Abstract
Background:
Self-management of blood glucose (BG) is considered a norm for diabetes control. However, this invasive process is uncomfortable for patients, especially when intensive measurements with frequent finger pricks are required. Saliva, an alternative body fluid that is easily accessible and contains trace amount of glucose can be potentially used for the noninvasive monitoring of diabetes.
Methods:
As a solution for real-time glucose measurements using saliva for diabetic care, we have developed an on-chip disposable glucose nano-biosensor through a layer-by-layer assembly process. In this work, a clinical study of 10 healthy subjects was conducted to determine the potential usefulness of salivary glucose (SG) sensors for glycemic control.
Results:
Findings revealed (1) the individual BG/SG ratio at fasting was consistent over an entire year when there was no significant change of personal health; (2) the individual SG levels tracked closely with BG levels after meals; (3) a time difference of 15-30 minutes exists between peak levels of BG and SG; (4) 2 hours after a meal, the BG/SG ratio returned to a similar value at fasting.
Conclusions:
We propose to measure fasting and pre- and 2-hour postprandial SG levels for self-management of glycemic levels. As a result, this article is not intended to replace the common BG tests. With preliminary results, we believe SG itself could be used as means for reliable diabetes monitoring and a potential fluid for prognosis of future disease.
Keywords: biochemistry, diabetes, enzymology, oral diagnosis, saliva, salivary diagnostics
Total populations of diabetics have reached 371 million globally in 2012 according to a statistical report by International Diabetes Federation.1 In the United States alone, an estimated cost of 245 billion dollars placed a huge burden on the country.2 Recently, new cases of diagnosed diabetes among children and adolescents raised significant public concerns. The prevalence of diabetes is threatening life quality of entire populations. To date, blood glucose (BG) tests by finger pricks remains the standard self-management method for diabetes.3 However, those with high risks of diabetes were often unaware of their health conditions, or skipping BG tests frequently at home.4 Their unwillingness to measure BG was mostly due to annoying pains and massive scarring formation that was developed from finger pricks.5 Although health literacy and treatment regimens have been well established, the blood test procedure is still considered invasive and inconvenient for home use. Frequent glucose measurements are necessary for the effective glycemic control and appropriate therapeutic interventions, therefore, an innovative, nondisruptive method using saliva as an indicative fluid is being explored to manage diabetes.
First of all, a simple saliva test procedure is in need to eliminate interferences caused by high viscosity when using saliva. Saliva generated from each gland has unique rheological properties with different diagnostic capabilities that can be extracted. For instance, the parotid gland, which has lower viscosity (1-3 mPa), is usually separated and taken for clinical analysis.6 A positive correlation between BG and stimulated parotid salivary glucose (SG) in diabetics has been reported.7,8 However, it is inconvenient to collect parotid gland saliva because the process requires complicated equipment that can only be operated by highly trained professions. Alternatively, whole saliva can be collected noninvasively by individuals with limited training. In fact, some commercially available saliva-based devices include testing for drugs and alcohol, antibodies, the human immunodeficiency virus, and steroids.9,10 All these applications are just the tip of the iceberg for practical uses of saliva as a diagnostic fluid. Whole saliva offers distinctive advantages over blood: it is easily accessible and good for rapid tests; it contains multiple biomarkers enabling multiplex detection; the noninvasive tests can be brought to point-of-care locations, such as for home use and especially for children and elders. Regarding to glycemic control, SG levels of healthy individuals vary from 0.5 to 2 mg/dL.11 The SG level is even lower in hypoglycemic subjects (0.3 mg/dL) and higher in hyperglycemic subjects (20 mg/dL). The main objective of this study is to measure SG levels accurately in real time by using developed sensors.
Numerous clinical studies all over the world have been carried out to assess the feasibility of using SG level as an indicator for the management of diabetes (Table 1).3,8,12-29 Of the 19 studies, 16 revealed a positive correlation between SG levels and BG/serum glucose levels. It is worth pointing out that SG values measured by groups from India, Iran, and Brazil23-29 vary a lot from the others. This finding indicates the criterion of SG levels are also impacted by regional disparities, which may be induced by the population’s ethnicity, dietary pattern, and lifestyle.30,31 The variability in SG levels of different workers may also be a reflection of different choice of study design, as well as the diversity of the methods and criteria for selecting the samples.32,33 Studies reveal diabetics are distinguished from healthy subjects based on their higher average SG levels of about 8.2 mg/dL for diabetics as compared to 1.7 mg/dL for healthy persons.8,12,13-22 Also, well-controlled diabetics (4 mg/dL) demonstrate lower SG levels than uncontrolled diabetics (9 mg/dL) in average.
Table 1.
