Abstract
Background:
The aim of this study is to identify whether plantar shear stress in neuropathic patients with diabetes with callus is increased compared with those without callus.
Method:
The differences in foot deformity, limited joint mobility, repetitive stress of walking, and ill-fitting shoes between patients with callus and those without callus were also determined. Subjects were recruited from the Diabetic Foot Outpatient Clinic. A newly developed in-shoe measurement system, which has flexible and thin insoles, enabled measurement of both plantar pressure and shear stress simultaneously when subjects walked as usual on a 10 m walkway.
Results:
It was found that plantar shear stress adjusted for weight during the push-off phase was increased by 1.32 times in patients with callus compared with those without callus (mean ± SD: 0.0500 ± 0.0160 vs 0.0380 ± 0.0144, P = .031). Moreover, hallux valgus deformity, reduction in dorsiflexion of the ankle joint and increase in plantar flexion were showed in feet with callus. Increased plantar shear stress may be caused by gait change that patients having callus push off with the metatarsal head instead of the toe as a result of foot deformity and limited joint mobility.
Conclusions:
It was found that plantar shear stress adjusted for weight during the push-off phase was increased in patients with callus compared with those without callus by using the newly developed measurement system. These results suggest that reduction of plantar shear stress during the push-off phase can prevent callus formation in neuropathic patients with diabetes.
Keywords: diabetic foot, diabetes mellitus, foot ulcer, gait, shear strength, shoes
A diabetic foot ulcer is one of the complications of diabetes mellitus, and is defined as cutaneous erosions characterized by a loss of epithelium that extends into or through the dermis to deeper tissues.1 Foot ulcers seriously affect the patient’s quality of life (QOL) and result in substantial health care cost.2-4 Therefore, prevention of diabetic foot ulcers is worthwhile to improve the QOL and to reduce the social and economic impact.
One of the most effective strategies to prevent foot ulcers is prevention of callus formation in patients with diabetes. Callus is a pathway proceeding to foot ulcers, and involves hyperkeratosis caused by excessive mechanical loading.5-7 Calluses are more frequent under bony prominence such as the metatarsal head.8 A relative risk of 11.0 for an ulcer developing under an area of callus has been reported since the hyperkeratinization may result in a breakdown of skin and tissue integrity.6
Callus formation is thought to be related to pressure and shear stress.7 In neuropathic patients with diabetes, foot deformity, limited joint mobility, repetitive stress of walking,9 and ill-fitting shoes10 may be risk factors of callus formation. However, it is unknown if there are different factors between patients with callus and patients without callus. Muscle atrophy caused by motor neuropathy results in foot deformity.11 Autonomic neuropathy also leads to the process of collagen glycation,11,12 which causes a decrease in joint mobility especially in the foot.13 It is known that foot deformity and limited joint mobility affect gait pattern.11,14
In the clinical setting, recently, plantar shear stress has been highlighted as one of the risk factors of foot ulcers, since pressure by itself has been considered as an insufficient predictor of foot ulcers.15,16 However, how plantar shear stress is related to callus formation has not been identified yet. This is mainly due to the difficulty of measurement for plantar shear stress.16-18
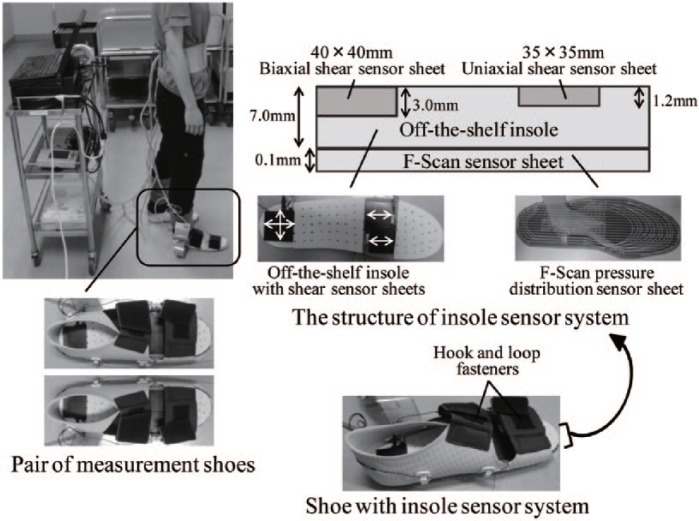
For this purpose, we developed a measurement system of plantar pressure and shear stress during gait (Figure 1). The design of the measurement system is based on the F-Scan pressure distribution sensor sheet, and shear sensor sheets using piezoelectric film. The system has 3 characteristics. First, in-shoe sensors can simultaneously measure plantar pressure and shear stress during gait. Second, the flexible thin insole containing the shear sensor sheets is arranged to enable measurements in patients with diabetes without damaging their skin. Third, the shear sensors are placed to measure the shear stress under the first/second and fifth metatarsal head where callus is frequently present.8 It was confirmed that shear sensor sheets have no influence on a result by F-Scan pressure distribution sensor sheet in advance. The data measured by the system showed the characteristics of the gait cycle such as normal gait, flat-floored walking, and walking while shuffling 1 foot.19 The reliability of the system was also confirmed for 5 adult subjects.19
Figure 1.
