Abstract
Background:
It has been suggested that dietary freedom in functional insulin therapy may be detrimental to glycemic control in type 1 diabetes. This study evaluates the effect of carbohydrate intake on glycemic control and postprandial blood glucose concentrations.
Methods:
Insulin pump data from 148 adults with type 1 diabetes, trained in functional insulin therapy, using pumps for ≥6 months, with ≥2 weeks of consecutive downloaded data, ≥80% use of a bolus calculator, ≥3 capillary blood glucose tests/day, and a concurrent HbA1C, were analyzed. More detailed periprandial data (pre- and postmeal glucose, carbohydrate intake, insulin bolus) were collected from a subset of 105 downloads (3495 meals).
Results:
Mean (± SD) age of contributors was 43 ± 13 years, HbA1C 7.84% ± 0.93 (62.19 mmol/mol); daily carbohydrate intake 166 ± 71 g. HbA1C reduced with increased meals/day (r = –.370, P < .0005) and increased with mean carbohydrate content/meal (r = .198, P = .043). However, total daily carbohydrate intake had a weak but significant negative association with HbA1C (r = –.181, P = .027). There was no association between standard deviation of carbohydrate intake and HbA1C (r = .021, P = .802) or between meal carbohydrate content and postprandial change in blood glucose (r = –.004, P = .939) for meals with early postprandial (1-3 hours; n = 390) readings. There was a weak positive correlation (r = .184, P = .008) between meal carbohydrate content and late (4-7 hours; n = 390) postprandial readings.
Discussion:
With appropriate training, patients using insulin pumps can accommodate a flexible diet with variable carbohydrate intake, without detriment to glycemic control. However, large carbohydrate meals may contribute to poorer outcomes, through impact on late postprandial glycemia.
Keywords: type 1 diabetes, carbohydrate counting, insulin pump therapy, functional insulin therapy
Functional insulin therapy (FIT), pioneered in Dusseldorf in 19781 is a widely adopted2-5 treatment regimen for type 1 diabetes based on multiple daily insulin injections with frequent self-monitoring of capillary blood glucose (CBG). It is taught by structured educational programs such as the UK-based Dose Adjustment for Normal Eating (DAFNE) and has demonstrated improved glycemic control with reductions in severe hypoglycemia and significant improvements in quality of life,2,6 related to dietary freedom and removal of guilt from eating,2,6 achieved by adjusting rapid acting insulin for estimated dietary carbohydrate and preprandial CBG. Some recent UK studies7 have not shown expected benefits with FIT and one proposed reason could be too much freedom in carbohydrate intake. The cross-sectional Eurodiab complications study8 found larger carbohydrate intake to be associated with a modest increase in HbA1C and intervention studies have found reduced carbohydrate diets to cause lasting reductions in HbA1C in motivated, adherent patients.9,10
It is therefore possible that larger carbohydrate content at each meal is detrimental to glycemic control. Larger meals are more likely to be associated with greater error in carbohydrate-counting than small meals (mainly manifest as underestimation),11,12 and higher carbohydrate meals are also likely to contain more fat, which slows glucose absorption leading to higher postprandial glucose and contributing to higher HbA1C. High postprandial glucose levels are also implicated as a putative mechanism for endothelial dysfunction13 as well as a contributing factor to HbA1C.
The aim of this study was to determine the effect of carbohydrate intake on overall glycemic control and on postprandial glucose control, as well as glycemic variability. We achieved this by reviewing anonymized, routinely collected electronic data downloads from a secondary care clinic supporting adults with type 1 diabetes using continuous subcutaneous insulin infusion (CSII).
Methods
All adults using CSII who attend clinic at our large secondary care were considered for this study. These patients are routinely offered structured education in FIT (DAFNE) prior to commencing CSII and those new to the clinic already established on CSII are offered pump-specific DAFNE. Pump data is routinely downloaded before the appointment with the health care professional. Inclusion criteria were clinical diagnosis of type 1 diabetes, use of a Medtronic pump (Medtronic Ltd, Northridge, CA, USA) for ≥6 months, age ≥18 years, with ≥2 weeks of consecutive pump data downloaded since January 2011. The study was restricted to users of Medtronic products to allow uniform collection of data using Medtronic Carelink Pro® software, which provides 2-4 weeks of data per download. We also restricted data collection to patients with ≥3 CBG readings per day and in whom ≥80% of boluses were given through the “bolus wizard.” This is an automated bolus calculator that uses CBG, carbohydrate intake (estimated by the patient) and parameters such as insulin:carbohydrate ratio, insulin sensitivity factor, target glucose range and “active insulin” (an estimation of remaining active insulin from a previous bolus). Exclusion criteria were pregnancy, hemoglobinopathies, hemolytic anemia, gastroparesis, coeliac disease, pancreatic insufficiency, moderate/severe renal impairment (GFR <60 mL/min/1.73 m2), and current continuous glucose monitoring and advanced bolus options such as dual or square wave boluses, due to the infrequency of their use in this cohort. A concurrent HbA1C was obtained from electronic patient records. In all, 148 patients satisfied these criteria.
