Abstract
In the present era of near-continuous glucose monitoring (CGM) and automated therapeutic closed-loop systems, measures of accuracy and of quality of glucose control need to be standardized for licensing authorities and to enable comparisons across studies and devices. Adequately powered, good quality, randomized, controlled studies are needed to assess the impact of different CGM devices on the quality of glucose control, workload, and costs. The additional effects of continuing glucose control on the general floor after the ICU stay also need to be investigated. Current algorithms need to be adapted and validated for CGM, including effects on glucose variability and workload. Improved collaboration within the industry needs to be encouraged because no single company produces all the necessary components for an automated closed-loop system. Combining glucose measurement with measurement of other variables in 1 sensor may help make this approach more financially viable.
Keywords: continuous glucose control, time in band, diabetes, intensive care
The story of glucose control in critically ill patients gained momentum in 2001 with publication of the results from the study by van den Berghe and colleagues in which surgical intensive care unit (ICU) patients who were managed according to a tight glucose control protocol had better survival rates compared to those managed according to standard practice at the time.1 This study focused the attention of intensivists worldwide on the fact that glucose control could be of greater importance than previously thought in all ICU patients, not just those with known diabetes. The results encouraged the development of new technologies to measure and monitor blood glucose more continuously in ICU patients and protocols to manage the tighter glucose control now targeted. Industry began to invest heavily in the field and ICUs began to integrate tighter glucose control into standard of care practice.
Then, in 2009, the results of the multicenter Normoglycemia in Intensive Care Evaluation-Survival Using Glucose Algorithm Regulation (NICE-SUGAR) study were published,2 showing, with a more pragmatic design, that a tight glucose target (81-108 mg/dL) was associated with higher mortality rates than a moderate blood glucose target (140-180 mg/dL). This and other studies also highlighted the increased risk of hypoglycemic episodes with tight glucose protocols.3-8 These results dampened enthusiasm for the tight glucose control approach and, indeed, created considerable confusion as to how glucose control should be managed in ICU patients and where the future should lie in terms of the ongoing research and development of glucose monitoring technologies and patient management protocols. Some have even suggested that “tight glucose control” should be “abandoned.”9 Scientific groups and opinion leaders interpreted the results to indicate that, while glucose management was still important, a less strict approach was acceptable and suggested varying targets including ≤180 mg/dL,10-14 140-200 mg/dL,15 and <150 mg/dL.16
In this review, we discuss the precise role of tight glucose control in today’s ICU, and how the industry can be encouraged to face concerns related to lack of return for investment. We also comment on current and future perspectives, including cost-effectiveness issues and development of semiautomated glucose control including closed-loop systems, and suggest key areas for ongoing research.
Can Continuous Glucose Monitoring Make a Difference?
The large studies cited above, assessing the possible place of tight glucose control in critical care, all used intermittent blood glucose monitoring.2,4,5 Yet, human physiology is continuous not sporadic, making continuous monitoring of any variable more physiologically relevant than intermittent measures. If monitoring is only intermittent, it is possible that certain acute changes in the parameter being measured will be missed if they occur between measurements. In addition, treatments, even when given intermittently, have long-lasting effects. Intermittent monitoring, depending on the frequency of the measurements, has limited ability to follow the effects of treatments and to accurately perform trending. In terms of blood glucose, continuous glucose monitoring (CGM) may, by facilitating more timely therapeutic intervention, potentially help reduce the numbers of hyper- and hypoglycemic episodes, known to be associated with worse patient outcomes, and also reduce glucose variability, another factor that has been associated with worse outcomes.17-19 Continuous monitoring may also offer advantages in terms of reduced nursing workload.20
But can CGM really make a difference to patient care or is it merely a research tool of use to map and trend glucose concentrations in critically ill patients? Does preventing hypo- and hyperglycemic episodes or reducing glycemic variability really make any difference to patient survival, length of ICU or hospital stay, rates of complications or need for organ support? There are relatively few data available to answer these questions, especially as glucose metrics are not standardized making comparison between studies difficult.21,22
In a study by Boom et al,20 there were no differences in the numbers of severe hypoglycemic episodes (defined as a blood glucose < 40 mg/dL) in patients managed using continuous (with a subcutaneous monitor) or intermittent glucose monitoring. Similarly, De Block et al showed that real-time CGM did not improve mean glycemia, parameters of glycemic variability, or hypoglycemic events compared to blinded CGM.23 However, in an earlier study, Holzinger et al24 noted that CGM (again using a subcutaneous device) was associated with a reduction in the rate of hypoglycemia, although there were no differences of glycemic performance between groups in terms of time in band. Other studies have recently been completed comparing different CGM devices with intermittent sampling and the results should provide more detail regarding the effectiveness of this approach on the quality of glucose control. Importantly, CGM will be shown to have an effect on outcomes only if the monitoring data retrieved from the device are appropriately and frequently used to adjust therapy and the chosen insulin protocol is effective. Insulin protocols are still largely based on intermittent measurements and CGM-specific protocols may need to be developed.25,26
Overview of Current Technologies
There are more than 15 continuous or semi-CGM devices for which published data are available, with different designs, measurement methods, frequencies of sampling, and probe location sites (Table 1); 5 currently have a CE mark and 1 (GlucoScout, International Biomedical, Austin, TX) is FDA approved for clinical measurement use in US hospitals. The development and/or commercialization of some of these devices has been paused.