Using Saliva as a Diagnostic Tool.
| Salivary glucose (mg/dL) | Nondiabeticsa | Diabetics |
Reference | |
|---|---|---|---|---|
| Well controlledb | Uncontrolledc | |||
| *Parotid (fasting & after glucose dose) | 0.76 ~ 2.88 (n = 26) | — | 1.54 ~ 7.00 (n = 26) | 8 |
| *Whole (fasting) | 0.813 ± 0.07 | 2.1 ± 0.4 | 12 | |
| (n = 30) | (n = 30) | |||
| *Unstimulated whole (fasting) | 1.23 ± 0.52 | 4.22 ± 3.59 | 13 | |
| (n = 15) | (n = 106) | |||
| *Unstimulated whole (nonfasting) | Undetectable | 10 ~ 33 | 14 | |
| (n = 25) | (n = 135) | |||
| *Stimulated whole (fasting) | 0 ~ 7 | 9 ~ 15 | 15 | |
| (n = 30) | (n = 30) | |||
| *Whole (fasting & after OGTT) | 0.63 ~ 1.67 | — | — | 16 |
| (n = 2) | ||||
| Unstimulated whole (fasting) | 1.03 ± 1.03 | 2.05 ± 1.91 | 17 | |
| (n = 21) | (n = 20) | |||
| *Whole (nonfasting) | 0.7 ~ 18.9 | 0.2 ~ 7.7 | 1.5 ~ 25.6 | 18 |
| Median = 3 | Median = 2 | Median = 6.8 | ||
| (n = 50) | (n = 50) | (n = 50) | ||
| *Unstimulated whole (fasting & after OGTT) | 0.19 ~ 3.82 | — | — | 19 |
| (n = 6) | ||||
| Unstimulated whole (nonfasting) | 0 ~ 6.27 | 0.036 ~ 17.26 | 20 | |
| Median = 0.38 | Median = 0.76 | |||
| (n = 34) | (n = 41) | |||
| Unstimulated whole (fasting) | 1.3 ± 0.3 | 3.9 ± 0.8 | 21 | |
| (n = 22) | (n = 40) | |||
| *Stimulated whole (nonfasting) | — | 3.52 ± 2.95 | 5.41 ± 3.17 | 22 |
| (n = 21) | (n = 43) | |||
| *Whole (fasting) | 6.08 ± 1.16 | 10.93 ± 1.93 | 23 | |
| (n = 40) | (n = 40) | |||
| *Whole (fasting) | 4.32 ± 0.62 | 12.11 ± 6.97 | 24 | |
| (n = 10) | (n = 20) | |||
| *Unstimulated whole (nonfasting) | 4.58 ± 1.32 | 11.87 ± 3.01 | 13.34 ± 1.61 | 25 |
| (n = 30) | (n = 30) | (n = 30) | ||
| *Unstimulated whole (fasting & after OGTT) | 1.38 ~ 7.87 | — | — | 26 |
| (n = 30) | ||||
| *Unstimulated whole (fasting) | 7.65 ± 0.82 | 20.14 ± 9.86 | 27 | |
| (n = 100) | (n = 100) | |||
| *Stimulated whole (fasting) | 6.35 ± 6.02 | 14.03 ± 16.76 | 28 | |
| (n = 40) | (n = 40) | |||
| *Unstimulated whole (fasting) | 7.1 ~ 12.1 | 12.06 ~ 46.99 | 29 | |
| Median = 9.174 | Median = 30.54 | |||
| (n = 50) | (n = 50) | |||
A correlation between SG levels and BG/serum glucose levels is claimed from the study.
Nondiabetic controls have fasting BG values < 100 mg/dL. bWell-controlled diabetics have random nonfasting BG values > 120 mg/dL and ≤ 200 mg/dL. cUncontrolled diabetics have random nonfasting BG values > 200 mg/dL.
These evidences elucidate real-time SG monitoring tests as a noninvasive way for early warning and management of diabetes. Nevertheless, for better interpretation of the diabetes progression, clinical studies with patients are required to understand the relationship between real-time SG levels and diabetic conditions. Moving to the next step, the research goal is focus on detecting extremely low/high SG levels in patients with diabetes to alert them of dangerous hypoglycemia/hyperglycemia conditions.
Materials and Methods
Sensor Fabrication
SG sensors were previously developed, validated, and published in our other research articles.34-37 DS550 screen-printed electrodes (Dropsens incorporated, Asturias, Spain) were used as sensor chips. A layer-by-layer self-assembly modification process was applied to functionalize the working electrode surface.34-37 Multilayer films were composed of single-walled carbon nanotubes functionalized with carboxylic groups (SWNT-COOH, diameter: 1~2 nm; length: 2~5 μm, Brewer Science, Rolla, CA) and three repeated layers of chitosan (CS)/gold nanoparticles (GNp)/glucose oxidase (GOx) to achieve the best glucose sensing performance.34 All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). 2 mg/dL CS were prepared by dissolving the CS powder thoroughly into acetate buffer solution (pH 4.65). GNp (20nm diameter) was used as received. GOx (type II lyophilized powder with at least 17,300 units/g solid, EC 1.1.3.4 from Aspergillus niger) was dissolved in phosphate buffered saline (PBS, pH 7.4) to produce 1 mg/mL enzyme stock solution. After fabrication, all sensors were packed in gel-boxes (Gel-Pak, Hayward, CA) and sealed in vacuum bags using a vacuum packaging machine (VACmaster pro110, Overland Park, KS). Sensors were preserved at 4°C when not in use.
Measurements of BG and SG in healthy subjects
Human subject research were undertaken to understand the relationship between BG and SG with healthy subjects under the protocol (identified as #12-11-31) approved by the Institutional Review Board in Northeastern University. Ten healthy volunteers between the ages of 20 and 60 years participated in the study anonymously. Diabetic and prediabetic subjects were excluded. Each subject signed consent forms after fully understanding the purpose, procedure, and risks of the study and received $12 compensation to purchase a lunch box at completion of the session. All subjects were required to fast overnight without drinking or eating anything (except water) after 10 pm prior to the test date. In the morning, both blood and saliva samples of each subject were taken for analysis. Blood samples were measured through finger pricks by using a Freestyle Lite BG monitoring system (Abbott, Alameda, CA). Detailed clinical accuracy evaluation is available in Abbott’s 2007 report.38 0.5-1 mL saliva samples were collected following the saliva collection protocol as described in the Supplementary material and measured immediately using a SG sensor (Figure 1). Subjects who consented to an additional 2-hour glucose monitoring test were split into three groups: oral glucose tolerance test (OGTT), regular meal, or standardized meal (as described in Supplementary material). Subjects’ blood and saliva samples were taken before and 15 minutes, 30 minutes, 1 hour, 2 hours after the food/glucose beverage intake. The viscosity of each saliva sample was recorded using a portable viscometer (Core-Parmer, Hills, IL).
