Abstract
The purpose of this study was to determine the in vitro and ex vivo susceptibility of human corneal cells to West Nile virus (WNV) infection and evaluate the ability of the virus to disseminate to the corneas of infected mice. Human corneal epithelial cells were challenged with WNV, incubated for 1–6 days, and tested for evidence of WNV infection. Viral RNA and antigen were detected at every time point, and the virus reached a peak titer of 2.5 × 107 plaque-forming units (pfu)/mL at 3 days postinoculation (PI). Corneas procured from donors were incubated in culture dishes containing WNV for 1–5 days and tested for evidence of WNV. Viral RNA and antigen were detected, and the virus reached a mean peak titer of 4.9 × 104 pfu/mL at 5 days PI. Mice were inoculated intraperitoneally with WNV, and their eyes were harvested at 2, 5, and 8 days PI and tested for evidence of WNV. Viral RNA was detected in corneas of four of nine systemically infected mice as early as 2 days PI. We conclude that human corneal cells support WNV replication in vitro and ex vivo, and WNV may disseminate into the corneas of experimentally infected mice. These findings indicate that corneal transmission cannot be ruled out as a novel mode of human-to-human WNV transmission and additional experiments should be conducted to assess this risk further.
Introduction
West Nile virus (WNV; family Flavivirdae, genus Flavivirus) is maintained in nature in an enzootic transmission cycle that primarily involves “hematophagous” mosquitoes and susceptible avian hosts.1–3 Humans, horses, and other mammals usually are incidental hosts. Approximately 75% of WNV infections in humans are asymptomatic, 25% develop West Nile fever (WNF), and < 1% are characterized by neuroinvasive disease.4 The first report of WNV in the United States (U.S.) occurred in New York in 1999.5 WNV is now endemic in most states and has been responsible for over 40,000 human cases (Centers for Disease Control and Prevention; http://www.cdc.gov/westnile/statsMaps/finalMapsData/index.html, accessed March 23, 2016); of these, almost 20,000 cases featured neuroinvasive disease and over 1,800 were fatal. However, the true impact of WNV on human health is unknown because WNF is often undiagnosed.4 An estimated 3.2 million individuals in the U.S. became infected with WNV from 1999 to 2010, of which 780,000 developed clinical disease.6 Despite the proposed scope of clinical disease, there have been no documented cases of corneal transmission of WNV from a donor to a recipient.
Although WNV is transmitted to humans primarily by the bite of infected mosquitoes, several modes of human-to-human transmission have been documented including solid organ transplantation,7 blood transfusion,8 breast feeding,9 and intrauterine transmission.10 Transmission through solid organ transplantation was first described in 2002 when four recipients of transplanted organs were infected from a single donor.7 Although transplantation-acquired WNV disease is rare, additional cases have since been reported.11–15 Transmission through blood transfusion was also first observed in 2002. Nationwide screening of blood donations for evidence of WNV infection by nucleic acid amplification technology (NAT) was implemented in 2003 and greatly reduced the risk of transfusion-acquired WNV disease.16,17 Nevertheless, sporadic cases of transfusion-acquired WNV disease continue to occur due to false-negative NAT results.18–20
Approximately 47,500 patients received corneal transplants in the U.S. in 2015 (Eye Bank Association of America [EBAA]; http://restoresight.org/wp-content/uploads/2016/03/2015-Statistical-Report.pdf, accessed March 30, 2016). Donors are screened using Food and Drug Administration (FDA) guidelines and EBAA criteria to ensure corneal tissue is suitable for transplantation. While FDA requires serologic analysis of postmortem samples from potential cornea donors to screen for hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV-1 and 2) (FDA, Human Cells, Tissues and Cellular and Tissue-Based Products, Section 1271.85; www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=1271&showFR=1, accessed March 30, 2016), routine screening for WNV currently is not required. Although performed at some eye banks, voluntary routine testing of cornea donors has not been embraced nationwide due to lacking evidence of viral transmission via donor corneal tissue, the seasonal nature of the risk for mosquito-borne transmission, and the occurrence of false-positive results of the FDA-approved test available currently (Procleix WNV Assay; Gen-Probe Inc., San Diego, CA). In addition, confirmatory WNV testing is not valid regarding donor suitability testing (Package insert, Procleix WNV Assay, 500630 Rev A). False-positive results generated by this test could have a significant impact on the availability of corneas for transplant in the U.S.
This study was conducted to evaluate whether human corneal cells support in vitro and ex vivo replication of WNV and determine whether WNV disseminates to the corneas of infected vertebrate animals, as a preliminary investigation into the potential for WNV transmission due to keratoplasty.
Materials and Methods
Virus and cell cultures.
WNV (strain NY99-35261-11) was obtained from the World Health Organization Center for Arbovirus Reference and Research maintained at the Center for Disease Control and Prevention Division of Vector-Borne Infectious Diseases (Fort Collins, CO). Herpes simplex virus 1 (HSV-1, strain HF) was obtained from the American Type Culture Collection (ATCC, Manassas, VA). Human corneal epithelial (HCE) cells (Invitrogen, Carlsbad, CA) were cultured in keratinocyte serum-free medium (KSFM; Invitrogen) supplemented with 25 μg/mL bovine pituitary extract (BPE), 0.1 ng/mL human recombinant epidermal growth factor (rEGF), 100 units/mL penicillin, 100 μg/mL streptomycin, and 2.5 μg/mL fungizone. African green monkey (AGM) kidney (Vero) cells (ATCC) were cultured in Dulbecco's modified Eagle medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin, and 2.5 μg/mL fungizone. HCE and Vero cells were cultured at 37°C with 5% CO2.
Human corneas.
Corneoscleral tissue was obtained, inspected, and stored in Optisol GS (Bausch & Lomb, Irvine, CA) at 4°C in accordance with EBAA-compliant protocols at Iowa Lions Eye Bank. Research consent was obtained for all donor corneas. All tissues were determined to be suitable for cornea transplantation but were diverted to this study because they were nearing expiration and had not yet been used for transplant surgery. Tissues were transported to Iowa State University on ice, stored at 4°C upon arrival, and used within 24 h of receipt and 14 days of preservation. Donor identities were kept confidential. Each cornea was assigned a unique identification code consisting of three letters and seven digits. The last two letters of every code was denoted as OD or OS for right eye and left eye, respectively. Paired corneas were assigned the same identification code aside from the final two letters.