Table 1.
Characteristics of Some Devices for Continuous Glucose Monitoring.
| Company name | Product name | Regulatory status—hospital use | Sample location | Glucose source | Sensor location | Measurement method | Measurement frequency |
|---|---|---|---|---|---|---|---|
| Edwards Lifesciences | GlucoClear | CE mark (commercialization paused) | Catheter in peripheral vein | Venous blood | Sensor in catheter lumen | Electrochemical/enzymatic | 5 min |
| International Biomedical | GlucoScout | FDA approved No CE mark |
Catheter in peripheral vein, central vein, or radial artery | Venous or arterial blood | External sensor with tubing | Electrochemical/enzymatic | 5 to 60 min |
| B. Braun | ? | Pending | Catheter in peripheral vein, central vein, or radial artery | Venous or arterial blood | External sensor with tubing | NIR absorption spectroscopy, electrochemical/enzymatic | 5 to 60 min |
| Cascade Matrix | CMI System | Pending | Catheter in peripheral vein, central vein, or radial artery | Venous blood | External sensor with tubing | NIR absorption spectroscopy, electrochemical/enzymatic | 5 to 60 min |
| Optiscan | Optiscanner | CE mark | Catheter in central vein | Venous blood transformed into plasma | External sensor with tubing | MIR absorption Spectroscopy |
15 min |
| Medtronic (GluMetrics) | GluCath | Pending | Optical fiber in radial artery, peripheral vein, or central vein | Venous or arterial blood | Sensor in artery or vein lumen | Boronic acid Quenched fluorescence |
1 to 5 min |
| GlySure | GlySure CGMS |
CE mark | Optical fiber in central vein | Venous or arterial blood | Sensor in artery or vein lumen | Diboronic acid Quenched fluorescence |
1 to 5 min |
| GlucoSet | GlucoSet CGMS |
Pending | Optical fiber in radial artery, peripheral vein, or central vein | Venous or arterial blood | Sensor in artery or vein lumen | Boronic acid, change in hydrogel volume | 1 to 5 min |
| Flowsion | Diramo System | Pending | Microdialysis catheter in central vein | Dialysate from venous or arterial blood | External sensor with tubing | Quenched fluorescence | 5 to 10 min |
| Maquet Critical Care | Eirus System | CE mark | Microdialysis catheter in central vein, peripheral vein, or radial artery | Dialysate from venous or arterial blood | External sensor with tubing | Electrochemical/enzymatic | 5 to 10 min |
| Probe Scientific | MicroEye | Pending | Microdialysis catheter in central vein, peripheral vein, or radial artery | Dialysate from venous or arterial blood | External sensor with tubing | Electrochemical/enzymatic | 5 to 10 min |
| A. Menarini Diagnostics | GlucoDay | Pending for blood | Microdialysis catheter in central vein, peripheral vein, or radial artery | Dialysate from venous or arterial blood | External sensor with tubing | Electrochemical/enzymatic | 5 to 10 min |
| A. Menarini Diagnostics | GlucoDay | CE mark for ISF | Microdialysis catheter in subcutaneous tissue | Dialysate from interstitial fluid | Sc tissue | Electrochemical/enzymatic | 5 min |
| Roche Diagnostics | Seven Plus | Pending | Electrochemical electrode in subcuntaneous tissue | Interstitial fluid | Sc tissue | Multiple electrodes Electrochemical/enzymatic/fluorescence |
1 min |
| Medtronic MiniMed | Sentrino | CE mark (commercialization paused) |
Electrochemical electrode in subcuntaneous tissue | Interstitial fluid | Sc tissue | Multiple electrodes Electrochemical/enzymatic |
1 min |
| DexCom | DexCom G4 | CE marked and FDA approved for management of diabetes | Electrochemical electrode in subcutaneous tissue | Interstitial fluid | Sc tissue | Electrochemical/enzymatic | 5 min |
| Abbott Diabetes | Navigator II | CE marked for management of diabetes | Electrochemical electrode in subcutaneous tissue | Interstitial fluid | Sc tissue | Electrochemical/enzymatic | 1 min |
| EchoTherapeutics | Symphony tCGM System | Pending | Electrochemical electrode on skin surface; noninvasive | Transdermal interstitial fluid | Skin surface | Electrochemical/enzymatic | 1 min |
Information was up to date at the time of publication.