Demographic data, including completion of DAFNE, were collected from patient records. Data on total daily carbohydrate intake was obtained from all 148 pump downloads. More detailed analysis was undertaken in a random subset of 105 subjects, including total daily carbohydrate intake, number of carbohydrate intakes, mean breakfast, lunch and dinner carbohydrate intake (from 06:00-10:00, 11:00-15:00 and 16:00-22:00, respectively) over the past 14 days as well as pre- and postmeal glucose for a total of 3495 meals. A “meal” was defined as a bolus wizard event with preprandial glucose, insulin and carbohydrate intake and postprandial glucose readings all available. This could include any size of carbohydrate intake, including snacks. As many meals as possible were collected from each patient, up to a maximum of 42 (based on 3 meals per day for 2 weeks) to avoid overrepresentation of frequent eaters. Preprandial glucose was recorded as being below, within or above the patient’s individualized bolus wizard target and postprandial glucose readings were recorded as being either early (1-3 hours) or late (4-7 hours), following the bolus wizard event. Our clinic follows DAFNE protocols, and routine postprandial testing is not advocated. Although our analysis may include a testing bias, we felt that this should be equally spread across high or low glucose values. Postprandial glucose was required to have no interim insulin or carbohydrate activity recorded following the meal and only the first postprandial CBG reading was recorded and categorized as to when this occurred. Preprandial blood glucose was recorded quantitatively as well as categorized as within, below or above the individual’s programmed target blood glucose range for that time of day. Meals with preprandial hypoglycemia (≤3.9 mmol/L) were excluded.
Pearson’s correlation was used to determine significance of associations. Analyses of variance and chi-squared tests were used to compare means and proportions. Where homogeneity of variance was violated (as ascertained using the Levene test), the Welch ANOVA was used with a suitable post hoc test.
Results
Insulin pump downloads from 148 out of 467 pump patients at our clinic fitted inclusion and exclusion criteria. Main reasons for exclusions were non-Medtronic pumps, <3 CBG/day or <80% usage of the bolus wizard. Demographics of the main data set (n = 148) and the subset providing individual meal data (n = 105) are presented in Table 1 and were not different. The population was a broad sample with a wide range of age (19-76 years), carbohydrate intake (26-391 g), and HbA1C (5.2-11.0% [33-97 mmol/mol]).
Table 1.
Characteristics of the Full Cohort (n = 148) and Subcohort (n = 105).
| Variable | Full cohort (n = 148) |
Subcohort (n = 105) |
P value | ||
|---|---|---|---|---|---|
| Mean ± SD / percentage | Range | Mean ± SD / percentage | Range | ||
| Age (years) | 43 ± 13 | 19-76 | 44 ± 13 | 22-76 | .566 |
| Female (%) | 65.5 | — | 65.7 | — | .977 |
| Completed DAFNE (%) | 92.7a | — | 90.0b | — | .724 |
| Duration pump use (months) | 48 ± 30 | 10-196 | 53 ± 31 | 17-196 | .192 |
| HbA1C (%) | 7.84 ± 0.93 | 5.2-11.0 | 7.77 ± 0.88 | 5.2-10.4 | .536 |
| Units insulin/kg/day | 0.56 ± 0.17 | 0.232-1.409 | 0.56 ± 0.17 | 0.28-1.41 | .893 |
| BMI (kg/m2) | 26.92 ± 4.44 | 18.22-48.12 | 26.75 ± 4.02 | 20.19-37.45 | .766 |
| Triglyceride (mmol/L) | 0.90 ± 0.52 | 0.30-3.20 | 0.90 ± 0.47 | 0.30-2.40 | .907 |
| Blood glucose tests per day | 5.47 ± 2.05 | 3.0-14.0 | 5.50 ± 1.98 | 3.0-14.0 | .910 |
| Mean daily carbohydrate intake (g) | 166 ± 71.06 | 26-391 | 166.5 ± 71.1 | 50-375 | .943 |
| Bolus wizard usage (%) | 97 ± 5.02 | 80.9-100.0 | 96.9 ± 5.06 | 80.9-100 | .829 |
85.8% had completed DAFNE, 6.8% had not; 7.4% did not have this data available. b87.6% had completed DAFNE, 9.7% had not; 6.7% did not have this data available.