Polymerase chain reaction.
Total RNA was extracted from cell monolayers and corneas using Trizol Reagent (Invitrogen) and analyzed by reverse transcription polymerase chain reaction (RT-PCR) using primers specific for WNV, the human ribosomal protein L11 (RPL11) gene, or the AGM β-actin gene. The WNV-specific primers, WN233 and WN640c, target a 408-nt region of capsid–membrane genes.21 The RPL11-specific primers, RPL11-F (5′-CTCCATCATGGCGCAGGATCAAGG-3′) and RPL11-R (5′-CAACACCTTGGCTGCTCGCGTCAG-3′), were designed to target a 127-nt region of the RPL11 gene and used as positive controls. The AGM β-actin-specific primers, AGMβa-F (5′-CTCACGTTATGGATGATGATATCG-3′) and AGMβa-R (5′-CGGCCAGCCAGGTCCAGACGCAGG-3′), were designed to target a 556-nt region of the β-actin gene and also used as positive controls. Complementary DNAs were generated using Superscript III reverse transcriptase (Invitrogen), and PCRs were performed using Taq DNA polymerase (Invitrogen). PCR products were examined by 1% agarose gel electrophoresis and visualized with ethidium bromide. WNV RNA expression was quantified using real-time quantitative PCR with the 2ΔΔCT method (http://tools.thermofisher.com/content/sfs/manuals/cms_041440.pdf).22 The mouse ribosomal protein L19 (RPL19) gene was used as a reference for normalization. The WNV-specific primers target a region of the prM-E genes (forward: 5′-TTCAACTGCCTTGGAATGAGC-3′; reverse 5′-AGCGTGCACGTTCACGGGAGAG-3′), RPL19 primers, and PCR conditions have been reported previously.21
Preparation of protein lysates.
Protein lysates were prepared from cultured cells and corneas. Cultured cells were scraped from flasks, clarified by centrifugation and resuspended in lysing buffer (10 mM Tris-HCl pH 7.5, 150 mM NaCl, 5 mM EDTA, 1% sodium deoxycholate, 1% Triton X-100, 0.1% SDS, and a cocktail of protease inhibitors [Sigma; St. Louis, MO]). Corneas were homogenized in lysing buffer using a mortar and pestle on ice. A different mortar and pestle was used for each sample. Homogenized cultured cells and corneal tissues were placed on ice for 15 minutes then microfuged at 4°C for 15 minutes. Supernatants were collected and stored at −80°C.
Western blots.
Protein samples were mixed with an equal volume of sample buffer, heated (95°C for 5 minutes), and resolved on 8–16% Tris-glycine gels (Invitrogen). Proteins were transferred to 0.45-μm nitrocellulose membranes (Invitrogen) following published protocols.23 Membranes were blocked overnight at 4°C in phosphate-buffered saline (PBS, pH 7.2) with 5% (w/v) nonfat dried milk. Membranes were incubated with 1/100 pooled suspension of mouse anti-WNV envelope (E) protein monoclonal antibodies 3.67G and 3.91D (Millipore, Billerica, MA) or 1/1,000 of rabbit antihuman β-tubulin polyclonal antibody (Invitrogen) for 1 hour at 25°C. Membranes were then washed and incubated with 1/2,000 horseradish peroxidase–conjugated anti-mouse IgG antibody (Invitrogen) or 1/2,000 horseradish peroxidase–conjugated anti-rabbit IgG antibody (Invitrogen) for 1 hour at 25°C. Specifically bound antibody was visualized using 3,3′-diaminobenzidine (0.05% in PBS with 0.018% H2O2).
Plaque assays.
Supernatants were subjected to serial 10-fold dilutions, inoculated onto confluent monolayers of Vero cells in 35-mm culture dishes, and incubated at 37°C for 60 minutes. To each well, 3 mL of neutral red-deficient minimum essential medium (Invitrogen) supplemented with 2% FBS, antibiotics, and 1% agar was added. Plates were incubated at 37°C for 2 days (HSV-1) or 3 days (WNV). Next, 3 mL of the same medium containing 0.22% neutral red was added to each well. Plaques were counted 24 hours later, and viral titers were expressed as plaque-forming units per milliliter (pfu/mL).
In vitro experimental infections.
HCE and Vero cell monolayers, approaching confluence in triplicate 25-cm2 flasks, were inoculated with WNV or HSV-1 at a multiplicity of infection (MOI) of 0.1. HSV-1 was used as a positive control because previous studies have demonstrated that this virus replicates in HCE cells.24 Vero cells were included because this cell line is susceptible to WNV infection.25 HCE and Vero cell monolayers inoculated with virus that had been heat inactivated (95°C for 2 hours) were used as negative controls (i.e., to assess whether the RT-PCR assays could detect input virus RNA). Cells were incubated for 1 hour at 25°C on an orbital shaker to allow virus attachment. The virus inoculum was removed, and the cell monolayers were rinsed twice using PBS and incubated in cell culture medium. At 1, 2, 3, 4, 5, and 6 days postinoculation (PI), cells were scraped from the flasks and clarified by centrifugation (10,000 g, 10 minutes, 4°C). Supernatants were collected and stored at −80°C. Cell pellets were resuspended in Trizol (Invitrogen) or lysing buffer and analyzed using RT-PCR or western blot, respectively.
Ex vivo experimental infections.
Human corneas were placed into individual 35-mm culture dishes containing 5 mL KSFM culture media supplemented with BPE, rEGF, antibiotics, and fungizone and incubated for 4 days at 37°C with 5% CO2 unless stated otherwise. Corneas were inoculated with WNV or HSV-1 at MOIs of 15 (in a total volume of 200 μL) and incubated for 1 hour at 25°C on an orbital shaker to allow virus attachment. Media was not removed from the culture dishes before virus inoculation due to the large number of free-floating cells that had detached from corneal preparations. Corneas were incubated for 5 days, and supernatant aliquots (100 μL) were harvested from each culture dish at 2, 3, 4, and 5 days PI. Each aliquot was clarified by centrifugation to remove free-floating cells from the preparation. At the final time point, selected corneas were collected, divided into equal halves, and homogenized in Trizol (Invitrogen) or lysing buffer using a mortar and pestle. Free-floating cells present in the culture dishes at the final time point were clarified by centrifugation and combined with the corneal tissues before homogenization and cell lysis.