Simultaneous measurement system of plantar pressure and shear stress.
The main aim of this study is to identify whether plantar shear stress in neuropathic patients with diabetes with callus is increased compared with patients without callus by using a simultaneous measurement system of plantar pressure and shear stress. Moreover, we clarify the differences in foot deformity, limited joint mobility, repetitive stress of walking, shoe heel height, and hardness of the soles of heels between patients with callus and patients without callus.
Material and Methods
Setting and Subject Recruitment
This was a cross-sectional observational study conducted from July to November 2011. All data were collected at the Diabetic Foot Outpatient Clinic at the University of Tokyo Hospital. Individuals with the diagnosis of diabetes mellitus and neuropathy were included. The exclusion criteria were patients who could not walk without aid, those who had difficulty in wearing the measurement shoes (foot length was over 26.5 cm), or those who had a cutaneous disease. The study protocol was approved by the Ethical Committee of the Graduate School of Medicine, the University of Tokyo (#3433). Written informed consent was obtained from all subjects.
Demographic Data
Information on age, sex, the type and duration of diabetes, hemoglobin A1c (HbA1c), neuropathy, angiopathy, height, mass, and body mass index (BMI) was obtained from the medical records. BMI values were divided into 3 categories.20 Neuropathy was defined if motor neuropathy or sensory neuropathy existed. Motor neuropathy was defined by the absence of the Achilles tendon reflex. Sensory neuropathy was diagnosed if monofilament perception5 and/or vibratory perception21 were abnormal.22 Angiopathy was determined based on ankle brachial pressure index (ABI) <0.9 or toe brachial pressure index (TBI) <0.7.23
Weekly Walking Time and Footwear
Parameters relating to walking habit were recorded. Weekly walking time was obtained based on an interview regarding daily outdoors. The shoes subjects wore on visiting the clinic were measured and were considered as the shoes they usually wear according to the interviews. The shoe heel height was evaluated with a measuring tape and a rigidometer (Durometer, Elastron, Inc, Kyoto, Japan) was used to examine the hardness of the soles of heels. The rigidometer indicates 0 when the needle is maximally stuck out, while 100 represents the state that the point of the needle is flush with a pressurization face.
Foot Deformity and Joint Mobility
Hallux valgus was evaluated as an indicator of foot deformity. The presence of hallux valgus deformity was defined when the interaction angle between the longitudinal axes of the first metatarsal and the first proximal phalanx was over 30°.24
Dorsiflexion/plantar flexion of the ankle joint and inversion/eversion of the subtalar joint were evaluated. The passive range of joint mobility was measured in the supine position with a goniometer, and dorsiflexion was examined in knee flexion.
Callus Assessment
Callus was defined as hyperkeratosis caused by excessive mechanical loading,5 and localized proliferation of the horny layer of the epidermis was determined as a callus. The presence of callus under the metatarsal head was assessed by the researcher. Furthermore, it was confirmed that this assessment was consistent with 2 nurses attending the clinic.
Plantar Pressure and Shear Stress Measurement
Simultaneous measurement system of plantar pressure and shear stress
Figure 1 depicts the measurement system. The shoes that we designed for measurements accommodated an F-Scan pressure distribution sensor sheet, 35 × 35 × 1.2 mm uniaxial shear sensor sheets under the medial (the first/second) and lateral (the fifth) metatarsal head, and a biaxial shear sensor sheet under the heel. Uniaxial shear sensor sheets were arranged to measure anteroposterior shear stress during gait. The anterior component of the sensors was set in the positive direction. The shoes had hook and loop fasteners to avoid slipping back and forth of the feet in the shoes. We prepared 5 sizes of insoles (22-26 cm) to enable shear stress measurement under the first/second and the fifth metatarsal head regardless of foot size.