Figure 1.
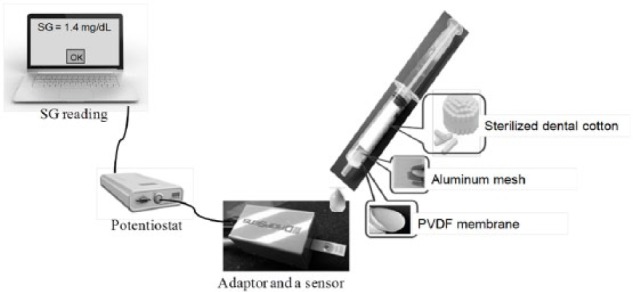
Laboratory setup of saliva sensing system. PVDF: polyvinylidene fluoride.
Sensor Test
The laboratory setup for saliva sensing systems consists of a single-use sensor, a boxed adapter (Dropsens incorporated), a DY2100 mini potentiostat (Digi-ivy, Austin, TX), and a laptop with preinstalled software for post data analysis (Figure 1). 100 μL sample is dropped using pipette to cover all three electrodes on sensors. For amperometric tests, a constant 0.05 V was applied between WE and RE for 30 seconds, and the output current between WE and CE was recorded. Data between 27 and 30 seconds were collected, integrated, and processed through the use of matlab programming. The output signal was converted into SG values by using the formula SG = (Signal+0.2511)/0.066 mg/dL, which was derived from a linear regression curve based on 281 saliva samples as reported in the human subject research section. Final SG values were displayed in mg/dL.
SG Analysis With Reference Method
A spectrophotometric method for quantitative detection of glucose was conducted to validate sensor measured SG levels. Ultraviolet-visible (UV-vis) spectroscopy (mini1240, Shimadzu, Kyoto, Japan) was used along with colorimetric glucose assay kits (K606-100, Biovision, Milpitas, CA) based on enzymatic reactions. Saliva samples were boiled at 100°C in a BSH200 mini dry bath (Benchmark scientific, Edison, NJ) for 30 to 60 minutes to kill reactive bio-molecules residua. After cooling down, cell debris was removed by centrifuging at 12,000 × g for 6 minutes using an Eppendorf 5424 micro-centrifuge (Hauppauge, NY). Clear supernatant was collected and analyzed immediately by UV method. Otherwise, samples were stored at −20°C (no longer than 24 h) and thawed at room temperature before analysis. Pooled saliva was gathered from 2 healthy subjects and treated using the same procedure as for saliva samples. Six standard glucose solutions plus 1 blank sample (without reaction reagent) were prepared along with saliva samples to provide a calibration curve. Standard glucose solutions were prepared by spiking pooled saliva with different glucose concentrations to eliminate a matrix effect. Samples were then mixed with reagents from glucose assay kits following the manufacturer’s protocol and incubated at 37°C for 40 minutes. Absorbance measured at 570 nm wavelength was proportional to glucose levels. SG levels were then calculated using the calibration curve from this batch test.
Results
Sensors Characterizations
Cyclic voltammetry (CV) tests were conducted with the voltage between WE and RE sweeping from -0.2V to 0.4V at a scan rate of 50 mV/s. A suitable voltage as low as 0.05 V was determined under which the output current reflected the change of glucose concentrations in PBS with highest sensitivity and linearity in the steady-state calibration curve (Figure 2A). For electrochemical sensing, a sample’s viscosity, molecular weight, and ionic strength may impact applied working potential.39 As a result, same tests were conducted using filtered saliva samples which were spiked with elevated concentrations of glucose and final concentrations ranged from 0.7 to 35 mg/dL. Glucose was spiked as concentrated solution by adding 1 part solution to 40 parts saliva to eliminate dilution effect. Results revealed the working potential remained unchanged, which indicated the matrix effect was eliminated (Figure 2B). By comparing CV results from buffer solution and spiked saliva samples, the absolute values of current density were proportional to glucose concentrations under fixed working potential. However, the direction of current flow was different with these 2 test media, and the Ep/2 (potential when the current was one half of that at the peak) was 0.17 V and −0.14 V with buffer solution and spiked saliva samples, respectively. It is because the working potential is picked in a region where anodic current dominates (forward sweep) with buffer tests, while with spiked saliva tests it drops in a region where cathodic current dominates (backward sweep).
Figure 2.

Cyclic voltammetry tests determined steady-state calibration curve of SWNT/(CS/GNp/GOx)3 functionalized Pt electrode at an applied potential by testing (A) glucose in PBS; (B) spiked salivary glucose. Error bars, ± standard deviation and n = 3.