Daily Carbohydrate Intake and HbA1C
Higher total daily carbohydrate intake was associated with lower HbA1C (r = –.181, P = .027; Figure 1). There were no associations between mean daily carbohydrate intake and standard deviation (SD) of blood glucose (r = –.131, P = .112), between SD of carbohydrate intake and SD of blood glucose (r = .060, P = .472) or HbA1C (r = .021, P = .802), or between mean daily carbohydrate intake and BMI (r = –.002, P = .981) or triglyceride levels (r = –.003, P = .974).
Figure 1.
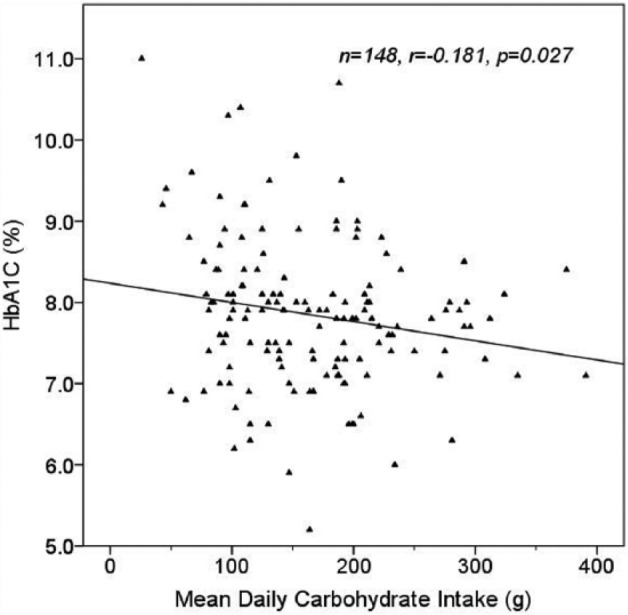
Effect of mean daily carbohydrate intake on HbA1C, using mean daily carbohydrate intakes over the course of 2- to 4-week pump downloads from 148 patients.
Meal Size and Frequency
The mean (± SD) number of meals per day was 3.85 (± 1.43) and mean carbohydrate per intake was 46.1 g (± 24.2). Mean (± SD) breakfast, lunch and dinner carbohydrate values were 39.9 g (± 21.4), 43.8 g (± 20.1) and 46.4 g (± 26.8), respectively. Greater number of meals/day were associated with lower HbA1C (r = –.370, P < .0005; Figure 2a), and with higher mean daily carbohydrate intake (r = .527, P < .0005). Higher carbohydrate intake per meal was weakly correlated with higher HbA1C (r = .198, P = .043; Figure 2b), and higher number of meals/day was associated with lower mean carbohydrate per meal (r = –.324, P = .001).
Figure 2a.
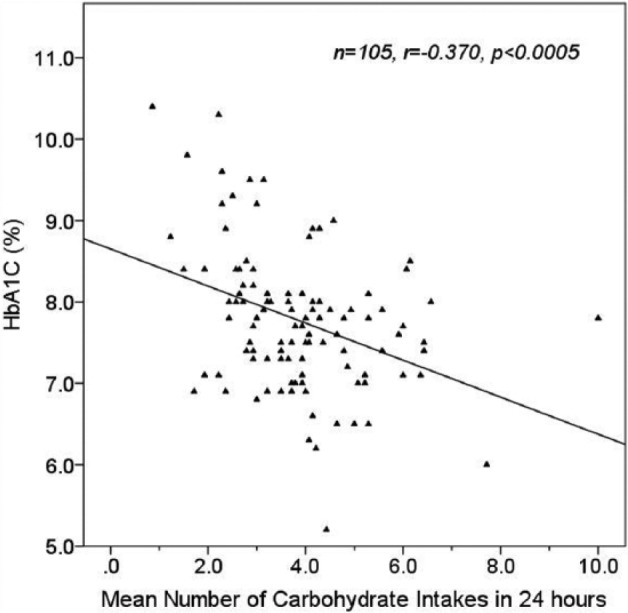
Effect of mean number of daily carbohydrate intakes on HbA1C.
Figure 2b.