The different methods of performing CGM have been discussed in detail previously.21 Essentially, glucose can be sampled from venous or arterial blood or interstitial fluid. Importantly, the glucose value will vary according to the fluid sampled, for example, being greater in arterial than in venous blood. Monitoring can be achieved by continuous or near-continuous withdrawal of the measurement fluid from a catheter to an external sensor or by placement of a sensor within the tissue or fluid of interest (Table 1). A fully noninvasive transdermal probe (Symphony, EchoTherapeutics, Iselin, NJ is also being developed.
All the current systems have specific limitations, including issues related to exposure to heparin and potential need for calibration. Systems that rely on intermittent withdrawal of blood from a vascular catheter carry a risk of foreign body reactions with thrombus formation and catheter occlusion necessitating replacement; this effect may be more frequent in venous than in arterial catheters.27 The definition of continuous in terms of glucose monitoring has been covered in a previous article,21 and the frequency of measurement for most current devices is between 1 and 5 minutes. Most of the studies have used the mean absolute relative difference (MARD) or mean absolute deviation in hypoglycemia, and the percentage of measurement in the Clarke error grid zone A to assess accuracy compared to intermittent sampling.21 Importantly, these devices need to be shown to be accurate through the full range of glucose values, including during hypo- and hyperglycemia, before they can receive FDA/CE approval. The life span of the various devices is also important as several may be needed during a patient’s ICU stay. Outcome data using these devices in clinical practice are still very limited, with most studies having focused on accuracy.
Indexes of Quality of Glucose Control
An index can be defined as a single number calculated from an “array” of clinical data to comprise a single value for comparison or action. Indexes are typically used to evaluate care in terms of eventual outcome (retrospective use) or as a diagnostic or status metric in determining care decisions (prospective). For the purposes of blood glucose control, the array typically comprises blood glucose values, but can also include insulin and/or nutrition data.
No 1 metric is going to be sufficient to monitor quality of glucose control; several different metrics need to be defined and measured and reported consistently across clinical trials. An ideal index should be measurable and assessable over time as well as retrospectively, should be linked to outcome (except when assessing workload), should allow robust statistical comparison between studies, and should be calculated and calculable from regularly interpolated measurements. Importantly, indexes should be equally applicable to individual patients and to patient cohorts. Indexes are needed to evaluate safety (from the perspective of hypoglycemia and extreme hyperglycemia), performance, and workload associated with any glucose control protocol or process. Various options have been proposed for these 3 purposes, but none is yet accepted and used as standard. As examples, indexes of safety could be the number of patients with at least 1 episode of severe hypoglycemia, the number of patients with at least 1 episode of mild hypoglycemia, or the percentage of time spent in severe/mild hypoglycemia; indexes of performance could be mean glucose, (cumulative) time in band (see later), glycemic penalty index,28 or hyperglycemic index;29 and indexes of workload could be time spent on manual interventions or calibration measurements per day. Whichever metrics are chosen, they should be linked to relevant clinical outcomes, must relate to known physiological effects, and must be easy to understand and interpret.