Data collection
Plantar pressure and shear stress were measured when subjects walked as usual on a 10 m walkway. Before the data collection, subjects practiced once on the same walkway to reproduce their typical gait. Data were recorded at 50 Hz by reference to the previous studies.16,17 In subjects with callus, measurements were conducted before callus removal.
Parameters of plantar pressure and shear stress
Pressure variables analyzed were maximum peak pressure, maximum mean pressure and pressure-time integral. All the variables were calculated for the area of the metatarsal head over the stance phase. Maximum peak pressure represented the maximum pressure value detected by the sensor. Maximum mean pressure was the maximum value among the mean pressure values under the metatarsal head. Pressure-time integral reflected the total load based on maximum mean pressure and contact time which was the ground reaction time of the metatarsal head.
Shear stress variables analyzed were posterior peak shear, anterior peak shear and peak-to-peak shear. Figure 2 describes shear stress of 1 gait cycle at the metatarsal head. Posterior and anterior peak shear were the maximal value over the stance phase. Peak-to-peak shear was determined by adding the values of maximum anterior and maximum posterior shear during the push-off phase.
Figure 2.
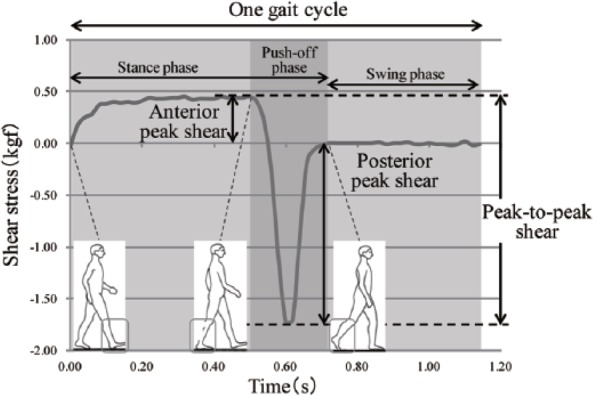
Shear stress variables.
After an initial step was removed, all pressure and shear stress variables were calculated from the 3 steps for each foot. In addition, the mean interval of the onset of pressure at the heel was used to determine gait cycle.
Data Analysis
Demographic data, weekly walking time, and footwear variables were compared between subjects with callus and subjects without callus. In this study, feet having callus are described callus feet, while feet without callus are noncallus feet. The hardness of the soles of shoes was categorized into <60 which usually reflects walking shoes or not. In addition, the presence of hallux valgus deformity and joint mobility were compared between callus feet and noncallus feet. Odds ratio (OR) and 95% confidence interval (95% CI) were calculated regarding hallux valgus deformity.
The association between pressure and shear stress variables with callus formation was then analyzed. The variables were compared between subjects with callus and subjects without callus. In subjects with callus under both metatarsal heads and in subjects without callus, the maximum pressure and shear stress values among all sensors were used. In subjects with callus under the metatarsal head of either left or right foot, pressure values from the foot with the callus were analyzed. In these subjects, the higher shear stress values between the medial and lateral metatarsal head on the foot with the callus were used for analysis as there were 2 shear sensor sheets on 1 side. The values were divided by the subjects’ body mass because normal reaction force is related to body mass.
All the comparisons were performed by using t-test or Fisher’s exact test. The statistical significance level was set at P < .05. All analyses were conducted using Statistical Analysis System software, version 9.2 (SAS Institute, Inc, Cary, NC, USA).
Results
Sixty-seven individuals were encountered at the Diabetic Foot Outpatient Clinic during the observation period. Sixty-three individuals fulfilled the inclusion criteria, but 13 were excluded because of a need for a walking stick or wheel chair (n = 11), difficulty in wearing the measurement shoes (n = 1), or a cutaneous disease (n = 1). Therefore, 50 patients were subjected to analyses. There were missing values of pressure variables for 1 subject because of a trouble of the F-Scan sensor.
The characteristics of 50 subjects are shown in Table 1. Of 9 subjects (18%) with callus under the metatarsal head, 6 had calluses under the metatarsal heads of both feet, while 3 had only under the right metatarsal head. The mass of subjects with callus was significantly lighter than that without callus (P = .001), and the proportion of obesity with a BMI over 25 was significantly lower in subjects with callus (P = .006). Other factors such as motor and sensory neuropathy were not significantly different between the groups.