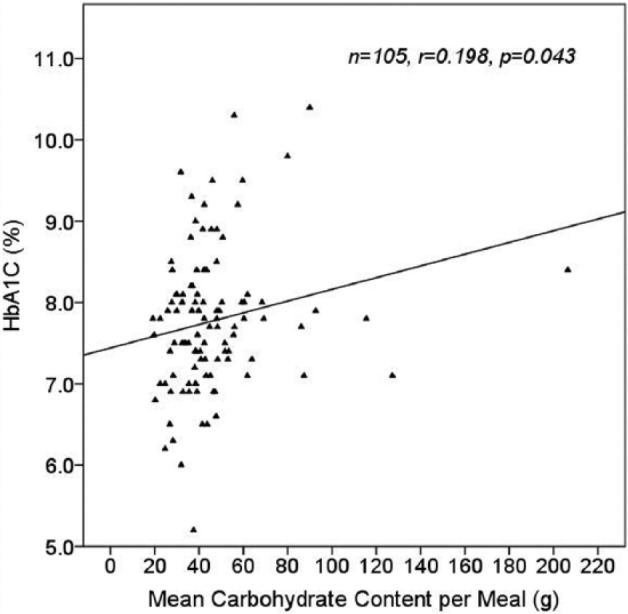
Effect of mean meal carbohydrate content on HbA1C using 14 days of individual data from 105 patients.
Prandial Blood Glucose Changes
Of the 3495 meals collected from the subset of 105 patients, 1993 meals (57%) had postprandial CBG at 1-3 hours. Of these, 19.6% (n = 390) had a preprandial CBG within target. For these 390 meals, mean change in blood glucose (ΔBG) was 3.11 mmol/L (± 3.084) at mean 2.08 hours postmeal and there was no association between meal carbohydrate content and ΔBG (r = –.004, P = .939) (Figure 3a). This was investigated further by dividing the meals into small (<40 g), average (40-80 g) and large (>80 g) carbohydrate content. There was no significant difference in the mean ΔBG for these carbohydrate categories (P = .453).
Figure 3a.
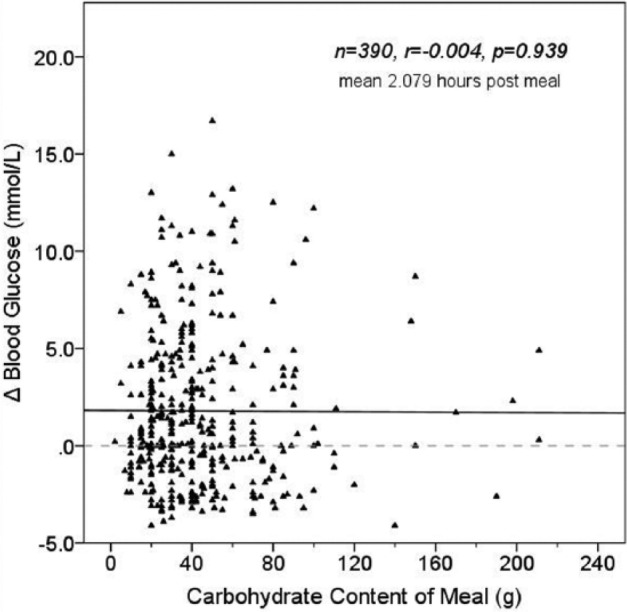
Effect of meal carbohydrate content on Δ blood glucose for meals with postprandial readings at 1-3 hours.
The remaining 1502 meals (43%) of the 3495 collected had a late (4-7 hour) postprandial CBG. Of these, 13.8% (n = 208) had preprandial glucose within target. Mean ΔBG was 3.05 mmol/L at mean 4.88 hours postmeal and there was a significant correlation between meal carbohydrate content and ΔBG ( = 0.184, P = .008) (Figure 3b). However, when ΔBG was compared between the carbohydrate categories <40 g, 40-80 g, and >80 g, the differences did not achieve significance (2.68, 3.36, and 3.79 mmol/L respectively, ANOVA P = .181).
Figure 3b.
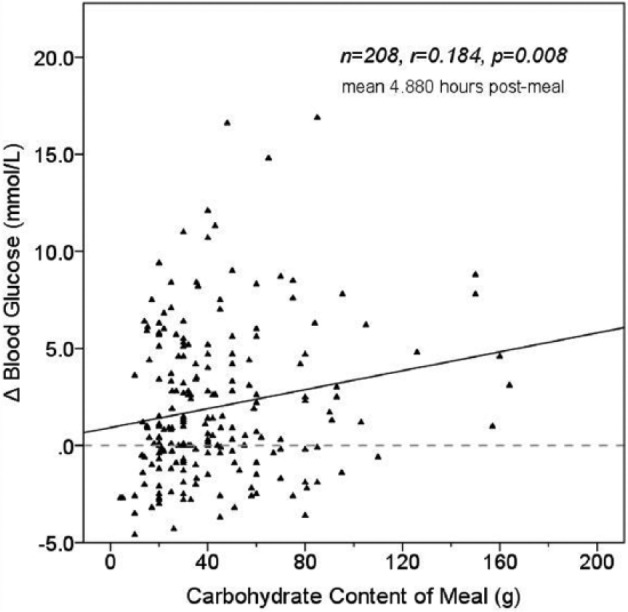
Effect of meal carbohydrate content on Δ blood glucose for meals with postprandial readings at 4-7 hours.