Time in Band
Several indexes are currently used, and all have strengths and weaknesses (Table 2). These have been discussed in detail in previous reports,21,30 and we will just mention 1 in a little more detail, the (cumulative) time in band. Time in band has been widely proposed as a potentially useful metric of performance of any glucose control program. The time in band calculates the proportion of time that blood glucose values fall within a specified range of glucose concentrations (indicative of well-regulated control) up to a point in time.31 This metric provides a combined indication of glycemia and variability, both known to be associated with outcome.18,19,32 This is an easy metric to calculate, with greater accuracy associated with higher measurement frequency (eg, near-continuous blood glucose measurement technology). The main other problem with using time in band as a metric at present, as indeed for many other indexes, is that the “optimal” band has not been clearly defined and may vary according to the specific patient population, for example, higher upper and lower cut-offs may be preferred in patients with preexisting, poorly controlled diabetes mellitus.31 Using a wider range will allow more variability in glucose concentrations. In a retrospective study using data from 2 randomized controlled trials, Penning et al31 used the cumulative time in band metric to compare the odds of survival for 3 different glucose “ranges” and demonstrated that in patients without diabetes, more time spent within the 72 to 126 mg/dL glycemic range was associated with a higher odds of survival compared with the 90 to 144 mg/dL and 72 to 144 mg/dL range. The odds of survival increased with increasing cumulative time in band. Krinsley and Preiser similarly reported that higher time in band (using 70 to 140 mg/dL as the range) was associated with improved outcomes in patients without diabetes.33 These associations may, however, reflect disease severity and glucose-modifying therapy interventions, and do not necessarily demonstrate causality.
Table 2.
Some of the Factors For and Against the Main Currently Used Indexes of Quality of Glucose Control.
| For | Against | |
|---|---|---|
| Glucose concentration—mean, median, … | • Simple • Easily measured • Can be monitored over time • Commonly used, so good for comparison with previous studies |
• Difficult to select a single level that is better or worse than another similar concentration (eg, 117-126 mg/dL) • Debate over nonparametric (median) measure of true “middle” and inaccurate parametric (mean) measure of nonnormal BG distributions • Measurements reflect results over a period of time—no real-time monitoring |
| Variability—standard deviation, interquartile range, coefficient of variation, … | • Easily measured • Can be monitored over time • Commonly used, so good for comparison with previous studies |
• Difficult to define variability • Can vary with measurement frequency • Measurements reflect results over a period of time—no real-time monitoring |
| Incidence or relative rates | • Easily measured • Easily counted, especially for hypoglycemia • Commonly used so good for comparison with previous studies |
• Range measurements can be skewed by measurement intervals • Hyperglycemia is hard to assess this way • CGMs with noise may over-/underreport incidence. |
| Time to an event | • Easily measured • Easily calculated |
• Not commonly or otherwise particularly associated with outcome • Protocol-dependent • Dependent on starting criteria of protocol • Does time to event matter clinically? |
| Exposure—time in event, (cumulative) time in range, area under the curve, glycemic penalty index,28 hyperglycemic index29 | • Easily calculated • Cumulatively can be calculated/used in real time • Has been associated with outcome (though not frequently) in ICU • All BG concentrations within the range are considered “equal” or similar, which likely has physiological merit |
• Not commonly used for association with outcome in previous studies, so comparisons difficult • Ranges are not standardized rendering comparison impossible without all the data • Typical measures do not account for frequency of exposure as well as severity • Some exposure indexes are hard to calculate and/or understand • Measurement frequency and/or interpolation dependent |
Meaningful Clinical Outcomes
We have discussed possible metrics for assessing the quality of glucose control, but meaningful clinical outcomes also need to be selected and defined for future clinical trials of these devices. Mortality remains an important outcome endpoint in almost all clinical trials in ICU patients, and indeed many other hospital specialties. Various mortality rates have been used including ICU, hospital, 28/30 days, 90 days, and longer. However, improvements in mortality are difficult to demonstrate in today’s ICU population and other endpoints are increasingly of importance. The occurrence of hypoglycemia is of course a major endpoint for reasons of patient safety. Other morbidity variables, including infectious complications and development of organ dysfunction, could also be used as outcomes, or combined with mortality to develop a composite outcome.34 Finally, protein economy has been suggested as a useful outcome indicator because the oxidant stress associated with hypoglycemic episodes leads to loss of lean muscle mass and poor long-term outcomes.35
Another important aspect when considering outcome data is whether tight glucose control protocols should be offered to all patients or restricted to those most likely to benefit and, if so, how can these patients be identified. In addition optimal blood glucose concentrations may vary among different groups of patients. In patients treated using an intensive insulin therapy protocol (targeting a blood glucose of 79-140 mg/dL), Waeschle et al36 reported that the rates of critical hypoglycemia (≤ 40 mg/dL) and hyperglycemia (above 180 mg/dL) were significantly different among different clinical subgroups (cardiac surgery, neurosurgery, abdominal, vascular, orthopedic, spinal, medical). The predisposing factors for these conditions also varied among the different groups. Clearly this is an area that needs further study.