Mouse experimental infections.
Nine 6- to 10-week-old C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were inoculated intraperitoneally with 300 pfu of WNV. Two mice inoculated with equal volumes of PBS were included as negative controls. At 2, 5, and 8 days postinfection, naive and WNV-infected mice were perfused with PBS. Eye, spleen, and brain tissues were collected and stored either in Trizol Reagent or RNAlater (Qiagen, Valencia, CA) followed by cobalt gamma irradiation (3 million rad) for virus inactivation. Spleen and brain tissues were also collected at days 5 and 8 and used as positive controls. These experiments were performed under biosafety level 3 conditions at the University of Texas Medical Branch. Eyes were transported on ice to the University of Iowa for processing. All animal experiments were carried out under a protocol (0902011) approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch. Upon arrival, to quantify viral genomes in different ocular compartments, each mouse eye was dissected into an anterior fraction containing cornea and posterior fraction containing retina, sclera, and choroid. Lens was not used in this analysis. Anterior and posterior fractions were diced with scissors, and RNA was extracted using the RNeasy Kit (Qiagen).
Results
Human corneal cells are susceptible to in vitro WNV infection.
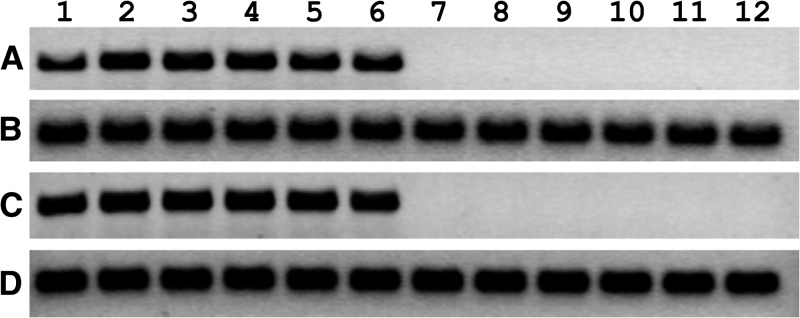
HCE cells were inoculated with WNV, incubated for 1–6 days, and assayed for evidence of viral infection using RT-PCR, western blot, and plaque assay. Cell cultures were monitored daily by phase contrast microscopy, and cytopathic effect was first observed at 3 days PI (data not shown). WNV RNA was detected by RT-PCR in supernatants collected from HCE cell cultures at every time point tested (Figure 1 ). The presence of WNV RNA was due to active viral replication rather than input virus from the initial inoculum because products of the expected size were not detected when cells were inoculated with heat-inactivated virus. As expected, WNV RNA was detected in all Vero cell cultures inoculated with viable virus, but not detected in any culture inoculated with heat-inactivated virus. Control RT-PCRs for human RPL11 and AGM β-actin were used to assure equal loading in each lane.
Figure 1.
Detection of West Nile virus (WNV) RNA using reverse transcription polymerase chain reaction (RT-PCR) in virus-inoculated human corneal epithelial (HCE) cells in vitro. Subconfluent monolayers of (A-B) HCE cells and (C-D) Vero cells in 25-cm2 flasks were inoculated with WNV at a multiplicity of infection of 0.1 (lanes 1–6) or they were inoculated with an equal amount of heat-inactivated virus (lanes 7–12). After 1 hour, the virus inocula were removed, and the cell monolayers were rinsed twice in phosphate-buffered saline and incubated in the appropriate cell culture media. Cells were homogenized in Trizol (Invitrogen) and total RNA was extracted at 1 (lanes 1 and 7), 2 (lanes 2 and 8), 3 (lanes 3 and 9), 4 (lanes 4 and 10), 5 (lanes 5 and 11), and 6 (lanes 6 and 12) days postinoculation. Equal amounts of total RNA (2 μg) were analyzed using RT-PCR with primers specific for (A–C) WNV, (B) human ribosomal protein RPL11, and (D) African green monkey β-actin.
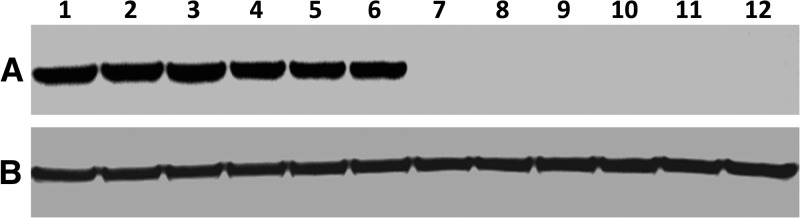
Cell lysates prepared from WNV-inoculated HCE cells were assayed by western blot using a pooled suspension of anti-WNV E protein monoclonal antibodies (Figure 2 ). Viral antigen was detected in all cell lysates harvested from cells inoculated with viable virus. In contrast, viral antigen was not detected when heat-inactivated virus was used. Western blots performed using anti-β-tubulin polyclonal antibody demonstrated that equal amounts of total protein were loaded into each lane. WNV antigen was also detected in all cell lysates prepared from Vero cells inoculated with viable virus, but not detected in any cell lysate inoculated with heat-inactivated virus (data not shown).
Figure 2.
Detection of West Nile virus (WNV) antigen using western blot in virus-inoculated human corneal epithelial (HCE) cells in vitro. Subconfluent monolayers of HCE cells in 25-cm2 flasks were inoculated with WNV at a multiplicity of infection of 0.1 (lanes 1–6) or an equal amount of heat-inactivated virus (lanes 7–12). After 1 hour, the virus inocula were removed, and the cells were rinsed twice in phosphate-buffered saline and incubated in the cell culture medium. Cell lysates were prepared at 1 (lanes 1 and 7), 2 (lanes 2 and 8), 3 (lanes 3 and 9), 4 (lanes 4 and 10), 5 (lanes 5 and 11), and 6 (lanes 6 and 12) days postinoculation. Equal amounts of total protein (8 μg) were resolved on 8–16% Tris-glycine gels and analyzed by western blot using (A) a pooled suspension of anti-WNV E protein monoclonal antibodies or (B) anti-human β-tubulin polyclonal antibody.
