Abstract
Zika virus (ZIKV) and chikungunya virus (CHIKV) are currently circulating in overlapping areas in the American continents and may both be transmitted by Aedes spp. mosquitoes. The first documented case, to the authors' knowledge, of sequential CHIKV and ZIKV infections diagnosed in a nonendemic area in a returning traveler is reported. The implications for heightened clinical surveillance for these infections and specific patient recommendations are emphasized.
Introduction
Zika virus (ZIKV), a flavivirus, and chikungunya virus (CHIKV), an alphavirus, may both be transmitted by Aedes spp. mosquitoes, and are currently circulating in overlapping areas in the American continents leading to possible coinfections or sequential infections.1
Case Report
A 25-year-old female presented with a 3-day history of fever, rash, headache, and arthralgia. The patient had no significant past medical history, was on no regular medication, and had not received specific travel-related vaccinations.
The patient, born in Honduras, had recently returned to Madrid, Spain, from a 2-month visit to her country where she had stayed in Tegucigalpa and rural areas of Choluteca. The patient suffered another self-limiting febrile episode with a rash and joint pains 20 days previously, which had resolved after 72 hours with symptomatic treatment. A close relative in Honduras had similar signs and symptoms. She had remained well until the second (current) episode.
Significant findings on examination included fever (39°C), a generalized macular rash with palmar and plantar involvement (no Koplik spots), conjunctival injection, mild facial edema, and hand and foot swelling (Figures 1 and 2 ). Blood test results were unremarkable except for an initial C-reactive protein of 36 mg/L (normal range = 0.0–5.0 mg/L) and mild lymphopenia of 760 cells/μL (normal range = 1,000–3,500 cells/μL), and on follow-up blood tests (day 7 of symptoms), there was a mild elevation of aspartate aminotransferase (52 U/L; normal range = 4–50 U/L) and a mild neutropenia of 1,480 cells/μL (normal range = 1,700–7,500 cells/μL). A pregnancy test in serum was negative. Blood cultures were negative and a serological workup was negative (for human immunodeficiency virus, hepatitis B and C viruses, syphilis, leptospirosis, Q fever, rickettsiosis, parvovirus B19, human T-cell lymphotropic virus, and varicella zoster virus) or consistent with past infection/immunization (cytomegalovirus, mumps, rubella, measles).
Figure 1.
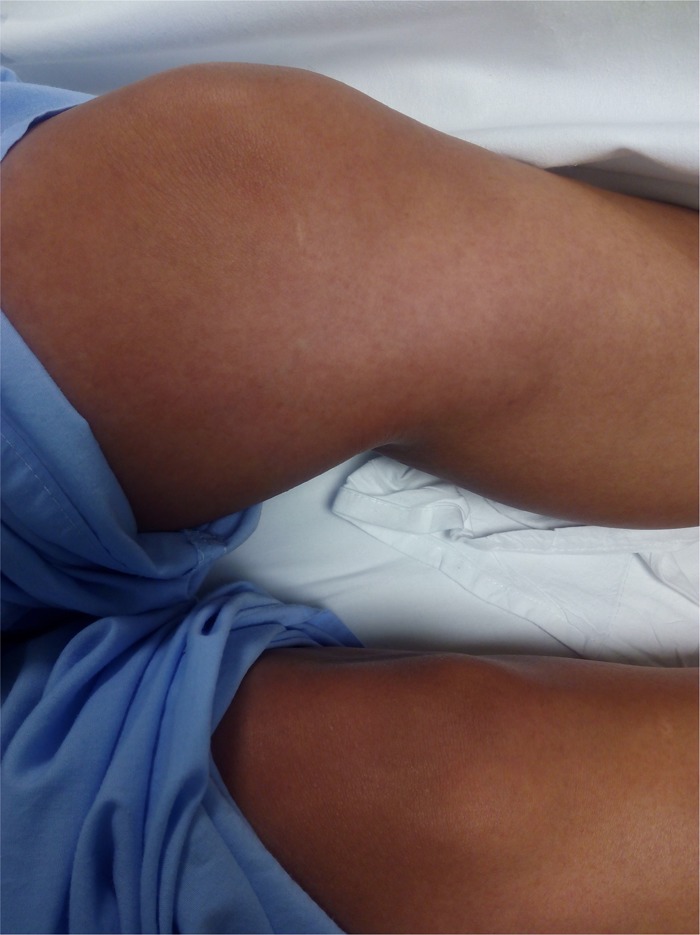
Rash on lower limbs of patient with sequential chikungunya and Zika virus infections.
