Abstract
Background
Blood biomarkers for ischemic and hemorrhagic stroke diagnosis remain elusive. Recent investigations suggested that apolipoprotein (Apo), matrix metalloproteinase (MMP), and paraoxonase-1 may be associated with stroke. We hypothesized that Apo A-I, Apo C-I, Apo C-III, MMP-3, MMP-9, and paraoxonase-1 are differentially expressed in ischemic stroke, hemorrhagic stroke, and controls.
Methods
In a single-center prospective observational study, consecutive stroke cases were enrolled if blood samples were obtainable within 12 hours of symptom onset. Age- (±5 years), race-, and sex-matched controls were recruited. Multiplex assays were used to measure protein levels. The Wilcoxon signed-rank test and the Mann–Whitney U-test were used to compare biomarker values between ischemic stroke patients and controls, hemorrhagic stroke patients and controls, and ischemic and hemorrhagic stroke patients. The 95% confidence intervals (CIs) for the difference of 2 medians were calculated.
Results
Fourteen ischemic stroke case–control pairs and 23 intracerebral hemorrhage (ICH) case–control pairs were enrolled. Median Apo A-I levels were lower in ischemic stroke cases versus controls (140 mg/dL versus 175 mg/dL, difference of 35 mg/dL, 95% CI −54 to −16) and in ischemic stroke versus ICH cases (140 mg/dL versus 180 mg/dL, difference of 40 mg/dL, 95% CI −57 to −23). Median paraoxonase-1 was lower in ischemic stroke cases than in both ICH cases and matched controls. Median Apo C-I was slightly lower in ischemic stroke cases than in ICH cases. There were no differences between groups for MMP-3, MMP-9, and Apo C-III.
Conclusion
Apo A-I and paraoxonase-1 levels may be clinically useful for ischemic stroke diagnosis and for differentiating between ischemic and hemorrhagic strokes.
Keywords: Ischemic stroke, intracerebral hemorrhage, biomarker, diagnosis, proteomics, plasma
Introduction
Every 40 seconds someone in the United States has a stroke.1 Ischemic stroke is misdiagnosed in over a third of cases during the initial presentation.2 This rises to two thirds when patients present with only nonspecific symptoms such as generalized weakness, altered mental status, abnormal gait, and dizziness.3 The current diagnostic and treatment paradigm includes neuroimaging to first distinguish ischemic from hemorrhagic stroke. This delay, along with early misdiagnosis and other factors, likely contributes to the 4% annual thrombolytic treatment rate in patients with acute ischemic stroke.4
Blood biomarkers that reliably diagnose stroke, and that could differentiate between hemorrhagic and ischemic stroke, have the potential to ameliorate the current diagnostic challenges and provide for the best possible stroke outcomes. Based on our prior findings5 and supportive evidence in the literature,6–16 we tested the following 6 potential biomarkers for their potential utility in stroke diagnosis: paraoxonase-1, matrix metalloproteinase (MMP)-3, MMP-9, apolipoprotein (Apo) A-I, Apo C-I, and Apo C-III.
Materials and Methods
The present study was an observational case–control study conducted between August 2013 and May 2014 at an urban tertiary care hospital. Study procedures were approved by the local institutional review board. Potential cases were identified from pages to the stroke team, as well as 24/7 screening of the emergency department population, neurology floor beds, and neurointensive care unit by hospital-based research assistants. Inclusion criteria were age 18 years or older, acute ischemic stroke, and a primary spontaneous intracerebral hemorrhage (ICH) diagnosed clinically and by neuroimaging (i.e., no radiographic features suggestive of aneurysmal subarachnoid hemorrhage, traumatic contusion, vascular malformation, tumor, or venous sinus thrombosis). Exclusion criteria included a recently documented (<2 weeks) history of myocardial infarction, ischemic or hemorrhagic stroke, surgery, or hospitalization; acute ischemic stroke patients already treated with thrombolysis or endovascular reperfusion therapy; known active malignant cancer, lymphoma, or leukemia; a history of a chronic inflammatory disorder; current treatment with anticoagulants; congenital/heritable disorder of coagulation/hemostasis; liver disease/cirrhosis; and participation in an acute interventional stroke trial. Consecutive cases were prospectively enrolled if blood samples were obtainable within 12 hours of symptom onset.
Controls were matched based on age (±5 years), race, and gender and were recruited from hospital staff and well visitors to the hospital using the same inclusion and exclusion criteria.
