia associated with ZIKV infection is thought to be relatively low titer and short lived, lasting only 2–3 days and waning with the onset of rash.30 This short window to detect ZIKV viremia greatly limits the diagnostic utility of serum PCR. The Food and Drug Administration has issued an Emergency Use Authorization for a serum RT-PCR assay developed by Quest Diagnostics (San Juan Capistrano, CA),32 which will expand the capability for diagnostic testing for ZIKV beyond state and federal public health facilities. For the above reasons, the negative predictive value of this serum PCR test will be low. However, the ability to detect acute ZIKV infection in urine, semen, and saliva offers critical and noninvasive opportunities to confirm ZIKV infection cases at later stages of infection, and there is growing evidence, supported by the findings in this case report, that urine PCR may be superior to serum PCR for the detection and confirmation of ZIKV infection.33 Health-care practitioners may also consider sending other bodily fluids or tissues for PCR or serological testing, depending upon the clinical presentation and clinical setting (e.g., CSF in the setting of neurological disease, amniotic fluid PCR for ZIKV-infected mothers with fetal anomalies).
The first case report of ZIKV and GBS was made during the 2013–2014 outbreak in French Polynesia8 during a concurrent epidemic of DENV (serotypes 1 and 3). The authors proposed that prior DENV exposure may play a role in potentiating GBS with subsequent ZIKV infection. A case-control study from this same outbreak subsequently found no significant difference in DENV seropositivity comparing ZIKV-infected patients with and without GBS; 42 ZIKV-associated GBS cases were identified in total.34 However, DENV seroprevalence was very high in the study population (95%) and power to detect an association was likely quite low. Other notable features of the French Polynesia outbreak included that the majority of Zika-associated GBS cases reported a history of transient illness preceding neurological decline (88%) and the finding that neurological disease began shortly after illness (median 6 days) and progressed rapidly (median 4 days to plateau). A total of 29% required respiratory assistance, with no deaths reported. This can be compared with other GBS literature, which documents typical evolution of neurological symptoms over 2–4 weeks,35 with a need for respiratory support in 17–30% of patients.36 In summary, and supported by this case report, there appears to be a shorter time from symptom onset to peak neurological symptoms in ZIKV-related GBS. The possible association of ZIKV-associated GBS with prior DENV infection remains an important area for further research at this time.
Conclusions
Clinicians should have a high index of suspicion for patients with recent travel to epidemic regions and be aware of the possible complications of ZIKV infection, including GBS.
Supplementary Material
ACKNOWLEDGMENTS
We thank HealthPartners Travel and Tropical Medicine Center (St. Paul, MN) and Elizabeth Schiffman at the Minnesota Department of Health.
Disclaimer: The opinions or assertions contained herein are the private views of the authors and are not to be construed as reflecting the official views of the U.S. Army or the U.S. Department of Defense.
Footnotes
Authors' addresses: Ryan G. Fabrizius and Kathryn Anderson, Department of Medicine, University of Minnesota, Minneapolis, MN, E-mails: fabri018@umn.edu and ande7622@umn.edu. Brett Hendel-Paterson, Salahudin Maalim, and Patricia F. Walker, Department of Medicine, University of Minnesota, Minneapolis, MN, and Travel and Tropical Medicine Center, HealthPartners Medical Group, Saint Paul, MN, E-mails: bhendel1@gmail.com, salahudin.m.maalim@healthpartners.com, and patricia.f.walker@healthpartners.com. Robyn M. Kaiser, Infectious Diseases, HealthPartners Medical Group, Saint Paul, MN, E-mail: robyn.m.kaiser@healthpartners.com.