Effect of Preprandial Blood Glucose
Post hoc analysis found meal size was significantly greater for meals with higher premeal glucose; CHO intake was 45.82 g versus 43.68 g versus 48.33 g (P = .002) for meals with preprandial blood glucose below (n = 550), within (n = 598) and above (n = 2347) target, respectively. The significant difference was found to be between meals with preprandial BG within and above target (43.68 g and 48.33 g respectively, P = .002). The proportions of postprandial glucose readings <4, 4-10, and >10 mmol/L were calculated for meals with preprandial blood glucose below, within and above target, regardless of carbohydrate content. High preprandial blood glucose was associated with the highest proportion of postprandial readings >10 mmol/L; 45.1% and 42.0% for readings at 1-3 hours and 4-7 hours, respectively. There was a significant association between preprandial blood glucose and both 1-3 hour (P < .001) and 4-7 hour (P < .001) postprandial glucose.
Discussion
This study aimed to investigate the link between dietary carbohydrate and glucose control in adults with type 1 diabetes, using FIT with insulin pumps, in real-life settings. Insulin pump data including CBG measurements, estimated carbohydrate content of meals, and insulin administration for those meals were examined. The major findings included evidence that frequent meals were associated with higher total daily carbohydrate intake and smaller carbohydrate intake at each meal, resulting in better control, while less frequent meals were associated with larger carbohydrate intake per meal and higher HbA1C. Analysis of individual meals demonstrated that higher carbohydrate meals had no effect on early postmeal glucose but resulted in higher late postmeal glucose. The SD of daily carbohydrate intake had no effect on HbA1C, suggesting that pump users effectively supported to use FIT can vary their diet in accordance with principles of DAFNE, without negative impact on glycemic control, but that large carbohydrate meals may indeed be more difficult to manage optimally. This may be due to the impact of fat and protein. The strongest contributor to postprandial glucose was preprandial glucose. This study used routine CBG readings and so of course may not demonstrate the whole picture as may be seen with continuous glucose monitoring. However, we feel that in line with the American Diabetes Association recommendations to test at 2 hours postmeal, CBG tests are how the majority of patients evaluate their bolus doses, and so our data are of clinical relevance.
The negative association between mean daily carbohydrate intake and HbA1C was unexpected, as carbohydrate restriction is a popular method for reducing insulin requirements and improving diabetic control. Nielsen found that limiting daily carbohydrate intake to 70-90 g/day in adherent patients with type 1 diabetes with suboptimal glucose control resulted in sustained reductions in HbA1C.10 In contrast, a 6-month study by Strychar et al into the effects of a high-carbohydrate, low-fat diet versus a eucalorific low-carbohydrate, high-monounsaturated fat diet in nonobese patients with type 1 diabetes found no significant differences in HbA1C.14 Our clinic is based on DAFNE principles which allow dietary freedom, and we do not routinely advise carbohydrate restriction, although we certainly have a proportion of patients who do carbohydrate restrict and are supported in this. Also, if very high carbohydrate meals are causing a specific problem, we would advise limiting these and also advocate the use of advanced bolusing options where appropriate. In fact, the lack of association of variability in carbohydrate intake with glucose variability or overall control (HbA1C) demonstrates effective carbohydrate counting in our group and implies that our subjects adjust their meal insulin appropriately to variations in meal size. Although FIT encourages flexible meal sizes, some patients can find a rigid diet easier to follow and thus restrict their diet due to fear of carbohydrate-miscalculation.15 The present data should reassure patients trained in FIT that they can vary their day-to-day and meal-to-meal carbohydrate intake without a detrimental effect on blood glucose. In our study, HbA1C showed a negative association with meal frequency, and more frequent meals had lower carbohydrate content than less frequent meals. However, overall daily carbohydrate intake correlated positively with meal frequency, indicating that those eating smaller meals were eating more carbohydrate overall per day. Although there are data to support low-carbohydrate diets, our data suggest that glycemic control can be maintained without carbohydrate restriction.