For amperometric tests, filtered saliva samples were spiked with elevated glucose concentrations. By comparing a single time point signal at 30 seconds and integration signal in a time window of 27-30 seconds, the later method displayed more accurate, reliable, and repeatable results (Figures 3A and B). With an appropriate post data processing method, sensors demonstrated repeatable measurements (coefficient of variance < 10%) with a wide linear range (1.1-45 mg/dL with R2 = 99%) for SG measurements. Here, extremely low SG levels (< 1.1 mg/dL) were not examined yet since saliva samples of patients with diabetes were excluded from this study.
Figure 3.
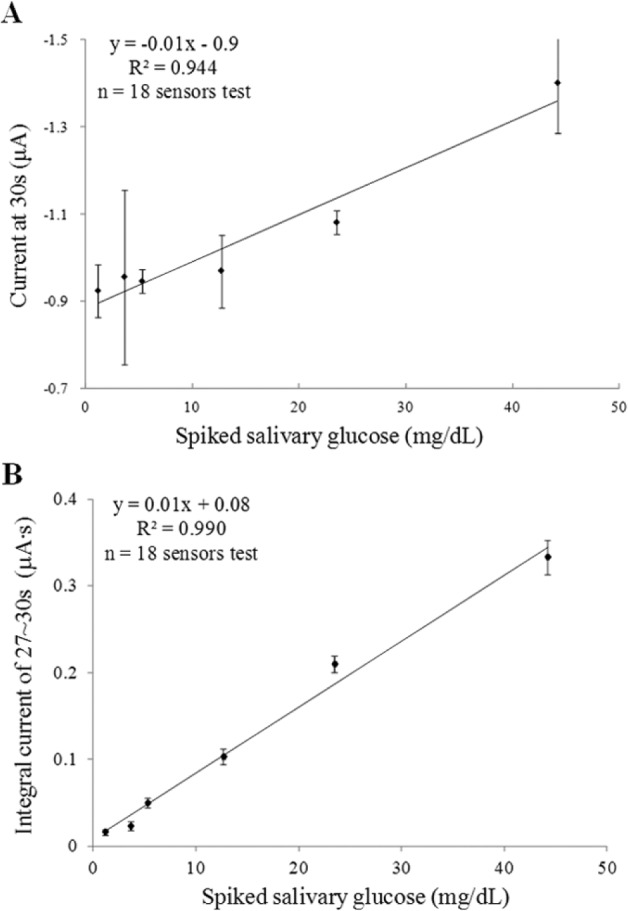
Amperometric tests of spiked salivary glucose with (A) current signal recorded at 30s, (B) integrated current signal of 27~30s with post data processing. Error bars, ± standard deviation and n = 3.
Matrix Effects for Sensor Testing
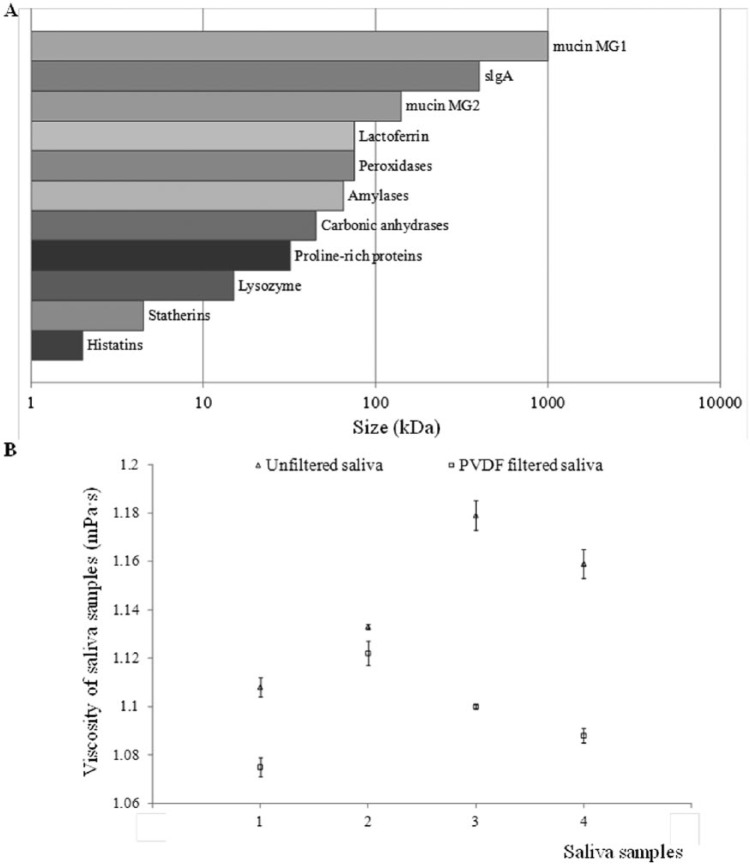
As aforementioned, CV test results with both buffer solutions and spiked saliva samples prove the implemented saliva collection system does provide a reliable filtering performance to remove large bio-molecules from saliva samples. The 0.2 micron pore size filter assembled in collection devices is comprised of a fine polyvinylidene fluoride (PVDF) plastic material (Sigma-Aldrich). This material is widely used for protein blotting due to its high protein binding capability (over 200 µg/cm2) with charged membrane surface.40 Although most proteins are < 0.1 μm (1000 kDa ≈ 1 μm) (Figure 4A),41 they aggregate consistently and therefore cannot pass through filters easily. So PVDF membrane is sufficient for the removal of aggregated mucins in saliva with size ranging from 0.5-10 μm.42 Experimental results of viscosity change in Figure 4B demonstrate good efficiency of applied filtration process, and the viscosity of filtered saliva samples is close to the viscosity of buffer solution (about 1.1 mPa·s). It proves matrix effects in saliva samples analysis are successfully eliminated for sensor testing.