Once participants or their legally authorized representatives provided signed, informed consent, they were asked about their medical history pursuant to stroke risk factors, including history of smoking, diabetes, hypertension, coronary artery disease, atrial fibrillation, and prior stroke. Venipuncture was performed for collection of a venous blood specimen.
Specimen Collection and Processing
Seven milliliters of venous blood was drawn into trace metal (Kendall Monoject) heparin tubes. Within 2 hours, the mixture was centrifuged for 10 minutes at 2000 × g at room temperature and the supernatant plasma was aliquoted into cryovials and frozen at −80°C until the time of laboratory analysis. Commercially available multiplex assays at the Clinical Laboratory Improvement Amendments-certified laboratory of Myriad RBM (Austin, TX) were used. The protein levels measured were paraoxonase-1, MMP-3, MMP-9, Apo A-I, Apo C-I, and Apo C-III. Given the known associations between Apo A-I and high-density lipoprotein cholesterol (HDL-C) and serum amyloid A (SAA), we also measured these variables to determine their likely impact on interpreting the association between Apo A-I and stroke. HDL-C was measured by the clinical laboratory. SAA levels were determined using commercially available enzymelinked immunosorbent assays (Abcam, Cambridge, MA).
Statistical Analyses
The Wilcoxon signed-rank test and the Mann–Whitney U-test were used to compare biomarker values between groups, and the 95% confidence intervals (CIs) for the difference of 2 medians were calculated.17 Matched pairs (dependent) t-tests and independent samples t-tests were used to compare high-density lipoprotein (HDL) and SAA levels groups. Pearson’s correlation coefficient was used to evaluate the strength of association between 2 variables. Analyses were conducted using SPSS 22.0 (IBM Corporation, Armonk, NY). Graphics were created using R (2.15.3), package “beeswarm.”18
Results
Fourteen ischemic stroke case–control pairs and 23 ICH case–control pairs were enrolled. The mean age for ischemic stroke patients was 66 (standard deviation [SD] 10) years, 43% were female, and 7% were African American. For ICH patients, the mean age was 63 (SD 15) years, 61% were female, and 52% were African American (Table 1). The median time from stroke symptom onset to blood draw for all cases was 4.5 hours (interquartile range 3.7–10.2 hours).
Table 1.
Demographics and past medical history by study group
| Ischemic | ICH | |||
|---|---|---|---|---|
| Characteristics | Control (n = 14) | Case (n = 14) | Control (n = 23) | Case (n = 23) |
| Age—mean (SD) | 63 (10) | 66 (10) | 63 (14) | 63 (15) |
| African-American—n (%) | 1 (7.1) | 1 (7.1) | 12 (52.2) | 12 (52.2) |
| Female—n (%) | 6 (42.9) | 6 (42.9) | 14 (60.9) | 14 (60.9) |
| Past medical history—n (%) | ||||
| Hypertension | 10 (71.4) | 9 (64.3) | 12 (52.2) | 17 (73.9) |
| Past or current smoker | 5 (35.7) | 7 (50.0) | 12 (52.2) | 13 (56.5) |
| Diabetes mellitus | 4 (28.6) | 7 (50.0) | 2 (8.7) | 8 (34.8) |
| Prior ischemic stroke | 2 (14.3) | 3 (21.4) | 0 (.0) | 2 (8.7) |
| Coronary artery disease | 1 (7.1) | 2 (14.3) | 1 (4.3) | 0 (.0) |
| Prior hemorrhagic stroke | 0 (.0) | 0 (.0) | 0 (.0) | 1 (4.3) |
Abbreviations: ICH, intracerebral hemorrhage; SD, standard deviation.