There are several mechanisms by which a smaller and more frequent meal pattern might drive better glycemic control. First, increased meal frequency may simply result in increased CBG frequency, providing opportunity to correct raised glucose with bolus insulin. The type 1 diabetes exchange registry of >20,000 patients found that frequency of self-testing was positively associated with better glycemic control.16 Second, amongst patients educated in dose-adjustment, dose error is commoner with larger meals, postprandial hyperglycemia being the most common outcome.17 It may be easier to control the smaller postprandial excursions associated with smaller carbohydrate intake; minimizing the size of inputs (carbohydrate and insulin) naturally reduces variability in output (postprandial glucose). Third, larger carbohydrate meals may be likely to contain more fat and defining meals just by carbohydrate takes away from the impact of fat and protein.18 High fat meals can slow gastric emptying, leading to late blood glucose elevations 5-14 hours after the meal19 and can increase free fatty acid levels,20 which impair insulin sensitivity leading to raised glucose. In patients with type 1 diabetes, higher fat meals required more insulin and caused more hyperglycemia than otherwise identical meals with lower fat.21 Recent data from Kordonouri et al shows that in young people with type 1 diabetes, consideration of fat and protein as well as carbohydrate in calculation of prandial insulin requirements resulted in significantly lower postprandial glucose than carbohydrate-counting alone.22 In reality, most patients do not adjust insulin for fat and protein and algorithms to do this are yet to be adequately tested. Last, large meals may also have a detrimental effect on the postprandial control of subsequent meals. In subjects with type 1 diabetes, an antecedent large lunch resulted in a larger area under the curve for postdinner glucose compared with a small lunch, despite almost identical predinner blood glucose.23
In our study we only included meals with premeal glucose in range, to exclude the effects of possible unrecorded hypoglycemia treatment or a correction component to the bolus, and to attempt to isolate the effect of meal size on postprandial glucose. We found no significant association between carbohydrate intake and early ΔBG (1-3 hours postmeal), with over 60% of postmeal readings falling <10 mmol/l, suggesting that appropriate bolus dosing can cover most of the variation in the early postmeal glucose response. These findings echo those of a study which randomized 9 highly motivated FIT-users with type 1 diabetes to low-carbohydrate and high-carbohydrate diets in a crossover design.24 Patients maintained good postprandial glucose profiles with both diets and the ΔBG (preprandial to 1 hour postprandial) was not affected by meal carbohydrate content (range 21-188 g). In our study, there was however a weak but significant association between carbohydrate intake and late ΔBG. By 4-7 hours, bolus insulin should have finished acting and the correlation with last glucose concentrations may relate to inadequate bolus dosing (which should also have affected the early response), a more prolonged absorption of carbohydrate from a larger meal (perhaps related to fat content), or inadequate basal insulin replacement. We cannot exclude from the present data the consumption of an extra snack, or a late postmeal hypoglycemic episode which had been treated.
DAFNE education, adopted in our centre, does not advocate routine postprandial testing and so the postprandial readings we have available to analyze in this study are not systematic readings, but those done for a variety of reasons by the patients. We categorized postprandial readings into early (1-3 hours) and late (4-7 hours) and have a relatively even split between them (57% early and 43% late). It is certainly possible that there may be bias in the tests performed early postmeal, where the patient was concerned about high or low readings, but we do not think there was a systematic bias in any direction. The late readings are most often readings done prior to the next bolus, or possibly before driving, activity or bed.
High postprandial glucose was most strongly associated with high preprandial glucose. This may reflect inadequate correction factors and/or inadequate basal insulin replacement. Meals with high preprandial glucose also had the largest carbohydrate content. The relationship may be that both are markers of poor control, associated with less effective self-management skills. However, postprandial glucose readings were within 4-10 mmol/L over 60% of the time when preprandial glucose was low or within target, suggesting that in our cohort, patients were actually quite good at carbohydrate estimation.
It is important to recognize that the present data were obtained from those trained in FIT, using CSII, engaged with their diabetes management (performing 5.5 CBG/day) and with reasonably good glycemic control (mean HbA1C 7.84%). They were supported by a multidisciplinary team with a special interest in type 1 diabetes, pump therapy and patient education. Mean daily carbohydrate intake in this study was 165.9 g, although there was a wide range from 26-391 g per day. This may demonstrate severe under-counting of carbohydrate or very low carbohydrate diets in a proportion of people. The Institute of Medicine recommend a minimum of 130 g carbohydrate per day and that it should account for 45-56% of total energy intake.25 Median intake is 180-230 g and 220-330 g in women and men, respectively. It makes sense for those with type 1 diabetes to follow this advice to avoid too much or too little fat/protein. Indeed, a cross-sectional study of carbohydrate-counting in adults with type 1 diabetes using either multiple daily injections or CSII found a mean daily carbohydrate intake of 275.6 g of carbohydrate.11 Therefore, intake of 165.9 g/day amongst our cohort may be considered relatively low. It is likely that those with daily carbohydrate <60 g were under-estimating their intake and we cannot account for any carbohydrate taken as hypoglycemia treatment, which is unlikely to be recorded.