Figure 2.
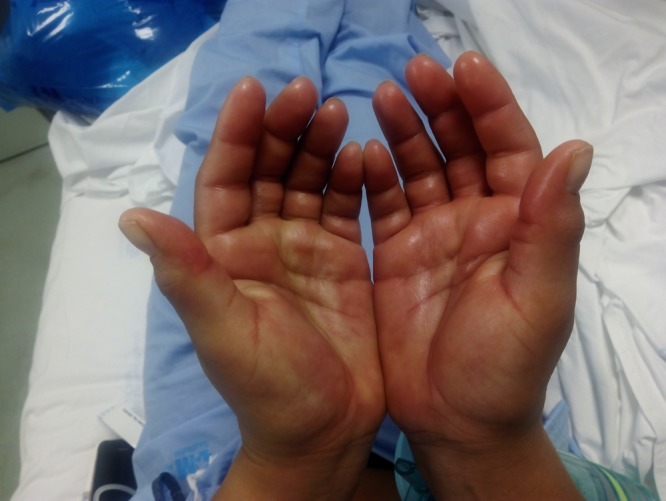
Palmar rash and hand swelling in a patient with sequential chikungunya and Zika virus infections.
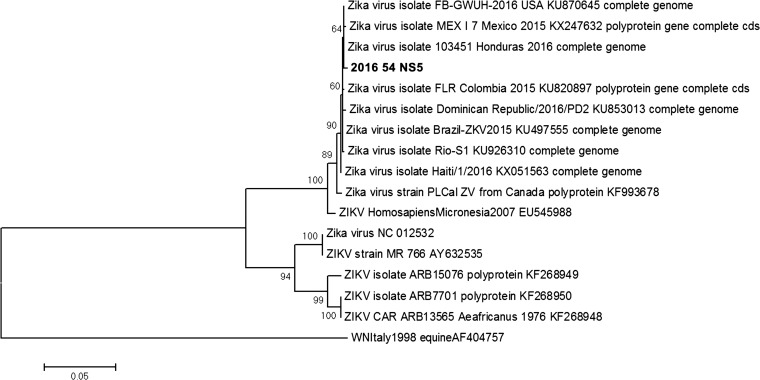
The diagnostic workup for arboviral infections is summarized in Table 1. Results were consistent with acute ZIKV infection, suggestive of recent acute infection with CHIKV and a probable past dengue virus (DENV) infection. The patient was afebrile at follow-up on day 6 with improvement of other symptoms. On day 17, polymerase chain reaction (PCR) remained positive in urine and samples of vaginal fluid and saliva were ZIKV-PCR negative. A 950–base pair amplicon of the NS5 gene was sequenced and found to be related to the ZIKV strains currently circulating in the American continents (Figure 3 ).4
Table 1.