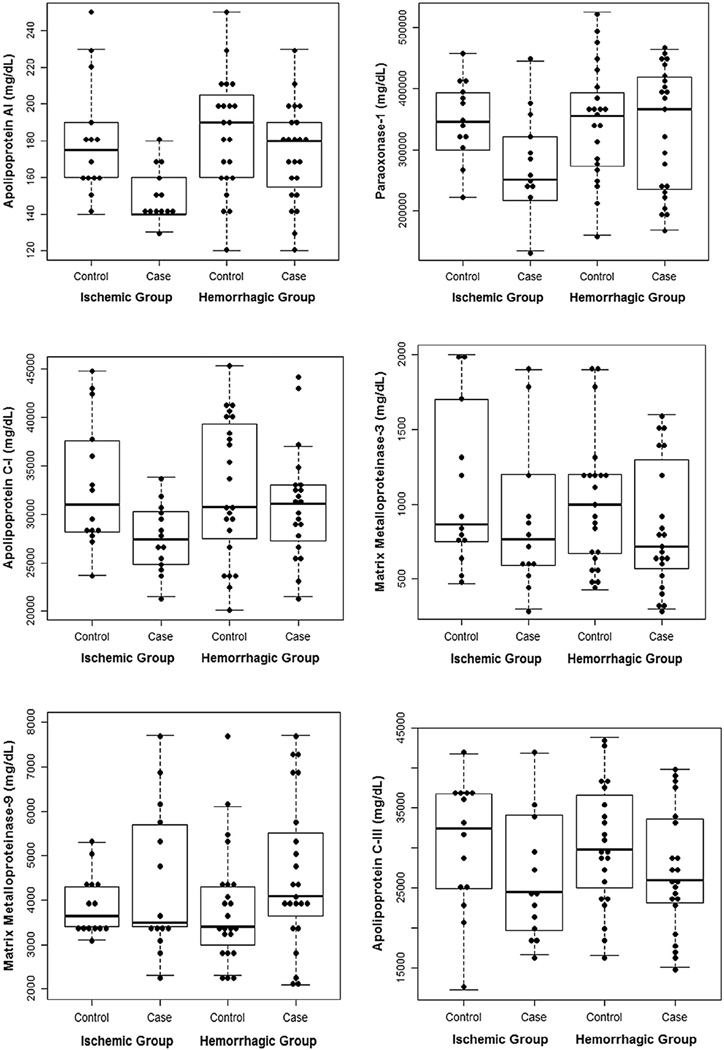
Median Apo A-I was lower in ischemic stroke cases (140 mg/dL) than in controls (175 mg/dL), with a difference of 35 mg/dL and a 95% CI of −54 to −16. Median Apo A-I was also lower in ischemic stroke cases (140 mg/dL) than in ICH cases (180 mg/dL) (difference 40 mg/dL, 95% CI −57 to −23; Fig 1, a). Median paraoxonase-1 level was lower in ischemic stroke cases (250,500 mg/dL) than in controls (345,500 mg/dL) (difference 95,000 mg/dL, 95% CI −166,960 to −23,040). Median paraoxonase-1 level was also lower in ischemic stroke cases (250,500 mg/dL) than in ICH cases (366,000 mg/dL) (difference 115,500 mg/dL, 95% CI −212,046 to −18,954; Fig 1, b). Median Apo C-1 was slightly lower in ischemic stroke cases (27,400 mg/dL) than in ICH cases (31,100 mg/dL) (difference 3700 mg/dL, 95% CI −7335 to −65; Fig 1, c). There were no differences between groups for MMP-3, MMP-9, and Apo C-III (Fig 1, d–f).
Figure 1.
Box plot + dot plot showing the distribution of protein levels by study group.
HDL-C levels were positively correlated with Apo A-I levels for all subjects. Pearson’s correlation coefficient for ischemic stroke cases was .78, for ischemic controls was .86, for ICH cases was .82, and for ICH controls was .89.
Mean HDL-C was lower in ischemic stroke cases (41 mg/dL, SD 8) than in controls (51 mg/dL, SD 10) (difference 10, 95% CI 3–17, P = .011), and marginally different between ischemic stroke cases (41 mg/dL, SD 8) and ICH cases (48 mg/dL, SD 12) (difference 7, 95% CI −.4 to 15, P = .063). There was no difference between ICH cases (48 mg/dL, SD 12) and ICH controls (54 mg/dL, SD 18) (difference 6, 95% CI −4 to 16, P = .208). Mean SAA levels were higher in ischemic stroke cases (38 ng/mL, SD 13) than in controls (29 ng/mL, SD 7) (difference 8, 95% CI .8–16, P = .033). Mean levels were not different between ICH cases (33 ng/mL, SD 25) and ICH controls (30 ng/mL, SD 9) (difference 4, 95% CI −16 to 7, P = .450), or between ischemic and ICH cases (difference 5, 95% CI −19 to 10, P = .538).
Discussion
We found that Apo A-I and paraoxonase-1 levels differed between ischemic stroke cases and controls, and between ischemic stroke cases and ICH cases. Apo C-I levels were also lower in ischemic stroke cases than in ICH cases. MMP-3, MMP-9, and Apo C-III levels did not differentiate between cases and controls. Importantly, our blood samples were drawn at a median time of 4.5 hours from symptom onset. Overall, our findings suggest that, in the setting of acute stroke, ascertainment of Apo A-I and paraoxonase-1 levels may be a promising approach for distinguishing ischemic stroke and hemorrhagic stroke in both controls.