We were able to include only a third of our pump patients, as only these had the comprehensive data we required. We excluded those with infrequent CBG tests, or insufficient bolus wizard usage to ensure that we had enough useable data from each patient, and to reduce patient-related variability. Patients are advised to used advanced bolus options for high fat meals, but in our cohort this was infrequently used, and given that protocols for the split of dual wave boluses and duration of the square component of the bolus are unclear and variably applied, we decided to exclude these meals from the analysis. Although our data are only representative of a specific cohort, we believe that it is sufficiently diverse, with a wide range of carbohydrate intake, glycemic control, insulin doses and insulin sensitivity. Limitations include using patient estimation of carbohydrate values. In all, 64% of Finnish patients with type 1 diabetes were found to estimate their prandial insulin requirements inaccurately, despite having had dose adjustment education,17 with hyperglycemia being the most common outcome. We didn’t have information for other factors that can affect glucose absorption such as glycemic index, fiber, protein, and fat. Antecedent calorie intake was not accounted for. It is also possible that subjects who recorded fewer meal boluses were taking snacks without entering carbohydrate information into their pumps and without bolusing for them. This may result in poorer glycemic control and falsely low total daily carbohydrate and carbohydrate intake frequency and may contribute to the seemingly counter-intuitive negative association between daily carbohydrate intake and HbA1C.
Despite these limitations, we feel our data provide a couple of important insights. First, they add to the data that small frequent meals may be better than larger infrequent meals, dispelling the still-held myth that people with diabetes need to eat at fixed times and challenging the suggestion that lower total carbohydrate intake is required for glycemic control. They show meal content and size can be altered without detriment to glucose control, and that starting with a premeal glucose in target seems to help maintain glucose within target at the time most patients are asked to test. However, large carbohydrate meals seem to have a late impact on blood glucose despite the early measurement being within range.
Acknowledgments
The authors would like to acknowledge members of the King’s diabetes team who look after these patients.
Footnotes
Abbreviations: BMI, body mass index; CBG, capillary blood glucose; CSII, continuous subcutaneous insulin infusion; DAFNE, Dose Adjustment for Normal Eating; ∆BG, change in blood glucose concentration; FIT, functional insulin therapy; SD, standard deviation.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article:PC has received speaker fees and travel support from Medtronic Ltd, Roche Ltd, Johnson and Johnson and Abbott and is a member of their advisory boards.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Muhlhauser I, Jörgens V, Berger M, et al. Bicentric evaluation of a teaching and treatment programme for type 1 (insulin-dependent) diabetic patients: improvement of metabolic control and other measures of diabetes care for up to 22 months. Diabetologia. 1983;25(6):470-476. [DOI] [PubMed] [Google Scholar]
- 2. DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: Dose Adjustment for Normal Eating (DAFNE) randomised controlled trial. BMJ. 2002;325:746-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dinneen SF, O’Hara MC, Byrne M, et al. The Irish DAFNE study protocol: a cluster randomised trial of group versus individual follow-up after structured education for type 1 diabetes. Trials. 2009;10:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McIntyre HD, Knight BA, Harvey DM, Noud MN, Hagger VL, Gilshenan KS. Dose Adjustment for Normal Eating (DAFNE)—an audit of outcomes in Australia. Med J Aust. 2010;192(11):637-640. [DOI] [PubMed] [Google Scholar]
- 5. Al-Adsani A, Al-Faraj J, Al-Sultan F, et al. Evaluation of the impact of the Kuwait Diabetes Care Program on the quality of diabetes care. Med Princ Pract. 2008;17(1):14-19. [DOI] [PubMed] [Google Scholar]
- 6. Speight J, Amiel SA, Bradley C, et al. Long-term biomedical and psychosocial outcomes following DAFNE (Dose Adjustment for Normal Eating) structured education to promote intensive insulin therapy in adults with sub-optimally controlled type 1 diabetes. Diabetes Res Clin Pract. 2010;89:22-29. [DOI] [PubMed] [Google Scholar]