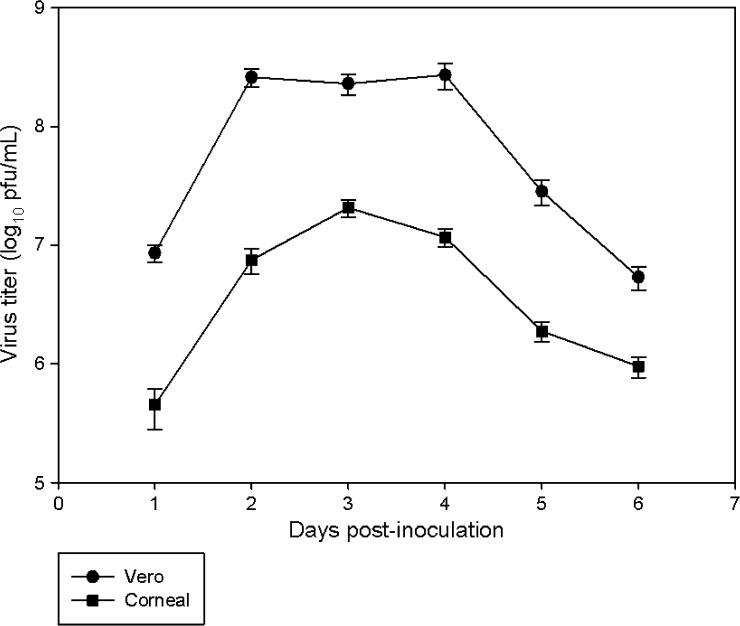
Plaque assays were performed to quantify the amount of WNV produced in HCE cells. The virus reached a peak titer of 2.5 × 107 pfu/mL at 3 days PI (Figure 3 ). When Vero cells were used, peak viral titers were approximately 10-fold higher as compared with HCE cells, and occurred at 2–4 days PI. HCE cells were also inoculated with HSV-1. Analysis of selected supernatants demonstrated that HSV-1 produced a peak titer of > 1 × 108 pfu/mL (data not shown).
Figure 3.
In vitro growth kinetics and yields of West Nile virus (WNV) in human corneal epithelial (HCE) and Vero cells. Subconfluent monolayers of HCE and Vero cells in 25-cm2 flasks were inoculated with WNV at a multiplicity of infection of 0.1 and incubated for 1–6 days. Supernatants were collected and viral titers were determined by plaque assay. The experiment was performed in triplicate, and each supernatant was tested six times. Data were used to calculate mean viral titers ±1 standard deviation.
Human corneal cells are susceptible to ex vivo WNV infection.
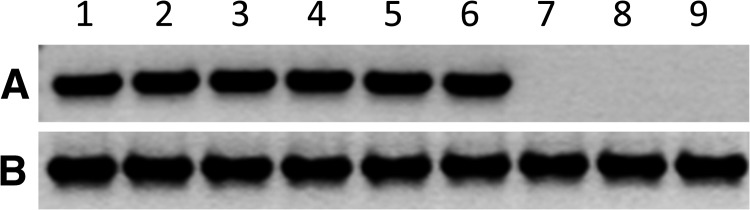
Ten paired corneas acquired from five donors (mean age = 64 years, range = 59–75 years), were placed into individual 35-mm culture dishes (one cornea per culture dish) and incubated in culture media for 4 days. Next, 3 × 104 pfu of WNV were added to each culture dish (MOI of 15 assuming 2 × 103 cells/cornea). Three additional corneas acquired from 74-year-old (N = 1) and 55-year-old (N = 2) donors were inoculated with heat-inactivated virus and used as negative controls. WNV RNA and antigen were detected by RT-PCR and western blot, respectively, in corneas inoculated with viable virus but not detected in corneas inoculated with heat-inactivated virus (Figures 4 ; data not shown).
Figure 4.
Detection of West Nile virus (WNV) RNA using reverse transcription polymerase chain reaction (RT-PCR) in virus-inoculated human corneal cells ex vivo. Intact human corneas were placed into individual 35-mm culture dishes that contained culture media and incubated for 4 days. Six corneas were inoculated with WNV at a multiplicity of infection of 15 (lanes 1–6). Another three corneas were inoculated with an equal amount of heat-inactivated virus (lanes 7–9). Corneas were incubated for 5 days and homogenized in Trizol (Invitrogen) using a mortar and pestle. Equal amounts of total RNA were subjected to RT-PCR using primers specific for (A) WNV or (B) human ribosomal protein RPL11.
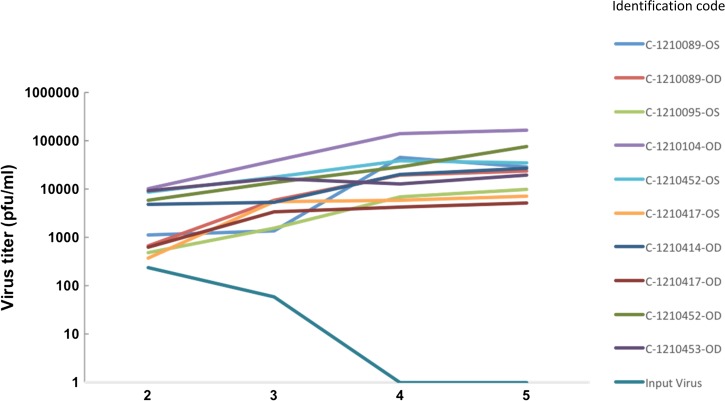
Plaque assays were performed to determine ex vivo growth kinetics and yields of WNV in human corneal cells. Every cornea inoculated with viable virus was susceptible to infection. Mean viral titers were 4.2 × 103, 1.1 × 104, 3.2 × 104, and 4.9 × 104 pfu/mL at 2, 3, 4, and 5 days PI, respectively, and peaked typically at 5 days PI (Figure 5 ). No virus was detected in corneas inoculated with heat-inactivated virus. Negative control culture dishes containing media and WNV but no corneal tissue yielded mean virus titers of 2.4 × 102 and 5.9 × 101 pfu/mL at 2 and 3 days PI, respectively, while no virus was detected at 4 and 5 days PI (Figure 5). Positive control corneas inoculated with HSV-1 demonstrated efficient HSV-1 replication with a mean peak viral titer of 2.3 × 106 pfu/mL at 5 days PI (data not shown).