What About Patients With Diabetes?
As seen earlier, various blood glucose targets have been recommended by experts and international societies since the NICE-SUGAR study. However, these provide global ranges for all patients, not taking into account individual patient factors including pre-ICU glycemic status. Observational and interventional data suggest that the association between blood glucose concentration and mortality is different in critically ill patients with and without preexisting diabetes mellitus,18,37-41 and that patients with diabetes with good premorbid glycemic control should have a “tighter” target than those with poor premorbid glycemic control.42,43 The associations between glucose concentrations and outcomes also differ in patients with known premorbid diabetes compared to those without diabetes.18,37,44 In a retrospective analysis comparing an electronic tight (80 to 110 mg/dL) with a moderate (90 to 140 mg/dL) glucose control protocol, there was a survival benefit with the tight target compared with the moderate target in patients without diabetes, but this finding was reversed in patients with diabetes.40 The HbA1c is now part of routine admission workup for critically ill patients in many units to assess premorbid glucose control. Elevated HbA1c at the time of ICU admission is associated with a higher insulin need during the ICU stay and with a higher risk of disturbed glucose metabolism 6-8 months after ICU admission, as recently shown in the DIabetes mellitus AFter Intensive Care admission (DIAFIC) study.45 An ongoing multicenter French study (clincialtrials.gov ID NCT02244073) is randomizing patients to different glucose targets based on their admission HbA1c value: an individualized target of (28.7 × A1C − 46.7) mg/dL or a standard target of <180 mg/dL. This study may help answer some of the questions related to the need to adapt glucose targets according to individual patient characteristics.
Cost-Effectiveness
One important consideration when assessing glucose control in the ICU is whether or not it is cost-effective, but there are few published data related to this parameter and most of those that are available used intermittent glucose monitoring. Importantly, glucose control will be cost-effective only if it is associated with improved relevant outcomes, such as ICU length of stay or nosocomial infections, which are known to be associated with increased costs. In an analysis of the original Leuven study, van den Berghe et al reported that intensive glucose control was associated with a cost-saving of 2638 euros/patient (data from 2000), driven predominantly by the reduced length of ICU stay in the treatment group.46 Krinsley and Jones reported a cost savings of $1580/patient (data from 2002-2004) in their before/after study in the United States,47 again driven by reduced ICU length of stay. In another multi-ICU before/after study, Sadhu et al reported significantly reduced ICU lengths of stay, but the decreases in costs ($5231, data from 2003-2005) were not significant.48 In a more recent before/after study in the Netherlands, van Hooijdonk et al49 reported that implementation of a strict glucose control guideline using point-of-care testing was associated with a 1.8% increase in total hospital costs. Using a sensitivity analysis, Krinsley reported that even a 5% reduction in nosocomial infections and in ICU length of stay associated with glucose control would yield considerable cost savings.50
Reduced nursing workload may also be associated with reduced costs and several studies have suggested that CGM can be associated with reduced nursing workload. CGM would certainly decrease the time input necessary for blood sampling. Aragon reported that, using hourly blood glucose monitoring, nearly 2 hours of direct nursing time was spent per patient per day to achieve tight glycemic control (80-110 mg/dL).51 Boom et al recently reported a 12 euro/patient saving (data from 2011/2012) with glucose control guided by subcutaneous CGM compared to point-of-care measurement, largely related to reduced daily (24 hour) nursing workload (17 vs 36 minutes; P < .001).20
When assessing the expenses associated with CGM, multiple “new” costs need to be taken into account compared to intermittent systems, including the purchase, installation, and maintenance of the CGM system; costs of disposable sensors, which will depend on how often these need to be changed; costs of nursing/physician time for insertion of CGM probe; and costs of a computerized algorithm for glucose control, if used. Studies are needed to demonstrate that CGM really improves morbidity and mortality before effects on costs can be fully evaluated.