Test results for arboviral infections
| Sample | ZIKV | DENV | CHIKV | MAYV |
|---|---|---|---|---|
| Day 3 of symptoms | ||||
| Peripheral blood PCR | Negative | Negative | Negative | Negative |
| Serology | IgM IFAT positive, ELISA negative | IgM ELISA negative | IgM IFAT positive | ND |
| IgG IFAT positive, ELISA positive | IgG ELISA positive | IgG IFAT positive | ND | |
| ZIKV microNT indeterminate | ND | CHIKV microNT positive 1/256 | ND | |
| Day 6 of symptoms | ||||
| Urine PCR | Positive | ND | Negative | Negative |
| Day 17 | ||||
| Saliva PCR | Negative | ND | ND | ND |
| Vaginal fluid PCR | Negative | ND | ND | ND |
| Urine PCR | Positive | ND | ND | ND |
| Day 34 | ||||
| Serology | IgM IFAT indeterminate, ELISA negative | ND | IgM IFAT positive | ND |
| IgG IFAT indeterminate, ELISA positive | ND | IgG IFAT positive | ND | |
| ZIKV microNT positive 1/512 | ND | CHIKV microNT positive 1/512 | ND | |
CHIKV = chikungunya virus; DENV = dengue virus; ELISA = enzyme-linked immunosorbent assay; IFAT = immunofluorescence antibody test; MAYV = Mayaro virus; microNT = microneutralization assay; ND = not done; PCR = polymerase chain reaction; ZIKV = Zika virus. ZIKV PCR = ZIKV PCR-RealStar® Zika Virus RT-PCR Kit 1.0 (Altona, Hamburg, Germany) and a modified version of;2 DENV PCR = in-house PCR; CHIKV PCR = in-house PCR; MAYV PCR = in-house PCR; ZIKV IgM and IgG = IIFT Arboviral Fever Mosaic 2 IgG/IgM (Euroimmun, Luebeck, Germany); CHIKV IgM and IgG = anti-chikungunya virus IIFT IgG/IgM (Euroimmun); DENV IgM and IgG = Panbio Dengue IgM capture ELISA and indirect Dengue IgG ELISA (Standard Diagnostics, Gyeonggi, Republic of Korea); ZIKV microNT = modified version of.3
Figure 3.
Phylogenetic analysis from partial NS5 Zika virus (ZIKV) gene. Maximum likelihood phylogenetic tree constructed by Mega 6 software using the Kimura 2-parameter model with 1,000 bootstrap replicates (tree rooted with West Nile virus). The sequence obtained in this study is marked in bold (GenBank KX377120).
Discussion
Clinical differentiation of infections produced by ZIKV, CHIKV, and DENV is often not possible. Sequential infections or coinfections by arboviruses circulating in epidemic regions may only be suspected after careful history-taking and the clinician may be faced with the retrospective interpretation of tests. The positive PCR for ZIKV in urine on days 6 and 17 supports the diagnosis of acute infection. Of interest were the positive IgM and IgG for ZIKV and the absence of neutralizing antibodies on the third day of overt symptoms. This may have been due to an early ZIKV IgM response and a cross-reactivity of ZIKV IgG with DENV IgG from a past infection.5 The early, weak and short ZIKV IgM response could be explained by the natural biological response to a second flaviviral infection, or by a low sensitivity of the assays used, since no experience on the performance characteristics of these assays are available. In this acute sample, neutralizing antibodies against ZIKV were not clearly observed possibly due to the short time elapsed from onset of symptoms, but neutralizing antibodies against ZIKV were clearly present in a follow-up paired sample (a microneutralization assay, similar to the plaque reduction neutralization test was used; however, in this case, these results merely supported the diagnosis of acute ZIKV infection which was already confirmed by PCR).
ZIKV infection may have preceded the CHIKV infection as ZIKV excretion in urine has been detected by PCR for a prolonged period of over 20 days.6 This seems unlikely due to the positive ZIKV microneutralization assay seroconversion and the negative CHIKV-PCR in biological samples. Since other alphaviruses, such as Mayaro virus, are circulating in the American continents, retrospective diagnosis of CHIKV infection based only on the immunofluorescence antibody test may not be considered definitive; but in this case, CHIKV infection was confirmed by the presence of neutralizing antibodies and Mayaro virus PCR was negative in initial peripheral blood and urine samples.
The patient was not pregnant and outcome was favorable with no apparent sequelae. Attending clinicians should remain vigilant as to the possible complications of sequential infections as other authors have raised the hypothesis of sequential arboviral immune stimulation as an explanation for the clustering of Guillain–Barré cases observed during previous arboviral outbreaks.7
Specific recommendations following diagnosis of imported arboviral infections should include deferral from blood donation and measures to avoid arthropod bites in areas where the vector is present. Aedes albopictus is present in several areas of continental Europe. Sexual transmission has been identified as a mode of acquisition of ZIKV infection, and ZIKV has previously been isolated from semen.8,9 To date, female-to-male ZIKV transmission has not been reported, and ZIKV has not been isolated in vaginal secretions, but further investigations should be considered to determine whether precautions to prevent sexual female-to-male transmission should be recommended. Restricting travel to epidemic areas if pregnant and postponing pregnancy if residing or returning from these areas have been proposed.