Apolipoprotein (Apo) is the protein component of lipoproteins, including HDL. Apo A-I is the primary lipoprotein associated with HDL in plasma.19 Lower Apo A-I levels were predictive of higher stroke risk in a large cohort study with over 175,000 patients,20 as well as ischemic stroke risk in patients with documented coronary artery disease.21 Apo C-I is associated with very lowdensity lipoprotein, low-density lipoprotein, and HDL.14,22 Our findings support prior studies that have investigated the utility of Apo for distinguishing ischemic and hemorrhagic strokes.14–16 In contrast to these prior studies that measured Apo levels days to weeks after symptom onset, our blood samples were obtained in the hyperacute setting.
Our finding that patients with ischemic stroke have both lower Apo A-I and HDL-C levels is consistent with their known antiatherogenic effects.19 The mechanisms of this antiatherogenicity include the ability of HDL-C to promote efflux of cholesterol from cells, as well as the antioxidant effects of HDL-C and Apo A-I.23 We also found an association between SAA and Apo A-I levels, as has been previously described.24,25 SAA levels were higher in ischemic stroke patients than in ICH patients. SAA, an acute phase reactant, can displace Apo A-I from HDL.26 Thus, in high inflammatory states, low plasma Apo A-I may be anticipated. Given that ICH typically leads to a strong inflammatory response,27,28 greater SAA levels observed in ischemic stroke in our study may reflect chronic inflammation and its known association with atherogenicity and ischemic stroke risk.29,30 Our findings on SAA, HDL-C, and Apo A-I levels support the notion that an acute inflammatory reaction superimposed on chronic low-grade inflammation accompanies acute ischemic stroke, as has been recently proposed.31
Paraoxonase-1 is a class A calcium-dependent, HDL-associated esterase. Its activities include the hydrolysis of toxic oxon metabolites and protection against vascular disease by metabolizing oxidized lipids.32 A strong association between the paraoxonase-1 Gln192Arg singlenucleotide polymorphism and stroke risk has been previously reported,6 and paraoxonase-1 activity has been shown to be decreased in ischemic stroke patients compared to controls.7 Paraoxonase-1 cotransports with HDL (and therefore Apo A-I) and is thought to account for at least some of the antioxidant properties of HDL.23,33 Thus, paraoxonase-1 may also be useful in a panel of markers for distinguishing ischemic and hemorrhagic strokes.
Apo C-I, like other members of the Apo C family, modifies the metabolism of triglyceride-rich lipoproteins.22 In contrast to our findings, Apo C-I levels were lower in hemorrhagic stroke patients than in ischemic stroke patients in a previous study.14 However, Apo C-I function is complex, and a “dual role” has been described. This includes prevention of premature atherosclerosis when Apo C-I is attached to HDL versus promotion of atherosclerosis when it is transferred to triglyceride-rich lipoproteins. The mechanisms of this transfer are largely unknown.22
Overall, ischemic stroke patients had lower levels of paroxonase-1, Apo A-I, and HDL-C compared to both matched control subjects and hemorrhagic stroke patients. Mechanistically, these findings are consistent with available literature in support of the antiatherogenic properties of these biomarkers.19,23,33 Thus, a profile suggestive of poor antiatherogenicity and chronic inflammation, while not specific for stroke, in the context of acute stroke symptoms may indicate ischemia. Further studies are warranted for this line of inquiry.
We found no significant difference in MMP-3 and MMP-9 levels between cases and controls. MMPs are zinc-dependent proteases that degrade the extracellular matrix.8 Three single-nucleotide polymorphisms in the MMP-3 gene have been associated with ischemic stroke,9 and MMP-3 has been shown to be increased in infarcted human brain tissue compared to the contralateral cerebral hemisphere.11 MMP-9 has been associated with NIHSS score and infarct volume, and lower MMP-9 levels have been associated with recanalization.12 MMP-9 levels have also been associated with hemorrhagic transformation after ischemic stroke.13 While these markers may inform stroke risk, severity, and prognosis, our study does not support their utility as diagnostic markers in the hyperacute time window.
Limitations of our study include the small sample size and the recruitment of patients at a single site. Also, whereas obtaining the samples within 12 hours of symptom onset includes a shorter time frame than many other studies, drawing the samples within an even shorter range, such as within 3 hours of symptom onset, could further delineate the relationship of these marker levels and stroke diagnosis. Serial measurements could also elucidate the evolution of stroke within the acute time window. Finally, we included well controls instead of stroke mimics. The clinical challenge is not distinguishing stroke patients from well controls, but from patients presenting with strokelike symptoms. We selected well controls to determine which markers might have utility in a setting where they would have the strongest signal. Next steps would be to assess diagnostic utility in routine clinical practice.