(Semi)automated Blood Glucose Control
Automation is widely used to improve productivity and quality in multiple areas involved in providing products and services. In medicine, automation has been adopted less rapidly. But blood glucose control in ICU patients could be an ideal area for an automated approach and steps have already been made in that direction. Original methods of glucose control used some form of paper-based glucose protocol to inform nurses when and how to adjust insulin concentrations, based on more or less frequent intermittent glucose measurements. This approach is simple to apply but is not patient specific and may introduce personal bias if not entirely prescriptive. Moreover, these early protocols rely on a reactive response to the current glucose value, which, depending on the frequency of measures, may be more or less relevant to the current trend in blood glucose. Noncompliance with the protocol is also a potential problem. Movement from paper- to computer-based protocols has been associated with reduced error rates and better glucose control,52 although this of course depends on the computer-based protocol under assessment and the paper protocol used as comparator.
As computer-based algorithms have developed, some have been integrated into clinical computerized decision support systems. Such algorithms can be adapted to patient-specific parameters, although the technology is not yet available in all ICUs. Various protocols have been developed for this approach, including the Stochastic TARgeted (STAR) approach,53 the LOGIC-Insulin algorithm,54 enhanced model predictive control (eMPC) algorithm,55 Glucose Regulation for Intensive care Patients (GRIP),56 Glucosafe,57 and Contrôle Glycémique Assisté par Ordinateur (CGAO).8 These systems, although the protocols are more complex, have the advantage of being more physiologically relevant and can adapt to inter- and intrapatient variability, including issues of insulin sensitivity, nutrition, and concomitant medication. When combined with CGM systems, these automated protocols could potentially enable more predictive glucose control by responding to real-time glucose values and evaluated trends. Importantly, the protocol appears to have a greater impact on outcomes than the measurement method,25,26 so currently available and new protocols need to be carefully validated in different patient populations. In addition, most widely used algorithms have been developed based on intermittent monitoring systems and will need to be adapted to be used with the more frequent measures available from CGM probes.58
The combination of CGM with an electronic protocol inevitably leads to the concept of automated “closed-loop” systems. Closed-loop systems, in which a variable is measured by some form of monitoring sensor and/or unit, which then applies an intervention based on the measured value, have been explored in various areas of critical care medicine including hemodynamic management and mechanical ventilation,59 but these systems are still not widely used. Concerns regarding patient safety and device regulation have slowed commercial development. Potential legal issues with liability also need to be evaluated and resolved as the responsibility for treatment-decisions is taken away from the physician to the device and, thus, potentially to the manufacturer.60 A final concern related to automated systems is that of the cybersecurity of programmable medical devices.
Nevertheless, glucose control does lend itself to an automated closed-loop approach that could regulate blood glucose in a more constant manner thus potentially reducing variability in glucose concentrations. This use of closed-loop systems in glucose control is not a new idea. Such systems (“artificial pancreas”) were first tested clinically in patients with diabetes almost 40 years ago61 and smaller, more advanced versions have recently been used in individuals with type 1 diabetes during free-living at home, with promising results.62-64 However, acutely ill patients may present greater variability in insulin requirements than people with diabetes and glucose control is just 1 aspect of multiple interventions, including nutritional intake, likely to be necessary at any 1 time in these patients. On the other hand, the use of intravenous insulin delivery in critically ill patients removes the absorption delays associated with subcutaneous insulin delivery in people with diabetes, thus potentially simplifying the process.
Several studies have now evaluated use of closed-loop systems in small groups of critically ill patients. Yatabe et al evaluated a closed-loop glycemic control device in 208 ICU patients and reported that the glucose concentration was kept in target for 50% of the study period with no hypoglycemic episodes.65 Leelarathna and colleagues66 randomized 24 critically ill patients to glucose control using a fully automated closed-loop system with a subcutaneous CGM sensor and intravenous insulin and dextrose infusions or using a local protocol with intravenous sliding-scale insulin for 48 hours targeting a blood glucose of 108-144 mg/dL. The closed-loop system was associated with increased time in band (54.3 [44.1-72.8]% vs 18.5 [0.1-39.9]%, P = .001) and lower mean glucose concentrations (140 [133-148] vs 164 [149-234] mg/dL; P = .001) (Figure 1). No patients in either group had a hypoglycemic episode (<72 mg/dL). In 450 patients who underwent surgical procedures for hepato-biliary-pancreatic disease, Okabayashi et al demonstrated that intensive insulin therapy with a closed-loop system decreased the incidence of surgical site infections.67
Figure 1.