References
- 1.MacNamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48:139–145. doi: 10.1016/0035-9203(54)90006-1. [DOI] [PubMed] [Google Scholar]
- 2.Robin Y, Mouchet J. Serological and entomological study on yellow fever in Sierra Leone. Bull Soc Pathol Exot. 1975;68:249–258. [PubMed] [Google Scholar]
- 3.Olson JG, Ksiazek TG, Suhandiman Triwibowo. Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg. 1981;75:389–393. doi: 10.1016/0035-9203(81)90100-0. [DOI] [PubMed] [Google Scholar]
- 4.Jan C, Languillat G, Renaudet J, Robin Y. A serological survey of arboviruses in Gabon [in French] Bull Soc Pathol Exot. 1978;71:140–146. [PubMed] [Google Scholar]
- 5.Saluzzo JF, Gonzalez JP, Hervé JP, Georges AJ. Serological survey for the prevalence of certain arboviruses in the human population of the south-east area of Central African Republic [in French] Bull Soc Pathol Exot. 1981;74:490–499. [PubMed] [Google Scholar]
- 6.Saluzzo JF, Ivanoff B, Languillat G, Georges AJ. Serological survey for arbovirus antibodies in the human and simian populations of the south-east of Gabon [in French] Bull Soc Pathol Exot. 1982;75:262–266. [PubMed] [Google Scholar]
- 7.Duffy MR, Chen TH, Hancock T, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel M, Lambert AJ, Laven J, Kosoy O, Panella A, Biggerstaff BJ, Fischer M, Hayes EB. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 8.Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastere S, Valour F, Baudouin L, Ghawche F. Zika virus infection complicated by Guillain-Barré syndrome: case report, French Polynesia, December 2013. Euro Surveill. 2014;19:20720. doi: 10.2807/1560-7917.es2014.19.9.20720. http://www.eurosurveillance.org/images/dynamic/EE/V19N09/art20720.pdf Available at. [DOI] [PubMed] [Google Scholar]
- 9.Guillain G, Barré JA, Strohl A. Sur un syndrome de radiculonévrite avec hyperalbuminose du liquide céphalo-rachidien sans réaction cellulaire: remarques sur les caractères cliniques et graphiques des réflexes tendineux. Bull Soc Med Hop Paris. 1916;40:1462–1470. [PubMed] [Google Scholar]
- 10.Poropatich KO, Walker CL, Black RE. Quantifying the association between Campylobacter infection and Guillain-Barré syndrome: a systematic review. J Health Popul Nutr. 2010;28:545–552. doi: 10.3329/jhpn.v28i6.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadden RDM, Karch H, Hartung HP, Zielasek J, Weissbrich B, Schubert J, Weishaupt A, Cornblath DR, Swan AV, Hughes RA, Toyka KV. Plasma Exchange/Sandoglobulin Guillain-Barre Syndrome Trial Group Preceding infections, immune factors, and outcome in Guillain-Barré syndrome. Neurology. 2001;56:758–765. doi: 10.1212/wnl.56.6.758. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs BC, Rothbarth PH, van der Meche FGA, Herbrink P, Schmitz PI, de Klerk MA, van Doorn PA. The spectrum of antecedent infections in Guillain-Barré syndrome: a case-control study. Neurology. 1998;51:1110–1115. doi: 10.1212/wnl.51.4.1110. [DOI] [PubMed] [Google Scholar]
- 13.Kang JH, Sheu JJ, Lin HC. Increased risk of Guillain-Barré syndrome following recent herpes zoster: a population-based study across Taiwan. Clin Infect Dis. 2010;51:525–530. doi: 10.1086/655136. [DOI] [PubMed] [Google Scholar]
- 14.Leis AA, Stokic DS. Neuromuscular manifestations of West Nile virus infection. Front Neurol. 2012;3:37. doi: 10.3389/fneur.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravi V, Taly AB, Shankar SK, Shenoy PK, Nagaraja D, Desai A, Gourie-Devi M, Chandramuki A. Association of Japanese encephalitis virus infection with Guillain-Barre syndrome in endemic areas of south India. Acta Neurol Scand. 1994;90:67–72. doi: 10.1111/j.1600-0404.1994.tb02681.x. [DOI] [PubMed] [Google Scholar]
- 16.Lebrun G, Chadda K, Reboux AH, Martinet O, Gaüzère BA. Guillain-Barré syndrome after chikungunya infection. Emerg Infect Dis. 2009;15:495–496. doi: 10.3201/eid1503.071482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solomon T, Dung NM, Vaughn DW, Kneen R, Thao LT, Raengsakulrash B, Loan HT, Day NP, Farrar J, Myint KS, Warrell MJ, James WS, Nisalak A, White NJ. Neurological manifestations of dengue infection. Lancet. 2000;355:1053–1059. doi: 10.1016/S0140-6736(00)02036-5. [DOI] [PubMed] [Google Scholar]
- 18.Pan American Health Organization (PAHO)/World Health Organization (WHO)–Regional Office for the Americas Epidemiological Alert: Zika Virus Infection: 7 May 2015. 2015. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=30075&lang=e Available at. Accessed June 9, 2016.
- 19.Centers for Disease Control and Prevention (CDC) All Countries and Territories with Active Zika Virus Transmission. 2016. http://www.cdc.gov/zika/geo/active-countries.html Available at. Accessed September 5, 2016.
- 20.Centers for Disease Control and Prevention (CDC) Zika Virus Disease in the United States, 2015–2016. 2016. http://www.cdc.gov/zika/geo/united-states.html Available at. Accessed September 5, 2016.