Figure 4.
(A) Saliva’s composition and size distribution. (B) Viscosity change of 4 individual saliva samples: unfiltered saliva (triangle); PVDF-filtered saliva (square). Error bars, ± standard deviation and n = 3.
Human Subject Research
Human subject research including 10 healthy subjects and 281 saliva samples demonstrated a strong linear correlation between sensor’s signal and UV measured glucose concentration in a range of 1.1 to 10.1 mg/dL (R2 = 93.6%; Figure 5A). As to each subject, multiple saliva samples were studied when the subject was at fasting, and after the food/glucose beverage intake. Each saliva sample was tested by a single-use SG sensor. A conversion formula SG = (Signal+0.2511)/0.066 mg/dL was derived from the linear regression curve (Figure 5A), and applied to convert sensors’ signal into SG levels. The Clarke Error Grid Analysis (EGA) was originally developed to quantify clinical accuracy of patient estimates of their BG, as compared to the BG value obtained in their meter.43 An EGA system for this research was developed based on SG analysis, assuming that hypoglycemia happens when SG is lower than 0.7 mg/dL, while hyperglycemia happens when SG is higher than 12 mg/dL. The grid breaks down a scatterplot of a reference glucose analysis result (UV method) and a sensor result into 5 regions. Based on EGA analysis of 281 SG sensors, data in region A (89.0%, clinically accurate) and B (11%, clinically acceptable) revealed comparable accuracy to standard BG measurements (Figure 5B).
Figure 5.

(A) Linear correlation between sensor and UV measured SG with obtained conversion formula. (B) Clark error grid analysis of SG sensors. Region A are those values within 20% of the reference values; region B contains points that are outside of 20% region but would not lead to inappropriate treatment so still are acceptable; region C contains points leading to unnecessary treatment; region D represents points indicating a potentially dangerous failure to detect hypoglycemia or hyperglycemia; region E includes points that would confuse treatment of hypoglycemia for hyperglycemia and vice versa.
One major objective of this study was to comprehend the meaning of glucose values at fasting. A fasting BG value is considered as a crucial index for diabetes diagnosis. So fasting SG levels could be a potential indicator of diabetes. With subjects A and B, the fasting SG levels during a year time ranged from 1.4 to 1.6 mg/dL and 1.1 to 1.4 mg/dL, respectively.34 There were some cases, however, when higher fasting SG levels were observed. Subjects were found either having a cold or fever, or were in menstrual cycles. Concurrently, 8 more subjects participated in the same test verified the calculated fasting BG (mg/dL)/SG (mg/dL) ratios were consistent from day to day (variance < 11%) when their health conditions were unchanged, and the ratio value was individually dependent.34 This finding proves the fasting SG level is a strong indicator of a person’s health, and could potentially be used to predict BG levels at fasting. From our own experience, a typical SG for a healthy person at fasting is about 1.5 mg/dL in average. With a known BG/SG ratio of 60-70 at fasting, a BG level between 90-105 mg/dL is in line with BG meter in the market.
Another major objective of this study was to infer the real-time correlation between BG and SG. When OGTT, regular meal, or standardized meal was given to subjects, the 2-hour postprandial BG/SG ratio was consistent with that at fasting when glucose was degraded and reached safe levels in the body (Figures 6A-6C). Also, a 15- to 30-minute time lag between BG and SG peak-to-peak values was observed. Considering time lags, a Pearson’s correlation test was employed to obtain the correlation between SG and BG in three healthy subjects between the ages of 20 and 60. Results including 7 glucose monitoring tests demonstrated the correlation between BG and SG is systematic significant (r = .74, P < .001) (Table 2).
Figure 6.
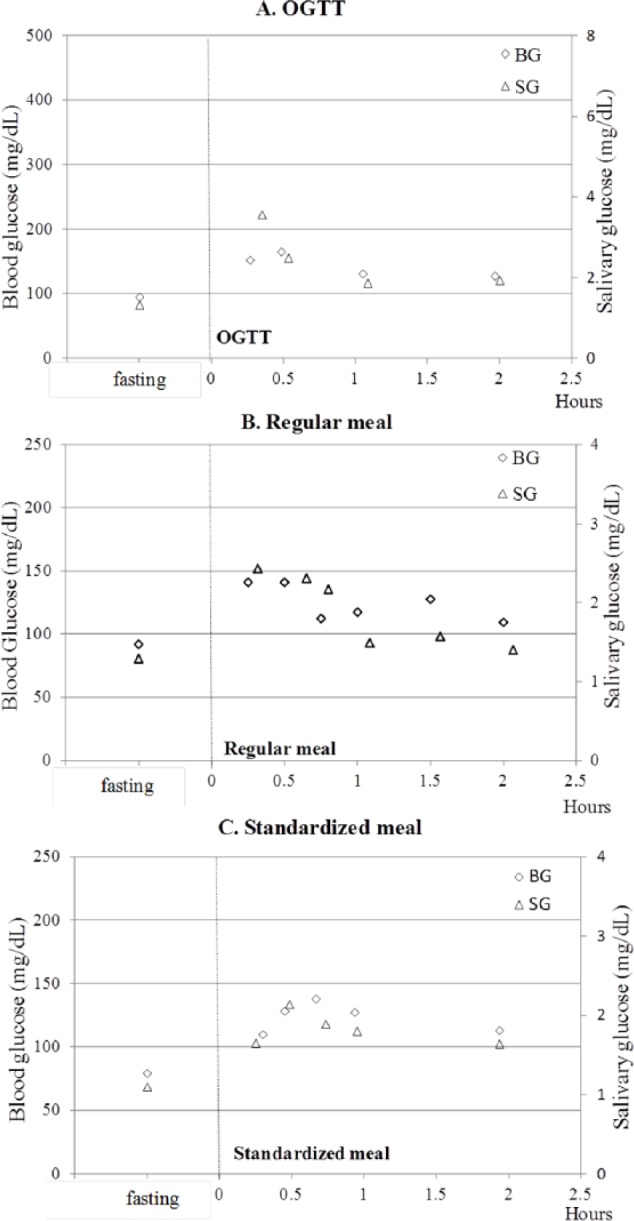
Glucose monitoring test of subject B before and after (A) OGTT, (B) regular meal, (C) standardized meal. The primary vertical axis on the left is used for BG values (rhombus), the secondary vertical axis on the right is used for SG values (triangle).