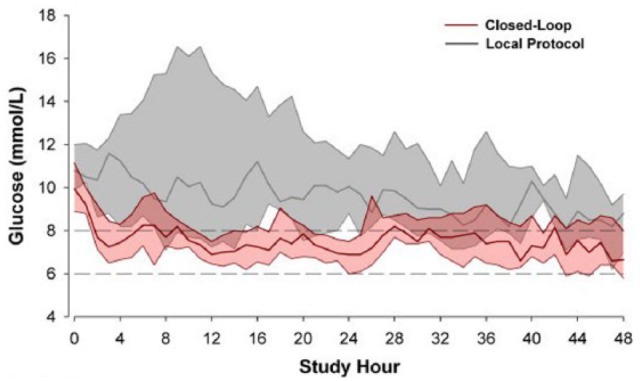
Glucose profiles (median and interquartile range) during closed-loop (pink) and manual sliding scale (gray) glucose control. Reproduced from Leelarathna et al.66
Simulation techniques have also been used to assess potential benefits of automated closed-loop compared to nonautomated protocol-driven glucose control25,26,68 and may represent a useful means of evaluating and adjusting device function and protocols, and for identifying minimal requirements for sensor accuracy before clinical testing. In recent consensus recommendations on blood glucose measurement in the ICU, it was suggested that for CGM, MARD values should not exceed 14%.30
Future Directions and Priorities for Research
Since the NICE-SUGAR study, there has been a general lapse in urgency surrounding the need to ensure that ICU patients achieve tight glucose targets. Yet there is good evidence that hypoglycemia and hyperglycemia are associated with worse outcomes in ICU patients. There is also evidence that CGM can improve glucose control in ICU patients by improving the time in band and reducing variability, and it is likely that CGM is also associated with reduced workload and costs. Finally, there is evidence that good glucose control is associated with better outcomes.
The next step is to clearly demonstrate that the better glucose control achieved with CGM and/or closed-loop systems is associated with improved clinical outcomes compared to intermittent monitoring and with a favorable cost-benefit ratio. These devices are expensive to develop and high quality studies are needed to prove that outcomes can be improved and that this approach can be economically viable for all involved.
Here we suggest some of the priorities (in no particular order) for future research in this field:
Measures of accuracy and of quality of glucose control need to be standardized to enable comparisons across studies and devices and for licensing authorities.
Adequately powered, good quality, randomized, controlled studies need to be conducted to compare the effects of CGM—preferably with closed-loop glucose control—and intermittent glucose monitoring on glucose metrics (avoidance of hypoglycemia, time in band, etc). The evaluation of the effects of CGM on clinical outcomes of morbidity, including rates of infection, and mortality measures would require large-scale studies.
Studies also need to be conducted in specific “at-risk” populations, for example, liver transplant recipients and patients with severe brain injury.69
The impact of different systems on workload and costs needs to be better defined.
The additional effects of continuing glucose control onto the general floor after the ICU stay need to be investigated to facilitate evaluation of effects on longer-term outcomes including infection rates.
Current algorithms are developed to work with intermittent monitoring systems and need to be adapted and validated for CGM.
Further understanding of glucose physiology in critically ill patients is needed to understand the impact of glucose control on insulin sensitivity and protein metabolism and their clinical relevance.
Automated closed-loop systems may prove to be beneficial in terms of reduced glucose variability and workload and should be tested clinically provided minimum sensor accuracy requirements are met.
Improved collaboration within the industry needs to be encouraged because no single company produces all the necessary components for an automated closed-loop system.
Combining glucose measurement with measurement of other variables in 1 sensor may help make this approach more financially viable.
Acknowledgments
The authors acknowledge the help of Dr Karen Pickett in writing the first draft of this review based on presentations by the authors at a meeting on glucose control endorsed by the Diabetes Technology Society and the European Society of Intensive Care Medicine.