Figure 5.
Ex vivo growth kinetics and yields of West Nile virus (WNV) in human corneal cells. Ten intact human corneas were placed into individual 35-mm culture dishes that contained culture media, incubated for 4 days, and inoculated with WNV at a multiplicity of infection of 15. Culture dishes that contained media in the absence of corneal cells were also inoculated with WNV to assess the longevity of the input virus. At 2, 3, 4, and 5 days postinoculation, an aliquot of each cell culture supernatant was collected from each culture dish, and virus titers were determined by plaque assay.
Dissemination of WNV to corneas of experimentally infected mice.
Nine WNV-infected mice and two uninfected mice were used to examine WNV expression in anterior and posterior ocular tissue fractions. Quantitative PCR detected WNV expression in both anterior and posterior fractions in four infected mice, and only the posterior fraction in one infected mouse. No WNV expression was detected in four infected mice and both uninfected mice.
Discussion
We provide evidence that human corneal cells are susceptible to WNV infection in vitro and ex vivo and report detection of viral nucleic acid in the anterior fraction of eyes harvested from experimentally infected mice. These findings indicate that corneal transplantation cannot be ruled out as a novel mode of human-to-human WNV transmission. The risk of acquiring WNV disease through corneal transplantation could be considered negligible given that no such cases have been documented. However, our data indicate that additional experiments should be conducted to assess this issue further and that screening of potential donors for evidence of WNV infection may warrant further consideration. There have been no reports to date of viral detection in corneas of WNV-seropositive donors. However, other viruses have been transmitted by corneal transplantation including HSV-1,26 HBV,27 and rabies virus,28,29 and evidence suggests that HCV, HIV-1, and chikungunya virus—a mosquito-borne alphavirus—also have the potential to be transmitted by this route.30–32
WNV has been associated with various human ophthalmic manifestations including optic neuritis, chorioretinitis, retinal hemorrhage, vitritis, retinal vascular leakage, and retinal detachment.33–38 It has been proposed that WNV disseminates from the central nervous system to the outer retina, retinal pigment epithelium (RPE), and choroid via optic nerve fibers.37
However, WNV has never been detected in optic nerve fibers or corneas procured from deceased humans with systemic infection. This may indicate that WNV cannot replicate in or disseminate into these tissues. Ocular tissues from deceased WNV patients need to be acquired and assayed for evidence of viral infection to better understand the pathogenesis of ocular involvement in WNV infection and more conclusively determine the likelihood of WNV transmission during corneal transplantation. We contacted several eye banks in regions of the U.S. where the incidence of WNV disease is particularly high in an attempt to acquire corneas from patients that died as a result of WNV disease, but were unable to obtain corneas from any patients that met this criterion. We did obtain and test a pair of corneas from a 70-year-old man diagnosed with WNF in 2006, who died of cardiac arrest in 2014. WNV RNA was not detected in the corneas by qRT-PCR (data not shown). This result was expected because WNV was not the cause of death and the infection was remote.
Our experiments do not demonstrate directly that WNV can traverse the optic nerve and disseminate into the cornea of infected donors. WNV was applied directly to corneal cells in culture dishes, allowing immediate contact between the virus and cells to take place. Previous studies have demonstrated that human RPE cell cultures are also susceptible to WNV infection.39 To address this issue, we evaluated WNV's ability to disseminate into corneas of mice infected systemically. The murine cornea provides a suitable surrogate because it is also avascular.40 However, our murine experiment also has limitations. It is possible that anterior fractions were WNV positive, because they were contaminated with trace amounts of infected vascular tissue. Although the availability of a stable green fluorescent protein (GFP)–expressing WNV complementary DNA infectious clone can be exploited to define the cellular distribution of the virus within murine ocular tissues,41–44 such clones are not suitable for in vivo murine work because the GFP insertion is unstable and attenuates the virus.45,46 While immunohistochemical (IHC) staining can be used to define more accurately the distribution of viral antigens within tissues, our IHC analysis demonstrated that several different anti-WNV antibodies cross-reacted with endogenous mouse proteins (data not shown). Performing keratoplasty using WNV-infected donor mice and uninfected recipient mice may determine more conclusively the likelihood of WNV transmission due to corneal transplantation.
WNV has been associated with visual impairment and ocular lesions including optic neuritis, anterior uveitis, and chorioretinitis in birds with naturally acquired infections.47–49 Studies have also documented the presence of WNV antigen and live virus in eyes harvested from infected birds.47–53 For instance, WNV antigen was detected in the retinas of six of eight red-tailed hawks and three of three Cooper's hawks.47 Retinas were the only ocular tissues to test positive. In another study, 16.7% of eyes harvested from North American owls contained viral antigen, which was usually detected in fibrocytes of the deep scleral layers.53 The corneas of many owls stained weakly positive for WNV antigen by IHC analysis, but these were considered to be false-positive results. There are no reports describing the detection of WNV in the corneas or optic nerves of infected birds of any species.
Efficient replication of WNV occurred in corneas procured from deceased donors, and the virus produced a mean peak titer 5 days PI. The peak titer occurred at the final time point tested, and therefore higher titers may have been reported had samples been collected over a longer period. Of note, we found that virus replication was more efficient when corneas are preincubated in culture media for 4 days before virus challenge, and the virus inocula not removed from the culture dishes, compared with assays performed without these measures. Fifteen corneas (from 12 patients) that had not been preincubated were inoculated with WNV for 5 days. Three corneas yielded maximum viral titers of 63–322 pfu/mL while the others did not support virus replication (data not shown). Two corneas (from two patients) were inoculated with HSV-1 without the preincubation measures; one yielded a maximum viral titer of 7.2 × 104 pfu/mL while the other did not support virus replication. When the preincubation step was included, HSV-1 titers were approximately 1,000-fold higher (data not shown). By the end of the preincubation period, many cells had detached from the cornea and were floating in the culture media. It is likely that the free-floating cells were more accessible to the virus compared with the cells clumped tightly together in a solid mass, thereby resulting in more efficient virus replication. The clinical impact of these findings is unknown currently.