Table 2.
Correlation of BG and SG in 10 Healthy Subjects
| Test | Sample size | BG range (mg/dL) | SG range (mg/dL) | Pearson correlation value (r) | P value |
|---|---|---|---|---|---|
| OGTT | 15 | 92 ~ 260 | 1.3 ~ 10.1 | .74 | <.001 |
| Regular meal | 14 | 87 ~ 141 | 1.0 ~ 2.4 | ||
| Standardized meal | 9 | 79 ~ 145 | 1.1 ~2.2 |
Hence, current results provide us a prospective approach to monitor health conditions through a noninvasive saliva test. It is effective to measure SG levels several times per day: once upon waking up, before and 2 hours after each meal, and before sleep, to indicate diabetes conditions. For well-controlled diabetics, predicted BG levels at sampling points should be in a normal range. Any higher values should raise attention and a follow up test is required in 15 minutes to confirm the result.
Discussion
Saliva Pretreatment
An effective saliva pretreatment method is a prerequisite for accurate sensor tests. Mucins in saliva may aggregate and form aqueous gels overlying on the sensor’s surface. The consequent bio-fouling severely interferes with glucose detection, especially when glucose levels are extremely low. To combat this problem, a saliva collection protocol is illustrated in Figure 1 of the Supplementary Materials). Through this process, saliva’s viscosity is greatly reduced (Figure 3B).
For the reference (UV) method, 2 major uncertainties, sample’s stability and matrix effects, may incur harmful interferences. Glucose in saliva is continually consumed by acid-producing bacteria (especially Mutans streptococci and Lactobacilli species), even though the saliva collection protocol is followed.44 According to our experiments more than 20% of glucose was consumed and lost when the saliva samples were kept at room temperature more than 2 hours. Thus, saliva is treated with boiling and centrifugation processes beyond the filtration procedure for UV analysis. The boiling process is to kill all reactive bio-molecules in saliva, while the centrifugation process is to obtain clear supernatant. Besides, matrix effects are considered for assay analysis. The manufacturer’s protocol is adapted for SG analysis by preparing standard glucose solutions using pooled saliva instead of buffer solutions. A blank (pooled saliva without reaction reagent) is used to create the baseline of the calibration curve. These adjustments have significantly increased UV method’s accuracy when measuring “real” SG levels.
Limitations and Next Steps
According to results from healthy human subject research, the lowest SG level obtained was 1.1 mg/dL, while the highest was 10.1 mg/dL. However, since patients with diabetes’ SG level can reach down to 0.3 mg/dL during hypoglycemia condition, and raise higher than 20 mg/dL during hyperglycemia condition, a broader SG measurement range is in need to be verified to warn patients from these dangerous situations. Also, a shelf life of at least 6 months is required to apply sensors in clinical exercise. In the future, rugged packaging needs to be designed to protect sensors and retain bio-reactivity during storage, so as to achieve longer shelf life for such biosensors.
Conclusions
In this study, researchers developed a simple and disposable saliva-based biosensor for real-time SG monitoring. It contains a screen-printed sensor chip and the WE is functionalized with SWNT and 3 layers of (CS/GNp/GOx) multilayer films. SG sensors displayed a linear detection range of 0.5-20 mg/dL glucose in PBS with a detection limit of 0.41 mg/dL. Also, the sensors detected 1.1-45 mg/dL glucose in saliva samples. A study of 10 healthy subjects accomplished a SG detection range of 1.1-10.1 mg/dL with acceptable accuracy against standard reference method as evaluated by EGA. 89% of sensor tests demonstrated less than 20% difference against reference values, and the remaining 11% of sensor tests were considered acceptable for their intended uses. Results indicated (1) the individual BG/SG ratio at fasting was relatively consistent over time if subjects’ health conditions were unchanged; (2) the individual SG levels tracked closely with BG levels after meals; (3) a time difference of 15-30 minutes between peak levels of BG and SG was observed due to physiology of the body metabolism; (4) the individual BG/SG ratio 2 hours after meals returned to a similar value at fasting. These discoveries showed great potential for diabetics to manage glycemic levels by simply measuring their SG levels at fasting, before and 2 hours after each meal, and before sleep. The saliva test has great potential to be applied as an adjunct diagnostic method for glucose monitoring in the future. Eventually, with a full clinical validation involving patients with diabetes, medical health providers and patients could use the SG sensors for diabetes screening.