Table 1.
Characteristics of the patients (N = 50).
| With callus (n = 9) | Without callus (n = 41) | P value | |
|---|---|---|---|
| Age (y), mean ± SD | 69 ± 8 | 67 ± 10 | .430a |
| Sex, n (%) | |||
| Male | 5 (55.6) | 27 (65.9) | .705b |
| Female | 4 (44.4) | 14 (34.1) | |
| Type of diabetes, n (%) | |||
| Type1 | 1 (11.1) | 1 (2.4) | .331b |
| Type2 | 8 (88.9) | 40 (97.6) | |
| Diabetes duration (y), mean ± SD (n = 8) | 15 ± 8 | 17 ± 11 | .634a |
| HbA1c (%), mean ± SD | 6.6 ± 0.8 | 6.7 ± 1.0 | .620a |
| Neuropathy, n (%) | |||
| Motor neuropathy | 7 (77.8) | 37 (90.2) | .293b |
| Sensory neuropathy | 7 (77.8) | 26 (63.4) | .699b |
| Angiopathy, n (%) (n = 40) | 0 (0.0) | 4 (10.0) | 1.000b |
| Height (m), mean ± SD | 1.61 ± 0.10 | 1.63 ± 0.09 | .477a |
| Mass (kg), mean ± SD | 55.8 ± 6.8 | 68.7 ± 17.8 | .001a* |
| BMI, n (%) | |||
| <18.5 | 1 (11.1) | 3 (07.3) | .006b* |
| 18.5-25.0 | 8 (88.9) | 17 (41.5) | |
| >25.0 | 0 (0.0) | 21 (51.2) | |
t-test. bFisher’s exact test.
P < .05.
Weekly walking time was not significantly different between subjects with callus and subjects without callus (mean ± SD: 13.1 ± 11.0 h/week vs 7.6 ± 10.5 h/week, P = .162). Subjects with callus wore significantly higher heel shoes compared with subjects without callus (2.4 ± 0.8 cm vs 1.7 ± 0.6 cm, P = .002). The hardness of the soles of shoes (<60, ≥60, with callus: 6 [66.7%], 3 [33.3%] vs without callus: 20 [48.8%], 21 [51.2%], P = .467) were not significantly different between the groups.
Hallux valgus deformity showed an increased risk for callus formation (OR: 5.0, CI: 1.47-17.03). Dorsiflexion of the ankle joint was significantly reduced in callus feet compared with noncallus feet, and plantar flexion was significantly increased in callus feet (Table 2). On the other hand, there were no significant differences in subtalar joint mobility (Table 2).
Table 2.
Comparison of joint mobility between Callus-Feet and NonCallus-Feet.
| Callus feet (n = 15) | Noncallus feet (n = 85) | P value | |
|---|---|---|---|
| Ankle joint mobility (°), mean ± SD | |||
| Dorsiflexion | 14.3 ± 7.0 | 20.2 ± 6.8 | .003* |
| Plantar flexion | 52.3 ± 8.8 | 48.0 ± 6.9 | .035* |
| Subtalar joint mobility (°), mean ± SD | |||
| Inversion | 32.7 ± 8.8 | 31.9 ± 7.4 | .734 |
| Eversion | 20.3 ± 8.3 | 18.7 ± 6.4 | .387 |
t-test.
P < .05.
There were no significant differences in duration of gait cycle between subjects with callus and subjects without callus (1.23 ± 0.14 s vs 1.24 ± 0.16 s, P = .830). Table 3 shows the comparison of pressure and shear stress variables between subjects with callus and subjects without callus after being adjusted for mass. Peak-to-peak shear was increased by 1.32 times in subjects with callus compared with subjects without callus (0.0500 ± 0.0160 vs 0.0380 ± 0.0144, P = .031), while pressure variables were not significantly different between the groups.
Table 3.