In conclusion, the experiments reported in this study indicate that human corneal cells support WNV replication in vitro and ex vivo, and that WNV may disseminate into the corneas of experimentally infected mice. However, it is not possible to conclude from this study that WNV can be detected in corneal tissue from infected human donors or can disseminate from a donor cornea to a transplant recipient, and thus the risk of WNV transmission to human patients undergoing corneal transplantation remains unknown. To provide a more informative risk assessment, corneal tissues need to be acquired from deceased WNV patients and assayed for the presence of this virus. The ability to maintain an adequate supply of corneas that are safe for transplantation without adding unnecessary costs to the health-care system is crucial to protecting the public. To increase our understanding of the potential risks associated with tissue transplantation and help guide future policies regarding WNV infection and corneal transplantation, we recommend additional preliminary investigations to address this important public health concern.
ACKNOWLEDGMENTS
We thank Adam Stockman and Greg Schmidt from the Iowa Lions Eye Bank for providing technical assistance.
Footnotes
Financial support: This study was supported by a grant from the Iowa Lions Eye Bank.
Authors' addresses: Bradley J. Blitvich, Department of Veterinary Microbiology and Preventive Medicine, College of Veterinary Medicine, Iowa State University, Ames, IA, E-mail: blitvich@iastate.edu. Tian Wang, Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston, TX, Department of Pathology, University of Texas Medical Branch, Galveston, TX, and Sealy Center for Vaccine Development, University of Texas Medical Branch, Galveston, TX, E-mail: ti1wang@utmb.edu. Vandana Saxena, Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston, TX, and Department of Immunology, National AIDS Research Institute, Pune, Maharashtra, India, E-mail: vandysaxena@gmail.com. Shemin Zeng and Robert F. Mullins, Department of Ophthalmology and Visual Sciences, Carver College of Medicine, University of Iowa, Iowa City, IA, and The Stephen A. Wynn Institute for Vision Research, University of Iowa, Iowa City, IA, E-mails: shemin-zeng@uiowa.edu and robert-mullins@uiowa.edu. Karen M. Harmon, Department of Veterinary Diagnostic and Production Animal Medicine, College of Veterinary Medicine, Iowa State University, Ames, IA, E-mail: kharmon@iastate.edu. Matthew D. Raymond, Department of Veterinary Diagnostic and Production Animal Medicine, College of Veterinary Medicine, Iowa State University, Ames, IA, and Wisconsin National Primate Research Center, University of Wisconsin-Madison, Madison, WI, E-mail: mdraymond@wisc.edu. Kenneth M. Goins, Department of Ophthalmology and Visual Sciences, Carver College of Medicine, University of Iowa, Iowa City, IA, and Iowa Lions Eye Bank, Coralville, IA, E-mail: kenneth-goins@uiowa.edu. Cynthia R. Reed, Iowa Lions Eye Bank, Coralville, IA, E-mail: cynthia-reed@uiowa.edu. Mark A. Greiner, Department of Ophthalmology and Visual Sciences, Carver College of Medicine, University of Iowa, Iowa City, IA, The Stephen A. Wynn Institute for Vision Research, University of Iowa, Iowa City, IA, and Iowa Lions Eye Bank, Coralville, IA, E-mail: mark-greiner@uiowa.edu.
References
- 1.Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310:308–315. doi: 10.1001/jama.2013.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciota AT, Kramer LD. Vector-virus interactions and transmission dynamics of West Nile virus. Viruses. 2013;5:3021–3047. doi: 10.3390/v5123021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colpitts TM, Conway MJ, Montgomery RR, Fikrig E. West Nile virus: biology, transmission, and human infection. Clin Microbiol Rev. 2012;25:635–648. doi: 10.1128/CMR.00045-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou S, Foster GA, Dodd RY, Petersen LR, Stramer SL. West Nile fever characteristics among viremic persons identified through blood donor screening. J Infect Dis. 2010;202:1354–1361. doi: 10.1086/656602. [DOI] [PubMed] [Google Scholar]
- 5.Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe KE, Crabtree MB, Scherret JH, Hall RA, MacKenzie JS, Cropp CB, Panigrahy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage HM, Stone W, McNamara T, Gubler DJ. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 6.Petersen LR, Carson PJ, Biggerstaff BJ, Custer B, Borchardt SM, Busch MP. Estimated cumulative incidence of West Nile virus infection in US adults, 1999–2010. Epidemiol Infect. 2013;141:591–595. doi: 10.1017/S0950268812001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwamoto M, Jernigan DB, Guasch A, Trepka MJ, Blackmore CG, Hellinger WC, Pham SM, Zaki S, Lanciotti RS, Lance-Parker SE, DiazGranados CA, Winquist AG, Perlino CA, Wiersma S, Hillyer KL, Goodman JL, Marfin AA, Chamberland ME, Petersen LR. West Nile Virus in Transplant Recipients Investigation Team Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med. 2003;348:2196–2203. doi: 10.1056/NEJMoa022987. [DOI] [PubMed] [Google Scholar]
- 8.Pealer LN, Marfin AA, Petersen LR, Lanciotti RS, Page PL, Stramer SL, Stobierski MG, Signs K, Newman B, Kapoor H, Goodman JL, Chamberland ME. West Nile Virus Transmission Investigation T Transmission of West Nile virus through blood transfusion in the United States in 2002. N Engl J Med. 2003;349:1236–1245. doi: 10.1056/NEJMoa030969. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Possible West Nile virus transmission to an infant through breast-feeding—Michigan, 2002. MMWR. 2002;51:877–878. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Intrauterine West Nile virus infection—New York, 2002. MMWR. 2002;51:1135–1136. [PubMed] [Google Scholar]