Conclusion
Complementary to a growing number of studies that have reported on Apo as a potential stroke biomarker, and a larger body of literature describing Apo as an important factor in the pathophysiology of cardiovascular disease, we show that Apo A-I, paraoxonase-1, and Apo C-1 are associated with stroke. Further, Apo A-I and paraoxonase-1 levels may be useful for both diagnosis of ischemic stroke and differentiating between ischemic and hemorrhagic strokes. Our results suggest that a multibiomarker panel might help differentiate between stroke subtypes based on association with an inflammatory state.
Acknowledgments
Financial support: This work was supported by (1) an institutional Clinical and Translational Science Award, NIH/NCRR Grant Number 8UL1-TR000077, and (2) a grant for resident physician research from the Department of Emergency Medicine, University of Cincinnati.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Nor AM, Davis J, Sen B, et al. The Recognition of Stroke in the Emergency Room (ROSIER) scale: development and validation of a stroke recognition instrument. Lancet Neurol. 2005;4:727–734. doi: 10.1016/S1474-4422(05)70201-5. [DOI] [PubMed] [Google Scholar]
- 3.Lever NM, Nystrom KV, Schindler JL, et al. Missed opportunities for recognition of ischemic stroke in the emergency department. J Emerg Nurs. 2013;39:434–439. doi: 10.1016/j.jen.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Adeoye O, Albright KC, Carr BG, et al. Geographic access to acute stroke care in the United States. Stroke. 2014;45:3019–3024. doi: 10.1161/STROKEAHA.114.006293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kodali P, Chitta KR, Landero Figueroa JA, et al. Detection of metals and metalloproteins in the plasma of stroke patients by mass spectrometry methods. Metallomics. 2012;4:1077–1087. doi: 10.1039/c2mt20092a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranade K, Kirchgessner TG, Iakoubova OA, et al. Evaluation of the paraoxonases as candidate genes for stroke: Gln192Arg polymorphism in the paraoxonase 1 gene is associated with increased risk of stroke. Stroke. 2005;36:2346–2350. doi: 10.1161/01.STR.0000185703.88944.7d. [DOI] [PubMed] [Google Scholar]
- 7.Kim NS, Kang K, Cha MH, et al. Decreased paraoxonase-1 activity is a risk factor for ischemic stroke in Koreans. Biochem Biophys Res Commun. 2007;364:157–162. doi: 10.1016/j.bbrc.2007.09.119. [DOI] [PubMed] [Google Scholar]
- 8.Kim EM, Hwang O. Role of matrix metalloproteinase-3 in neurodegeneration. J Neurochem. 2011;116:22–32. doi: 10.1111/j.1471-4159.2010.07082.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim SK, Kang SW, Kim DH, et al. Matrix metalloproteinase-3 gene polymorphisms are associated with ischemic stroke. J Interferon Cytokine Res. 2012;32:81–86. doi: 10.1089/jir.2011.0022. [DOI] [PubMed] [Google Scholar]
- 10.Gurney KJ, Estrada EY, Rosenberg GA. Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol Dis. 2006;23:87–96. doi: 10.1016/j.nbd.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Cuadrado E, Rosell A, Penalba A, et al. Vascular MMP-9/TIMP-2 and neuronal MMP-10 up-regulation in human brain after stroke: a combined laser microdissection and protein array study. J Proteome Res. 2009;8:3191–3197. doi: 10.1021/pr801012x. [DOI] [PubMed] [Google Scholar]
- 12.Clark AW, Krekoski CA, Bou SS, et al. Increased gelatinase A (MMP-2) and gelatinase B (MMP-9) activities in human brain after focal ischemia. Neurosci Lett. 1997;238:53–56. doi: 10.1016/s0304-3940(97)00859-8. [DOI] [PubMed] [Google Scholar]
- 13.Castellanos M, Leira R, Serena J, et al. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003;34:40–46. [PubMed] [Google Scholar]
- 14.Allard L, Lescuyer P, Burgess J, et al. ApoC-I and ApoC-III as potential plasmatic markers to distinguish between ischemic and hemorrhagic stroke. Proteomics. 2004;4:2242–2251. doi: 10.1002/pmic.200300809. [DOI] [PubMed] [Google Scholar]