Comparison of plantar pressure and shear stress variables adjusted for mass between subjects with callus and subjects without callus under the metatarsal head.
| With callus (n = 9) | Without callus (n = 41) | P value | ||
|---|---|---|---|---|
| Pressure | ||||
| Maximum peak pressure, (/m2), mean ± SD | n = 8 | 855 ± 415 | 748 ± 281 | .370 |
| Maximum mean pressure, (/m2), mean ± SD | n = 8 | 266 ± 105 | 272 ± 76 | .860 |
| Pressure-time integral, (s), mean ± SD | n = 8 | 0.27 ± 0.10 | 0.29 ± 0.08 | .431 |
| Shear stress | ||||
| Peak-to-peak shear, mean ± SD | n = 9 | 0.0500 ± 0.0160 | 0.0380 ± 0.0144 | .031* |
| Posterior peak shear, mean ± SD | n = 9 | –0.0404 ± 0.0090 | –0.0322 ± 0.0125 | .069 |
| Anterior peak shear, mean ± SD | n = 9 | 0.0109 ± 0.0078 | 0.0074 ± 0.0029 | .220 |
t-test.
P < .05.
Discussion
This is the first report to measure plantar shear stress during gait in neuropathic patients with diabetes with callus, and to compare it with patients without callus. The newly developed measurement system, which has flexible and thin insoles containing shear sensor sheets made it possible to evaluate plantar shear stress. It is first clarified by actual measurement of plantar shear stress that shear stress has the potential to be one of the risk factors of callus formation. Plantar shear stress adjusted for mass during the push-off phase was increased by 1.32 times in patients with callus compared with patients without callus. Lord and Hosein indicated that increased shear stress under first/second metatarsal head represented characteristics of gait in neuropathic patients with diabetes and that shear stress might be a predictive factor of callus formation not just a parameter of gait change in neuropathic patients with diabetes.
There are several instruments to measure shear stress. One of the instruments is a platform to measure the distribution of forefoot pressure and shear stress using strain gauge.16,17 These results suggested that both plantar pressure and shear stress need to be considered to clarify the status of the plantar surface of the foot in patients with diabetes. However, it is difficult to determine if pressure and shear stress sufficiently reflect changes of gait pattern such as distribution and speed in patients with diabetes since their instruments could assess only 1 stance of gait. Lord and Hosein used flat inlays to measure plantar shear stress, in contrast, which had magneto-resistive transducers of a rigid circular disc at 3 points.18 In their study, patients with diabetes exhibited higher magnitudes of shear stress under the first/second metatarsal head where foot ulcers were frequently present.18 It was difficult to evaluate shear stress under callus area which usually ranges in the broad area of the sole of the foot due to the characteristics of hard and stiff transducers.
The current study found that callus feet had hallux valgus deformity more frequently than noncallus feet. Moreover, dorsiflexion of the ankle joint was decreased in callus feet compared with noncallus feet, whereas plantar flexion was increased. An increased plantar flexion represents equinus foot. Mobility in other joints such as the first metatarsophalangeal joint might have been limited in the subjects of the current study as the neuropathic patients with diabetes in a previous report.25 This study confirmed the belief that foot deformity and limited joint mobility might lead to callus formation.5
An increase in plantar shear stress may be caused by gait change that patients push off with the metatarsal head instead of the toe as a result of foot deformity and limited joint mobility. In a normal gait, the ground contact point of the soles shifts from the heel to toe successively and smoothly.26 In contrast, the results of this study indicate that foot deformity and limited joint mobility cause gait change, which is one of the pathways of callus formation in patients with diabetes.
Patients with callus had a significantly smaller mean mass and BMI compared with patients without callus. The thickness of the sole of the foot over the first and second metatarsal heads has been shown to be greater in healthy subjects than in patients with diabetes, especially in patients with foot ulcers or a history of foot ulcers.27 Therefore, a thinner fat pad thickness observed in patients with a smaller mean mass and BMI may be one of the risk factors of callus formation and subsequent ulceration. Relationship among body mass, walking time, and callus formation needs to be considered although weekly walking time is not significantly different between subjects with callus and subjects without callus. Patients with callus also wore higher heel shoes. By wearing higher heel shoes, it is possible that plantar pressure and shear stress under the medial metatarsal head were increased.28
Pressure variables adjusted for mass were not significantly different between patients with callus and patients without callus in contrast to shear stress, which at least indicated that the main factor associated with callus is not only pressure but also shear stress. Pataky et al compared plantar pressure under only the third metatarsal head, and reported that patients with callus under the third metatarsal head had higher peak pressure than patients without callus.29 In this study, on the other hand, it was difficult to statistically separately compare pressure every metatarsal head because of the small proportion of subjects having callus in all the subjects. It was shown that there may be differences of the association between pressure with callus formation and shear stress with callus formation by simultaneous measurement of pressure and shear stress during gait. We consider that measuring larger samples of patients with callus may enable investigation of different influence between pressure and shear stress on region-specific callus formation.