To the authors' knowledge, this is the first report of sequential CHIKV and ZIKV infections diagnosed in a nonendemic area in a returning traveler, and it illustrates the difficulties in establishing the etiologic diagnosis of these imported diseases.
Footnotes
Financial support: This work was supported by the Spanish Ministry of Science and Innovation and the Instituto de Salud Carlos III within the Network of Tropical Diseases Research RICET [RD06/0021/0020].
Authors' addresses: Francesca F. Norman, Sandra Chamorro, and María-Jesús Vivancos, Tropical Medicine, Infectious Diseases Department, Hospital Universitario Ramón y Cajal, Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS), Madrid, Spain, E-mails: ffnorman@gmail.com, sandracht@gmail.com, and mjesus.vivancos@gmail.com. Ana Vázquez and Fernando de Ory, National Microbiology Centre, CiberEsp, Instituto de Salud Carlos III, Madrid, Spain, E-mails: a.vazquez@isciii.es and fory@isciii.es. María Paz Sánchez-Seco, Alert and Emergency Unit, Instituto de Salud Carlos III, Madrid, Spain, E-mail: paz.sanchez@isciii.es. José-Antonio Pérez-Molina, Begoña Monge-Maillo, and Rogelio López-Vélez, Tropical Medicine, Infectious Diseases Department, Hospital Ramón y Cajal, Instituto Ramón y Cajalde Investigación Sanitaria (IRYCIS), Madrid, Spain, E-mails: jose.perezmolina@gmail.com, begomongem@gmail.com, and rogelio.lopezvelez@salud.madrid.org. Mario Rodríguez-Dominguez and Juan-Carlos Galán, Microbiology Department, Hospital Universitario Ramon y Cajal, Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS), Madrid, Spain, E-mails: mariojose.rodriguez@salud.madrid.org and juancarlos.galan@salud.madrid.org.
References
- 1.World Health Organization Mosquito-Borne Diseases. 2016. http://who.int/neglected_diseases/vector_ecology/mosquito-borne-diseases/en/ Available at. Accessed August 9, 2016.
- 2.Balm MND, Lee CK, Lee HK, Chiu L, Koay ESC, Tang JW. A diagnostic polymerase chain reaction assay for Zika virus. J Med Virol. 2012;84:1501–1505. doi: 10.1002/jmv.23241. [DOI] [PubMed] [Google Scholar]
- 3.Figuerola J, Jiménez-Clavero MA, López G, Rubio C, Soriguer R, Gómez-Tejedor C, Tenorio A. Size matters: West Nile Virus neutralizing antibodies in resident and migratory birds in Spain. Vet Microbiol. 2008;132:39–46. doi: 10.1016/j.vetmic.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Vázquez A, Sánchez-Seco MP, Palacios G, Molero F, Reyes N, Ruiz S, Aranda C, Marqués E, Escosa R, Moreno J, Figuerola J, Tenorio A. Novel flaviviruses detected in different species of mosquitoes in Spain. Vector Borne Zoonotic Dis. 2012;12:223–229. doi: 10.1089/vbz.2011.0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maria A, Maquart M, Makinson A, Flusin O, Segondy M, Leparc-Goffart I, Le Moing V, Foulongne V. Zika virus infections in three travellers returning from South America and the Caribbean respectively, to Montpellier, France, December 2015 to January 2016. Euro Surveill. 2016;21:30131. doi: 10.2807/1560-7917.ES.2016.21.6.30131. [DOI] [PubMed] [Google Scholar]
- 6.Gourinat AC, O'Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis. 2015;21:84–86. doi: 10.3201/eid2101.140894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastère S, Valour F, Baudouin L, Mallet H, Musso D, Ghawche F. Zika virus infection complicated by Guillain-Barré syndrome: case report, French Polynesia, December 2013. Euro Surveill. 2014;19:20720. doi: 10.2807/1560-7917.es2014.19.9.20720. [DOI] [PubMed] [Google Scholar]
- 8.Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17:880–882. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis. 2015;21:359–361. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]