- 21.Ministério da Saúde (Brazil) Ministério da Saúde divulga novos dados de microcefalia. 2015. http://portalsaude.saude.gov.br/index.php/oministerio/principal/secretarias/svs/noticias-svs/21020-ministerio-da-saude-divulga-novos-dados-demicrocefalia Available at. Accessed June 6, 2016.
- 22.Mlakar J, Korva M, Popovic M, Pojsak-Prijatelj M, Mraz J, Kolenc M, Rus KR, Vipotnik TV, Vodusek VF, Vizjak A, Pizem J, Petrovec M, Zupanc TA. Zika virus associated with microcephaly. N Engl J Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen SA, Jamieson DJ, Honein MA, Peterson LR. Zika virus and birth defects: reviewing the evidence for causality. N Engl J Med. 2016;374:1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 24.Sejvar JJ, Kohl KS, Gidudu J, Amato A, Bakshi N, Baxter R, Burwen DR, Cornblath DR, Cleerbout J, Edwards KM, Heininger U, Hughes R, Khuri-Bulos N, Korinthenberg R, Law BJ, Munro U, Maltezou HC, Nell P, Oleske J, Sparks R, Velentgas P, Vermeer P, Wiznitzer M. Brighton Collaboration GBS Working Group Guillain-Barré syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29:599–612. doi: 10.1016/j.vaccine.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Bui YG, Trepanier S, Milord F, Blackburn M, Provost S, Gagnon S. Cases of malaria, hepatitis A and typhoid fever among VFRs, Quebec. J Travel Med. 2011;18:373–378. doi: 10.1111/j.1708-8305.2011.00556.x. [DOI] [PubMed] [Google Scholar]
- 26.LaRocque RC, Deshpande BR, Rao SR, Brunette GW, Sotir MJ, Jentes ES, Ryan ET. Global TravEpiNet Consortium Pre-travel health care of immigrants returning home to visit friends and relatives. Am J Trop Med Hyg. 2013;88:376–380. doi: 10.4269/ajtmh.2012.12-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention (CDC) Arboviral Diseases, Neuroinvasive and Non-Neuroinvasive 2015 Case Definition. 2015. https://wwwn.cdc.gov/nndss/conditions/arboviral-diseases-neuroinvasive-and-non-neuroinvasive/case-definition/2015/ Available at. Accessed May 4, 2016.
- 29.Roze B, Najioullah F, Ferge J, Apetse K, Brouste Y, Cesaire R, Fagour C, Fagour L, Hochedez P, Jeannin S, Joux J, Mehdaoui H, Valentino R, Signate A, Cabie A. GBS Zika Working Group Zika virus detection in urine from patients with Guillain-Barré syndrome on Martinique, January 2016. Euro Surveill. 2016;21:1–4. doi: 10.2807/1560-7917.ES.2016.21.9.30154. [DOI] [PubMed] [Google Scholar]
- 30.Gourinat AC, O'Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis. 2015;21:84–86. doi: 10.3201/eid2101.140894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korhonen EM, Huhtamo E, Smura T, Kallio-Kokko H, Raassina M, Vapalahti O. Zika virus infection in a traveller returning from the Maldives, June 2015. Euro Surveill. 2016;21:pii:30107. doi: 10.2807/1560-7917.ES.2016.21.2.30107. [DOI] [PubMed] [Google Scholar]
- 32.Emergency Use Authorizations: Zika Virus Emergency Use Authorization. 2016. http://www.fda.gov/MedicalDevices/Safety/EmergencySituations/ucm161496.htm#zika Available at. Accessed June 6, 2016.
- 33.Bingham AM, Cone M, Mock V, Heberlein-Larson L, Stanek D, Blackmore C, Likos A. Comparison of test results for Zika virus RNA in urine, serum, and saliva specimens from persons with travel-associated Zika virus disease: Florida, 2016. MMWR. 2016;65:475–478. doi: 10.15585/mmwr.mm6518e2. [DOI] [PubMed] [Google Scholar]
- 34.Cao-Lormeau VM, Blake A, Mos S, Lastere S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, Vial AL, Decam C, Choumet V, Halstead SK, Willison HJ, Musset L, Manuguerra JC, Despres P, Fournier E, Mallet HP, Musso D, Fontanet A, Neil J, Ghawche F. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis of Guillain-Barré syndrome and validation of Brighton criteria. Brain. 2014;137:33–43. doi: 10.1093/brain/awt285. [DOI] [PubMed] [Google Scholar]
- 36.Hughes RAC, Wijdicks EF, Benson E, Cornblath DR, Hahn AF, Meythaler JM, Sladky JT, Barohn RJ, Stevens JC. Multidisciplinary Consensus Group Supportive care for patients with Guillain-Barré syndrome. Arch Neurol. 2005;62:1194–1198. doi: 10.1001/archneur.62.8.1194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.