There are several limitations for interpreting the results of this study. First, measurements in the current study did not reflect walking wearing usual shoes because plantar pressure and shear stress were measured by special measurement shoes. However, this study expressively demonstrated gait characteristics in neuropathic patients with diabetes without the influence of footwear. Second, we were not able to evaluate shear stress under the toes and mediolateral shear stress because of the size of the sensor sheets. In addition, larger samples of patients who have various types of foot deformity and neuropathy and also texture of callus may elucidate pathology of callus formation in the future.
The results of this study suggest that increased plantar shear stress may effect callus formation in neuropathic patients with diabetes. We will longitudinally clarify the influence of shear stress on callus formation in the future research. In particular, in patients with foot deformity or limited joint mobility, shear stress during the push-off phase needs to be evaluated. Cushioned and low-heeled shoes can be considered a beneficial intervention. Another research is required to develop a way to reduce plantar shear stress such as gait modification and shear-reducing footwear.
Footnotes
Abbreviations: ABI, ankle brachial pressure index; BMI, body mass index; CI, confidence interval; HbA1c, hemoglobin A1c; OR, odds ratio; QOL, quality of life; TBI, toe brachial pressure index.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. International Diabetes Federation. Diabetes Atlas. 7th ed. 2015. Available at: http://www.diabetesatlas.org/
- 2. Brod M. Quality of life issues in patients with diabetes and lower extremity ulcers: patients and care givers. Qual Life Res. 1998;7:365-372. [DOI] [PubMed] [Google Scholar]
- 3. Reiber GE, Boyko EJ, Smith DG. Lower extremity foot ulcers and amputations in diabetes. In: Diabetes in America. 2nd ed. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 1995:409-428. [Google Scholar]
- 4. Tennvall GR, Apelqvist J, Eneroth M. Costs of deep foot infections in patients with diabetes mellitus. Pharmacoeconomics. 2000;18:225-238. [DOI] [PubMed] [Google Scholar]
- 5. International Working Group on the Diabetic Foot. Practical guidelines on the management and the prevention of the diabetic foot: based upon the international consensus on the diabetic foot (2007) prepared by the International Working Group on the Diabetic Foot. Diabetes Metab Res Rev. 2008;24(suppl 1):S181-S187. [DOI] [PubMed] [Google Scholar]
- 6. Murray HJ, Young MJ, Hollis S, Boulton AJ. The association between callus formation, high pressures and neuropathy in diabetic foot ulceration. Diabet Med. 1996;13:979-982. [DOI] [PubMed] [Google Scholar]
- 7. Freeman DB. Corns and calluses resulting from mechanical hyperkeratosis. Am Fam Physician. 2002;65:2277-2280. [PubMed] [Google Scholar]
- 8. Lázaro-Martínez JL, Aragón-Sánchez FJ, Beneit-Montesinos JV, González-Jurado MA, García Morales E, Martínez Hernández D. Foot biomechanics in patients with diabetes mellitus: doubts regarding the relationship between neuropathy, foot motion, and deformities. J Am Podiatr Med Assoc. 2001;101:208-214. [DOI] [PubMed] [Google Scholar]
- 9. Young MJ, Cavanagh PR, Thomas G, Johnson MM, Murray H, Boulton AJ. The effect of callus removal on dynamic plantar foot pressures in patients with diabetes. Diabet Med. 1992;9:55-57. [DOI] [PubMed] [Google Scholar]
- 10. Harrison SJ, Cochrane L, Abboud RJ, Leese GP. Do patients with diabetes wear shoes of the correct size? Int J Clin Pract. 2007;61:1900-1904. [DOI] [PubMed] [Google Scholar]
- 11. McGuire J. Transitional off-loading: an evidence-based approach to pressure redistribution in the diabetic foot. Adv Skin Wound Care. 2010;23:175-188. [DOI] [PubMed] [Google Scholar]