- 11.Winston DJ, Vikram HR, Rabe IB, Dhillon G, Mulligan D, Hong JC, Busuttil RW, Nowicki MJ, Mone T, Civen R, Tecle SA, Trivedi KK, Hocevar SN. West Nile Virus Transplant-Associated Transmission Investigation Team Donor-derived West Nile virus infection in solid organ transplant recipients: report of four additional cases and review of clinical, diagnostic, and therapeutic features. Transplantation. 2014;97:881–889. doi: 10.1097/TP.0000000000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention West Nile virus infection in organ donor and transplant recipients—Georgia and Florida, 2002. MMWR. 2002;51:790. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention West Nile virus infections in organ transplant recipients—New York and Pennsylvania, August–September, 2005. MMWR. 2005;54:1021–1023. [PubMed] [Google Scholar]
- 14.Rabe IB, Schwartz BS, Farnon EC, Josephson SA, Webber AB, Roberts JP, de Mattos AM, Gallay BJ, van Slyck S, Messenger SL, Yen CJ, Bloch EM, Drew CP, Fischer M, Glaser CA. WNV Transplant Investigation Team Fatal transplant-associated West Nile virus encephalitis and public health investigation—California, 2010. Transplantation. 2013;96:463–468. doi: 10.1097/TP.0b013e31829b4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yango AF, Fischbach BV, Levy M, Chandrakantan A, Tan V, Spak C, Melton L, Rice K, Barri Y, Rajagopal A, Klintmalm G. West Nile virus infection in kidney and pancreas transplant recipients in the Dallas-Fort Worth Metroplex during the 2012 Texas epidemic. Transplantation. 2014;97:953–957. doi: 10.1097/01.TP.0000438621.81686.ab. [DOI] [PubMed] [Google Scholar]
- 16.Busch MP, Caglioti S, Robertson EF, McAuley JD, Tobler LH, Kamel H, Linnen JM, Shyamala V, Tomasulo P, Kleinman SH. Screening the blood supply for West Nile virus RNA by nucleic acid amplification testing. N Engl J Med. 2005;353:460–467. doi: 10.1056/NEJMoa044029. [DOI] [PubMed] [Google Scholar]
- 17.Stramer SL, Fang CT, Foster GA, Wagner AG, Brodsky JP, Dodd RY. West Nile virus among blood donors in the United States, 2003 and 2004. N Engl J Med. 2005;353:451–459. doi: 10.1056/NEJMoa044333. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention West Nile virus transmission through blood transfusion—South Dakota, 2006. MMWR. 2007;56:76–79. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention Fatal West Nile virus infection after probable transfusion-associated transmission—Colorado, 2012. MMWR. 2013;62:622–624. [PMC free article] [PubMed] [Google Scholar]
- 20.Montgomery SP, Brown JA, Kuehnert M, Smith TL, Crall N, Lanciotti RS, Macedo de Oliveira A, Boo T, Marfin AA. West Nile Virus Transfusion-Associated Transmission Investigation T Transfusion-associated transmission of West Nile virus, United States 2003 through 2005. Transfusion. 2006;46:2038–2046. doi: 10.1111/j.1537-2995.2006.01030.x. [DOI] [PubMed] [Google Scholar]
- 21.Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng S, Hernandez J, Mullins RF. Effects of antioxidant components of AREDS vitamins and zinc ions on endothelial cell activation: implications for macular degeneration. Invest Ophthalmol Vis Sci. 2012;53:1041–1047. doi: 10.1167/iovs.11-8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Athmanathan S, Reddy SB, Nutheti R, Rao GN. Comparison of an immortalized human corneal epithelial cell line with Vero cells in the isolation of Herpes simplex virus-1 for the laboratory diagnosis of herpes simplex keratitis. BMC Ophthalmol. 2002;2:3. doi: 10.1186/1471-2415-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Way JH, Bowen ET, Platt GS. Comparative studies of some African arboviruses in cell culture and in mice. J Gen Virol. 1976;30:123–130. doi: 10.1099/0022-1317-30-1-123. [DOI] [PubMed] [Google Scholar]
- 26.Remeijer L, Maertzdorf J, Doornenbal P, Verjans GM, Osterhaus AD. Herpes simplex virus 1 transmission through corneal transplantation. Lancet. 2001;357:442. doi: 10.1016/S0140-6736(00)04011-3. [DOI] [PubMed] [Google Scholar]
- 27.Hoft RH, Pflugfelder SC, Forster RK, Ullman S, Polack FM, Schiff ER. Clinical evidence for hepatitis B transmission resulting from corneal transplantation. Cornea. 1997;16:132–137. [PubMed] [Google Scholar]
- 28.Houff SA, Burton RC, Wilson RW, Henson TE, London WT, Baer GM, Anderson LJ, Winkler WG, Madden DL, Sever JL. Human-to-human transmission of rabies virus by corneal transplant. N Engl J Med. 1979;300:603–604. doi: 10.1056/NEJM197903153001105. [DOI] [PubMed] [Google Scholar]
- 29.Javadi MA, Fayaz A, Mirdehghan SA, Ainollahi B. Transmission of rabies by corneal graft. Cornea. 1996;15:431–433. doi: 10.1097/00003226-199607000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Lee HM, Naor J, Alhindi R, Chinfook T, Krajden M, Mazzulli T, Rootman DS. Detection of hepatitis C virus in the corneas of seropositive donors. Cornea. 2001;20:37–40. doi: 10.1097/00003226-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Qavi HB, Green MT, SeGall GK, Lewis DE, Hollinger FB. Frequency of dual infections of corneas with HIV-1 and HHV-6. Curr Eye Res. 1992;11:315–323. doi: 10.3109/02713689209001785. [DOI] [PubMed] [Google Scholar]
- 32.Couderc T, Gangneux N, Chretien F, Caro V, Le Luong T, Ducloux B, Tolou H, Lecuit M, Grandadam M. Chikungunya virus infection of corneal grafts. J Infect Dis. 2012;206:851–859. doi: 10.1093/infdis/jis296. [DOI] [PubMed] [Google Scholar]