Supplementary Material
Acknowledgments
This research is original, not under publication consideration elsewhere, and free of conflict of interest. The authors are grateful for the volunteers who participated in the clinical studies.
Footnotes
Abbreviations: BG, blood glucose; CE, counter electrode; CS, chitosan; CV, cyclic voltammetry; EGA, Clarke error grid analysis; GNp, gold nanoparticles; GOx, glucose oxidase; OGTT, oral glucose tolerance test; PBS, phosphate buffered saline; Pt, platinum; PVDF, polyvinylidene fluoride; RE, reference electrode; SG, salivary glucose; SWNT-COOH, carboxylic groups functionalized single-walled carbon nanotubes; UV-vis, ultraviolet-visible; WE, working electrode.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially funded by Keyghobad Ventures LLC under grant 0731102.
Supplemental Material: The supplementary material is available at http://dst.sagepub.com/supplemental
References
- 1. International Diabetes Federation. IDF Diabetes Atlas. 6th ed. Brussels, Belgium: International Diabetes Federation; 2013. [Google Scholar]
- 2. Centers for Disease Control and Prevention. Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: CDC; 2014. [Google Scholar]
- 3. Mascarenhas P, Fatela B, Barahona I. Effect of diabetes mellitus type 2 on salivary glucose—a systematic review and meta-analysis of observational studies. PLOS ONE. 2014;9(7):e101706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cha E, Umpierrez G, Kim KH, Bello MK, Dunbar SB. Characteristics of American young adults with increased risk for type 2 diabetes: a pilot study. Diabetes Educ. 2013;39(4):454-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heinemann L. Finger pricking and pain: a never ending story. J Diabetes Sci Technol. 2008;2(5):919-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pfaffe T, Cooper-White J, Beyerlein P, Kostner K, Punyadeera C. Diagnostic potential of saliva: current state and future applications. Clin Chem. 2011;57(5):675-687. [DOI] [PubMed] [Google Scholar]
- 7. Borg A, Birkhed D. Secretion of glucose in human parotid saliva after carbohydrate intake. Scand J Dent Res. 1988;96(6):551-556. [DOI] [PubMed] [Google Scholar]
- 8. Englander HR, Jeffay AI, Fuller JB, Chauncey HH. Glucose concentrations in blood plasma and parotid saliva of individuals with and without diabetes mellitus. J Dent Res. 1963;42(5):1246. [DOI] [PubMed] [Google Scholar]
- 9. Yeh C, Christodoulides JN, Floriano NP, et al. Current development of saliva/oral fluid-based diagnostics. Tex Dent J. 2010;127(7):651-661. [PMC free article] [PubMed] [Google Scholar]
- 10. Wong NSJ, inventor; Wong NSJ, assignee. Oral fluid collector. US patent US 13/149.629. May 31, 2011. [Google Scholar]
- 11. de Almeida Pdel V, Grégio AM, Machado MA, de Lima AA, Azevedo LR. Saliva composition and functions: a comprehensive review. J Contemp Dent Pract. 2008;9(3):72-80. [PubMed] [Google Scholar]
- 12. Shahbaz S, Katti G, Ghali RS, Katti C, Diwakar DD, Guduba V. Salivary alterations in type 1 diabetes mellitus patients: salivary glucose could be noninvasive tool for monitoring diabetes mellitus. Indian J Dent. 2014;25(4):420-424. [DOI] [PubMed] [Google Scholar]
- 13. Abikshyeet P, Ramesh V, Oza N. Glucose estimation in the salivary secretion of diabetes mellitus patients. Diabetes Metab Syndr Obes. 2012;5:149-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amer S, Yousuf M, Siddqiui PQ, Alam J. Salivary glucose concentrations in patients with diabetes mellitus—a minimally invasive technique for monitoring blood glucose levels. Pak J Pharm Sci. 2001;14(1):33-37. [PubMed] [Google Scholar]
- 15. Mirzaii-Dizgah I, Mirzaii-Dizgah MR, Mirzaii-Dizgah MH. Stimulated saliva glucose as a diagnostic specimen for detection of diabetes mellitus. J Arch Mil Med. 2013;1(1):24-27. [Google Scholar]
- 16. Mitsumori M, Yamaguchi M, Kano Y. A new approach to noninvasive measurement of blood glucose using saliva analyzing system. IEEE Eng Med Biol Soc. 1998;20:1767-1770. [DOI] [PubMed] [Google Scholar]
- 17. López ME, Colloca ME, Páez RG, Schallmach JN, Koss MA, Chervonagura A. Salivary characteristics of diabetic children. Braz Den J. 2003;14(1):26-31. [DOI] [PubMed] [Google Scholar]
- 18. Sashikumar R, Kannan R. Salivary glucose levels and oral candidal carriage in type II diabetics. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;109(5):706-711. [DOI] [PubMed] [Google Scholar]
- 19. Yamaguchi M, Mitsumori M, Kano Y. Noninvasively measuring blood glucose using saliva. IEEE Eng Med Biol Mag. 1998;17(3):59-63. [DOI] [PubMed] [Google Scholar]
- 20. Darwazeh AM, MacFarlane TW, McCuish A, Lamey PJ. Mixed salivary glucose levels and candidal carriage in patients with diabetes mellitus. J Oral Pathol Med. 1999;20(6):280-283. [DOI] [PubMed] [Google Scholar]