- 12. Grant WP, Sullivan R, Sonenshine DE, Adam M, Slusser JH, Carson KA, et al. Electron microscopic investigation of the effects of diabetes mellitus on the Achilles tendon. J Foot Ankle Surg. 1997;36:272-278. [DOI] [PubMed] [Google Scholar]
- 13. Sacco IC, Bacarin TA, Canettieri MG, Hennig EM. Plantar pressures during shod gait in diabetic neuropathic patients with and without a history of plantar ulceration. J Am Podiatr Med Assoc. 2009;99:285-294. [DOI] [PubMed] [Google Scholar]
- 14. Kwon OY, Minor SD, Maluf KS, Mueller MJ. Comparison of muscle activity during walking in subjects with and without diabetic neuropathy. Gait Posture. 2003;18:105-113. [DOI] [PubMed] [Google Scholar]
- 15. Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJ. Predictive value of foot pressure assessment as part of a population-based diabetes disease management program. Diabetes Care. 2003;26:1069-1073. [DOI] [PubMed] [Google Scholar]
- 16. Yavuz M, Tajaddini A, Botek G, Davis BL. Temporal characteristics of plantar shear distribution: relevance to patients with diabetes. J Biomech. 2008;41:556-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yavuz M, Erdemir A, Botek G, Hirschman GB, Bardsley L, Davis BL. Peak plantar pressure and shear locations: relevance to patients with diabetes. Diabetes Care. 2007;30:2643-2645. [DOI] [PubMed] [Google Scholar]
- 18. Lord M, Hosein R. A study of in-shoe plantar shear in patients with diabetic neuropathy. Clin Biomech. 2000;15:278-283. [DOI] [PubMed] [Google Scholar]
- 19. Taketoshi M, Masako H, Hiroshi N, Makoto O, Hiromi S. Insole-type simultaneous measurement system of plantar pressure and shear force during gait for patients with diabetes. J Robotics Mechatronics. 2012;24:766-772. [Google Scholar]
- 20. Tanaka K, Yoshimatsu H. Diagnostic criteria of abdominal obesity: difference between Japan and other countries. J Blood Pressure. 2007;14:157-161. [in Japanese] [Google Scholar]
- 21. Japanese Diabetic Neuropathy Association. Practical diagnostic criteria for diabetic polyneuropathy. Peripheral Nerve. 2004;15:92-94. [in Japanese] [Google Scholar]
- 22. Takehara K, Oe M, Tsunemi Y, Nagase T, Ohashi Y, Iizaka S, et al. Factors associated with presence and severity of toenail onychomycosis in patients with diabetes: a cross-sectional study. Int J Nurs Stud. 2011;48:1101-1108. [DOI] [PubMed] [Google Scholar]
- 23. Steed DL, Attinger C, Brem H, Colaizzi T, Crossland M, Franz M, et al. Guidelines for the prevention of diabetic ulcers. Wound Repair Regen. 2008;16:169-174. [DOI] [PubMed] [Google Scholar]
- 24. Zettl R, Trnka HJ, Easley M, Salzer M, Ritschl P. Moderate to severe hallux valgus deformity: correction with proximal crescentic osteotomy and distal soft-tissue release. Arch Orthop Trauma Surg. 2000;120:397-402. [DOI] [PubMed] [Google Scholar]
- 25. Caselli A, Pham H, Giurini JM, Armstrong DG, Veves A. The forefoot-to-rearfoot plantar pressure ratio is increased in severe diabetic neuropathy and can predict foot ulceration. Diabetes Care. 2002;25:1066-1071. [DOI] [PubMed] [Google Scholar]
- 26. Sudo K, Shimada S, Iida Y, Takahashi Y, Ohtsuka S. Quantitative evaluation of walking style from the temporal and spatial tracks of sole pressure points. Electronics and Communications in Japan. 2006;89:42-54. [Google Scholar]
- 27. Gooding GA, Stess RM, Graf PM, Moss KM, Louie KS, Grunfeld C. Sonography of the sole of the foot. Evidence for loss of foot pad thickness in diabetes and its relationship to ulceration of the foot. Invest Radiol. 1986;21:45-48. [PubMed] [Google Scholar]
- 28. Cong Y, Cheung JT, Leung AK, Zhang M. Effect of heel height on in-shoe localized triaxial stresses. J Biomech. 2011;44:2267-2272. [DOI] [PubMed] [Google Scholar]
- 29. Pataky Z, Golay A, Faravel L, Da Silva J, Makoundou V, Peter-Riesch B, et al. The impact of callosities on the magnitude and duration of plantar pressure in patients with diabetes mellitus. A callus may cause 18,600 kilograms of excess plantar pressure per day. Diabetes Metab. 2002;28:356-361. [PubMed] [Google Scholar]