- 33.Garg S, Jampol LM. Systemic and intraocular manifestations of West Nile virus infection. Surv Ophthalmol. 2005;50:3–13. doi: 10.1016/j.survophthal.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Khairallah M, Ben Yahia S, Ladjimi A, Zeghidi H, Ben Romdhane F, Besbes L, Zaouali S, Messaoud R. Chorioretinal involvement in patients with West Nile virus infection. Ophthalmology. 2004;111:2065–2070. doi: 10.1016/j.ophtha.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 35.Sivakumar RR, Prajna L, Arya LK, Muraly P, Shukla J, Saxena D, Parida M. Molecular diagnosis and ocular imaging of West Nile virus retinitis and neuroretinitis. Ophthalmology. 2013;120:1820–1826. doi: 10.1016/j.ophtha.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Learned D, Nudleman E, Robinson J, Chang E, Stec L, Faia LJ, Wolfe J, Williams GA. Multimodal imaging of West Nile virus chorioretinitis. Retina. 2014;34:2269–2274. doi: 10.1097/IAE.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 37.Khairallah M, Ben Yahia S, Attia S, Zaouali S, Ladjimi A, Messaoud R. Linear pattern of West Nile virus-associated chorioretinitis is related to retinal nerve fibres organization. Eye (Lond) 2007;21:952–955. doi: 10.1038/sj.eye.6702355. [DOI] [PubMed] [Google Scholar]
- 38.Vandenbelt S, Shaikh S, Capone A, Jr, Williams GA. Multifocal choroiditis associated with West Nile virus encephalitis. Retina. 2003;23:97–99. doi: 10.1097/00006982-200302000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Cinatl J, Jr, Michaelis M, Fleckenstein C, Bauer G, Kabickova H, Scholz M, Rabenau HF, Doerr HW. West Nile virus infection induces interferon signalling in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:645–651. doi: 10.1167/iovs.05-1022. [DOI] [PubMed] [Google Scholar]
- 40.Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJC, Richter E, Sakurai E, Newcomb MT, Kleinman ME, Caldwell RB, Lin Q, Ogura Y, Orecchia A, Samuelson DA, Agnew DW, St Leger J, Green WR, Mahasreshti PJ, Curiel DT, Kwan D, Marsh H, Ikeda S, Leiper LJ, Collinson JM, Bogdanovich S, Khurana TS, Shibuya M, Baldwin ME, Ferrara N, Gerber HP, De Falco S, Witta J, Baffi JZ, Raisler BJ, Ambati J. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ackermann A, Guelzow T, Staeheli P, Schneider U, Heimrich B. Visualizing viral dissemination in the mouse nervous system, using a green fluorescent protein-expressing Borna disease virus vector. J Virol. 2010;84:5438–5442. doi: 10.1128/JVI.00098-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Das Sarma J, Scheen E, Seo SH, Koval M, Weiss SR. Enhanced green fluorescent protein expression may be used to monitor murine coronavirus spread in vitro and in the mouse central nervous system. J Neurovirol. 2002;8:381–391. doi: 10.1080/13550280260422686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manicassamy B, Manicassamy S, Belicha-Villanueva A, Pisanelli G, Pulendran B, Garcia-Sastre A. Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proc Natl Acad Sci USA. 2010;107:11531–11536. doi: 10.1073/pnas.0914994107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ebihara H, Theriault S, Neumann G, Alimonti JB, Geisbert JB, Hensley LE, Groseth A, Jones SM, Geisbert TW, Kawaoka Y, Feldmann H. In vitro and in vivo characterization of recombinant Ebola viruses expressing enhanced green fluorescent protein. J Infect Dis. 2007;196((Suppl 2)):S313–S322. doi: 10.1086/520590. [DOI] [PubMed] [Google Scholar]
- 45.Pierson TC, Diamond MS, Ahmed AA, Valentine LE, Davis CW, Samuel MA, Hanna SL, Puffer BA, Doms RW. An infectious West Nile virus that expresses a GFP reporter gene. Virology. 2005;334:28–40. doi: 10.1016/j.virol.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 46.McGee CE, Shustov AV, Tsetsarkin K, Frolov IV, Mason PW, Vanlandingham DL, Higgs S. Infection, dissemination, and transmission of a West Nile virus green fluorescent protein infectious clone by Culex pipiens quinquefasciatus mosquitoes. Vector Borne Zoonotic Dis. 2010;10:267–274. doi: 10.1089/vbz.2009.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pauli AM, Cruz-Martinez LA, Ponder JB, Redig PT, Glaser AL, Klauss G, Schoster JV, Wunschmann A. Ophthalmologic and oculopathologic findings in red-tailed hawks and Cooper's hawks with naturally acquired West Nile virus infection. J Am Vet Med Assoc. 2007;231:1240–1248. doi: 10.2460/javma.231.8.1240. [DOI] [PubMed] [Google Scholar]
- 48.Wunschmann A, Shivers J, Bender J, Carroll L, Fuller S, Saggese M, van Wettere A, Redig P. Pathologic findings in red-tailed hawks (Buteo jamaicensis) and Cooper's hawks (Accipiter cooper) naturally infected with West Nile virus. Avian Dis. 2004;48:570–580. doi: 10.1637/7170-022004R. [DOI] [PubMed] [Google Scholar]
- 49.Wunschmann A, Shivers J, Bender J, Carroll L, Fuller S, Saggese M, van Wettere A, Redig P. Pathologic and immunohistochemical findings in goshawks (Accipiter gentilis) and great horned owls (Bubo virginianus) naturally infected with West Nile virus. Avian Dis. 2005;49:252–259. doi: 10.1637/7297-103104R. [DOI] [PubMed] [Google Scholar]
- 50.Nemeth N, Gould D, Bowen R, Komar N. Natural and experimental West Nile virus infection in five raptor species. J Wildl Dis. 2006;42:1–13. doi: 10.7589/0090-3558-42.1.1. [DOI] [PubMed] [Google Scholar]
- 51.Nemeth NM, Hahn DC, Gould DH, Bowen RA. Experimental West Nile virus infection in eastern Screech Owls (Megascops asio) Avian Dis. 2006;50:252–258. doi: 10.1637/7466-110105R1.1. [DOI] [PubMed] [Google Scholar]
- 52.Lim AK, Dunne G, Gurfield N. Rapid bilateral intraocular cocktail sampling method for West Nile virus detection in dead corvids. J Vet Diagn Invest. 2009;21:516–519. doi: 10.1177/104063870902100414. [DOI] [PubMed] [Google Scholar]
- 53.Gancz AY, Smith DA, Barker IK, Lindsay R, Hunter B. Pathology and tissue distribution of West Nile virus in North American owls (family: Strigidae) Avian Pathol. 2006;35:17–29. doi: 10.1080/03079450500465676. [DOI] [PubMed] [Google Scholar]