- 21. Aydin St. A comparison of ghrelin, glucose, alpha-amylase and protein levels in saliva from diabetics. J Biochem Mol Biol. 2007;40(1):29-35. [DOI] [PubMed] [Google Scholar]
- 22. Karjalainen KM, Knuuttila MLE, Käär ML. Salivary factors in children and adolescents with insulin-dependent diabetes mellitus. Pediatr Dent. 1996;18(4):306-311. [PubMed] [Google Scholar]
- 23. Agrawal RP, Sharma N, Rathore MS, et al. Noninvasive method for glucose level estimation by saliva. J Diabetes Metab. 2013;4(5):1000266. [Google Scholar]
- 24. Satish BNVS, Srikala P, Maharudrappa B, Awanti MS, Kumar P, Hugar D. Saliva: a tool in assessing glucose levels in diabetes mellitus. J Int Oral Health. 2014;6(2):114-117. [PMC free article] [PubMed] [Google Scholar]
- 25. Kumar S, Padmashree S, Jayalekshmi R. Correlation of salivary glucose, blood glucose and oral candidal carriage in the saliva of type 2 diabetics: a case-control study. Contemp Clin Dent. 2014;5(3):312-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mirzaii-Dizgah MH, Mirzaii-Dizgah I, Mizaii-Dizgah MR. Oral glucose tolerance test in unstimulated saliva of healthy individuals. Eur J Gen Dent. 2016;5(1):15-18. [Google Scholar]
- 27. Arora KS, Binjoo N, Reddy GVR, Kaur P, Modgil R, Negi LS. Determination of normal range for fasting salivary glucose in type 1 diabetics. J Int Soc Prev Community Dent. 2015;5(5):377-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vasconcelos ACU, Soares MSM, Almeida PC, Soares TC. Comparative study of the concentration of salivary glucose in type 2 patients with diabetes. J Oral Sci. 2010;52(2):293-298. [DOI] [PubMed] [Google Scholar]
- 29. Nagalaxmi V, Priyanka V. Can saliva be a marker for predicting type 1 diabetes mellitus?—A pilot study. J Indian Acad Oral Med Radiol. 2011;23(4):579-582. [Google Scholar]
- 30. Payne EK. Genetic studies of salivary cortisol profiles and their influence on chronic disease risk factors. Ann Arbor: University of Michigan; 2013. [Google Scholar]
- 31. Balan P, Babu SG, Sucheta KN, et al. Can saliva offer an advantage in monitoring of diabetes mellitus? A case control study. J Clin Exp Dent. 2014;6(4):e335-e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patel BJ, Dave B, Dave D, Karmakar P, Shah M, Sarvaiya B. Comparison and correlation of glucose levels in serum and saliva of both diabetic and non-patients with diabetes. J Int Oral Health. 2015;7(8):70-76. [PMC free article] [PubMed] [Google Scholar]
- 33. Soares MSM, Batista-Filho MMV, Pimentel MJ, Passos IIA, Chimenos-Küstner E. Determination of salivary glucose in healthy adults. Med Oral Patol Oral Cir Bucal. 2009;14(10):e510-e513. [DOI] [PubMed] [Google Scholar]
- 34. Du Y, Zhang W, Wang LM. Sensing of salivary glucose using nano-structured biosensors. Biosensors. 2016;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang W, Du Y, Wang LM. Noninvasive glucose monitoring using saliva nano-biosensor. Sens Biosensing Res. 2015;4:23-29. [Google Scholar]
- 36. Zhang W, Du Y, Wang LM. On-chip ultra-sensitive glucose sensing using multilayer films composed of single-walled carbon nanotubes-gold nanoparticles-and glucose oxidase. Sens Biosensing Res. 2015;4:96-102. [Google Scholar]
- 37. Zhang W, Wang LM. inventors; Northeastern University, assignee. Saliva glucose monitoring system. US patent US 14/153.647. July 17, 2014. [Google Scholar]
- 38. Schwartz LS, Bernstein MR, Taylor E, Ward J, Alva S, NG R. Evaluation of the Freestyle® lite blood glucose monitoring system. 2007. Available at: https://freestylediabetes.co.uk/images/uploads/documents/evaluation_freestylelite.pdf. Accessed March 24, 2016.
- 39. Ciszkowska M, Stojek Z. Boltammetric and amperometric detection without added electrolyte. Anal Chem. 2000;72(23):754A-760A. [DOI] [PubMed] [Google Scholar]
- 40. Whatman Westran PVDF membranes. 2016. Available at: http://www.sigmaaldrich.com/catalog/product/aldrich/z671010?lang=en®ion=US.
- 41. Lopatin ED. 2011. Chemical composition and function of saliva. Available at: http://www.umich.edu/~bmsteach/lopatin/salivarygland/lectures/download/Chem_Comp_&_Funct.ppt.
- 42. Zalewska A, Zwierz K, Zółkowski K, Gindzieński A. Structure and biosynthesis of human salivary mucins. Acta Biochim Pol. 2000;47(4):1067-1079. [PubMed] [Google Scholar]
- 43. Clarke LW, Cox D, Gonder-Frederick AL, Carter W, Pohl LS. Evaluating clinical accuracy of system for self-monitoring of blood glucose. Diabetes Care. 1987;10(5):622-628. [DOI] [PubMed] [Google Scholar]
- 44. Hayes ML, Roberts KR. The breakdown of glucose, xylitol and other sugar alcohols by human dental plaque bacteria. Arch Oral Biol. 1978;23(6):445-451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.