- 7. Dinneen SF, O’Hara MC, Byrne M, et al. Group follow-up compared to individual clinic visits after structured education for type 1 diabetes: a cluster randomised controlled trial. Diabetes Res Clin Pract. 2013;100:29-38. [DOI] [PubMed] [Google Scholar]
- 8. Buyken AE, Toeller M, Heitkamp G, et al. Carbohydrate sources and glycaemic control in type 1 diabetes mellitus. EURODIAB IDDM Complications Study Group. Diabet Med. 2000;17:351-359. [DOI] [PubMed] [Google Scholar]
- 9. Nielsen JV, Joensson E, Ivarson A. A low-carbohydrate diet in type 1 diabetes: clinical experience—a brief report. Ups J Med Sci. 2005;110:267-273. [DOI] [PubMed] [Google Scholar]
- 10. Nielsen JV, Gando C, Joensson E, Paulsson C. Low carbohydrate diet in type 1 diabetes, long-term improvement and adherence: a clinical audit. Diabetol Metab Syndr. 2012;4(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brazeau A, Mircescu H, Desjardins K, et al. Carbohydrate counting accuracy and blood glucose variability in adults with type 1 diabetes. Diabetes Res Clin Pract. 2013;99:19-23. [DOI] [PubMed] [Google Scholar]
- 12. Smart CE, Ross K, Edge JA, King BR, McElduff P, Collins CE. Can children with type 1 diabetes and their caregivers estimate the carbohydrate content of meals and snacks? Diabet Med. 2010;27:348-353. [DOI] [PubMed] [Google Scholar]
- 13. Monnier L, Colette C, Owens DR. Integrating glycaemic variability in the glycaemic disorders of type 2 diabetes: a move towards a unified glucose tetrad concept. Diabetes Metab Res Rev. 2009;25(5):393-402. [DOI] [PubMed] [Google Scholar]
- 14. Strychar I, Meltzer S, Cohn J, et al. Effects of a diet higher in carbohydrate/lower in fat versus lower in carbohydrate/higher in monounsaturated fat on post-meal TG concentrations and other cardiovascular risk factors in type 1 diabetes. Diabetes Care. 2009;32(9):1597-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lawton J, Rankin D, Cooke D, Clark M, Elliot J, Heller S. Dose Adjustment for Normal Eating: a qualitative longitudinal exploration of the food and eating practices of type 1 diabetes patients converted to flexible intensive insulin therapy in the UK. Diabetes Res Clin Pract. 2011;91:87-93. [DOI] [PubMed] [Google Scholar]
- 16. Miller KM, Beck RW, Bergenstal RM, et al. Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care. 2013;36(7):2009-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahola A, Mäkimattila S, Saraheimo M, et al. Many patients with type 1 diabetes estimate their prandial insulin need inappropriately. J Diabetes. 2010;2:194-202. [DOI] [PubMed] [Google Scholar]
- 18. Bell KJ, Smart CE, Steil GM, et al. Impact of fat, protein, and glycemic index on postprandial glucose control in type 1 diabetes: implications for intensive diabetes management in the continuous glucose monitoring era. Diabetes Care. 2015;38(6):1008-1015. [DOI] [PubMed] [Google Scholar]
- 19. Lee S, Cao M, Sajid S, et al. The dual-wave bolus feature in CSII controls prolonged post-prandial (pp) hyperglycemia better than standard bolus in type 1 diabetes (TIDM) [abstract]. Diabetes. 2003;52(1):A438. [PubMed] [Google Scholar]
- 20. Nadeau R, Yearick E. Influence of meal composition on serum amino nitrogen, glucose and nonesterified fatty acids. Am J Clin Nutr. 1996;19:329-334. [DOI] [PubMed] [Google Scholar]
- 21. Wolpert H, Atakov-Castillo A, Smith S, Steil G. Dietary fat acutely increases glucose concentrations and insulin requirements in patients with type 1 diabetes: implications for carbohydrate-based bolus dose calculation and intensive diabetes management. Diabetes Care. 2013;36:810-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kordonouri O, Hartmann R, Remus K, Bläsig S, Sadeghian E, Danne T. Benefit of supplementary fat plus protein counting as compared with conventional carbohydrate counting for insulin bolus calculation in children with pump therapy. Pediatr Diabetes. 2012;13:540-544. [DOI] [PubMed] [Google Scholar]
- 23. Mehra R, Raman R, Bayless M, Sivitz W. Antecedent caloric intake and glucose excursion following a subsequent meal in type 1 diabetes. J Diabetes. 2009;1:273-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rabasa-Lhoret R, Garon J, Langelier H, Poisson D, Chiasson JL. Effects of meal carbohydrate content on insulin requirements in type 1 diabetic patients treated intensively with the basal-bolus (ultralente-regular) insulin regimen. Diabetes Care. 1999;22(5):667-673. [DOI] [PubMed] [Google Scholar]
- 25. Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. Washington, DC: National Academics Press; 2005. [DOI] [PubMed] [Google Scholar]