- 15.Lopez MF, Sarracino DA, Prakash A, et al. Discrimination of ischemic and hemorrhagic strokes using a multiplexed, mass spectrometry-based assay for serum apolipoproteins coupled to multi-marker ROC algorithm. Proteomics Clin Appl. 2012;6:190–200. doi: 10.1002/prca.201100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.As S, Sahukar S, Murthy J, et al. A study of serum apolipoprotein A1, apolipoprotein B and lipid profile in stroke. J Clin Diagn Res. 2013;7:1303–1306. doi: 10.7860/JCDR/2013/5269.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonett DG, Price RM. Statistical inference for a linear function of medians: confidence intervals, hypothesis testing, and sample size requirements. Psychol Methods. 2002;7:370–383. doi: 10.1037/1082-989x.7.3.370. [DOI] [PubMed] [Google Scholar]
- 18.Eklund A. The bee swarm plot, an alternative to stripchart. R Package Version 0.1.6. 2013 [Google Scholar]
- 19.Walldius G, Jungner I. Apolipoprotein A-I versus HDL cholesterol in the prediction of risk for myocardial infarction and stroke. Curr Opin Cardiol. 2007;22:359–367. doi: 10.1097/HCO.0b013e3281bd8849. [DOI] [PubMed] [Google Scholar]
- 20.Walldius G, Aastveit AH, Jungner I. Stroke mortality and the apoB/apoA-I ratio: results of the AMORIS prospective study. J Intern Med. 2006;259:259–266. doi: 10.1111/j.1365-2796.2005.01610.x. [DOI] [PubMed] [Google Scholar]
- 21.Koren-Morag N, Goldbourt U, Graff E, et al. Apolipoproteins B and AI and the risk of ischemic cerebrovascular events in patients with pre-existing atherothrombotic disease. J Neurol Sci. 2008;270:82–87. doi: 10.1016/j.jns.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Bjorkegren J. Dual roles of apolipoprotein CI in the formation of atherogenic remnants. Curr Atheroscler Rep. 2006;8:1–2. doi: 10.1007/s11883-006-0056-2. [DOI] [PubMed] [Google Scholar]
- 23.Barter PJ, Nicholls S, Rye KA, et al. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 24.Korita I, Bulo A, Langlois MR, et al. Serum amyloid A is independently related to apolipoprotein A-I but not to HDL-cholesterol in patients with angina pectoris. Clin Biochem. 2013;46:1660–1663. doi: 10.1016/j.clinbiochem.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 25.Wang DX, Liu H, Yan LR, et al. The relationship between serum amyloid A and apolipoprotein A-I in high-density lipoprotein isolated from patients with coronary heart disease. Chin Med J. 2013;126:3656–3661. [PubMed] [Google Scholar]
- 26.Pussinen PJ, Malle E, Metso J, et al. Acute-phase HDL in phospholipid transfer protein (PLTP)-mediated HDL conversion. Atherosclerosis. 2001;155:297–305. doi: 10.1016/s0021-9150(00)00568-2. [DOI] [PubMed] [Google Scholar]
- 27.Chen S, Yang Q, Chen G, et al. An update on inflammation in the acute phase of intracerebral hemorrhage. Transl Stroke Res. 2015;6:4–8. doi: 10.1007/s12975-014-0384-4. [DOI] [PubMed] [Google Scholar]
- 28.Klebe D, McBride D, Flores JJ, et al. Modulating the immune response towards a neuroregenerative peri-injury milieu after cerebral hemorrhage. J Neuroimmune Pharmacol. 2015;10:576–586. doi: 10.1007/s11481-015-9613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coull B. Inflammation and stroke introduction. Stroke. 2007;38:631. [Google Scholar]
- 30.Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke. 2003;34:2518–2532. doi: 10.1161/01.STR.0000089015.51603.CC. [DOI] [PubMed] [Google Scholar]
- 31.Dziedzic T. Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert Rev Neurother. 2015;15:523–531. doi: 10.1586/14737175.2015.1035712. [DOI] [PubMed] [Google Scholar]
- 32.Furlong CE, Suzuki SM, Stevens RC, et al. Human PON1, a biomarker of risk of disease and exposure. Chem Biol Interact. 2010;187:355–361. doi: 10.1016/j.cbi.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Movva R, Rader DJ. Laboratory assessment of HDL heterogeneity and function. Clin Chem. 2008;54:788–800. doi: 10.1373/clinchem.2007.101923. [DOI] [PubMed] [Google Scholar]