Footnotes
Abbreviations: CGM, continuous glucose monitoring, ICU, intensive care unit; MARD, mean absolute relative difference.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JCP is a consultant for Edwards, Medtronic and Optiscan. JGC has consulted with Medtronic. RH has received speaker honoraria from Minimed Medtronic, Eli Lilly, BBraun, and Novo Nordisk, served on advisory panels for Eli Lilly, Novo Nordisk and Merck, received license fees from BBraun and Medtronic, and served as a consultant to BBraun. JIJ has received research funding and/or has been a consultant for Edwards Lifesciences, Medtronic Diabetes, GluMetrics, Glysure, Roche Diagnostics, Thermalin Diabetes, and Echo Therapeutics. JSK is a consultant for Edwards, Medtronic, Roche Diagnostics and Optiscan. CD is a consultant for Abbott, A. Menarini Diagnostics, Medtronic, Roche Diagnostics. LF has received speaking fees from Edwards Lifesciences.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This document was supported by unrestricted educational grants from B Braun, Glucoset, Glysure, Medtronic, Menarini Diagnostics, Optiscan, and Roche. These companies had no influence on the content of the article or on the decision to publish.
References
- 1. van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359-1367. [DOI] [PubMed] [Google Scholar]
- 2. Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283-1297. [DOI] [PubMed] [Google Scholar]
- 3. De La, Rosa GDC, Donado JH, Restrepo AH, et al. Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial. Crit Care. 2008;12:R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125-139. [DOI] [PubMed] [Google Scholar]
- 5. Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35:1738-1748. [DOI] [PubMed] [Google Scholar]
- 6. Arabi YM, Dabbagh OC, Tamim HM, et al. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med. 2008;36:3190-3197. [DOI] [PubMed] [Google Scholar]
- 7. Annane D, Cariou A, Maxime V, et al. Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA. 2010;303:341-348. [DOI] [PubMed] [Google Scholar]
- 8. Kalfon P, Giraudeau B, Ichai C, et al. Tight computerized versus conventional glucose control in the ICU: a randomized controlled trial. Intensive Care Med. 2014;40:171-181. [DOI] [PubMed] [Google Scholar]
- 9. Marik PE. Classic critical care papers. In: Marik PE, ed. Handbook of Evidence-Based Critical Care. New York, NY: Springer; 2010:7-12. [Google Scholar]
- 10. Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32:1119-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80. [DOI] [PubMed] [Google Scholar]
- 12. Ichai C, Preiser JC. International recommendations for glucose control in adult non diabetic critically ill patients. Crit Care. 2010;14:R166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kavanagh BP, McCowen KC. Clinical practice. Glycemic control in the ICU. N Engl J Med. 2010;363:2540-2546. [DOI] [PubMed] [Google Scholar]
- 14. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580-637. [DOI] [PubMed] [Google Scholar]
- 15. Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;154:260-267. [DOI] [PubMed] [Google Scholar]
- 16. Jacobi J, Bircher N, Krinsley J, et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med. 2012;40:3251-3276. [DOI] [PubMed] [Google Scholar]
- 17. Egi M, Bellomo R, Stachowski E, French CJ, Hart G. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology. 2006;105:244-252. [DOI] [PubMed] [Google Scholar]
- 18. Krinsley JS, Egi M, Kiss A, et al. Diabetic status and the relation of the three domains of glycemic control to mortality in critically ill patients: an international multicenter cohort study. Crit Care. 2013;17:R37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lanspa MJ, Dickerson J, Morris AH, Orme JF, Holmen J, Hirshberg EL. Coefficient of glucose variation is independently associated with mortality in critically ill patients receiving intravenous insulin. Crit Care. 2014;18:R86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boom DT, Sechterberger MK, Rijkenberg S, et al. Insulin treatment guided by subcutaneous continuous glucose monitoring compared to frequent point-of-care measurement in critically ill patients: a randomized controlled trial. Crit Care. 2014;18:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wernerman J, Desaive T, Finfer S, et al. Continuous glucose control in the ICU: report of a 2013 round table meeting. Crit Care. 2014;18:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eslami S, de Keizer NF, de Jonge E, Schultz MJ, Abu-Hanna A. A systematic review on quality indicators for tight glycaemic control in critically ill patients: need for an unambiguous indicator reference subset. Crit Care. 2008;12:R139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Block CE, Gios J, Verheyen N, et al. Randomized evaluation of glycemic control in the medical intensive care unit using real-time continuous glucose monitoring (REGIMEN Trial). Diabetes Technol Ther. 2015;17:889-898. [DOI] [PubMed] [Google Scholar]
- 24. Holzinger U, Warszawska J, Kitzberger R, et al. Real-time continuous glucose monitoring in critically ill patients: a prospective randomized trial. Diabetes Care. 2010;33:467-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilinska ME, Hovorka R. Glucose control in the intensive care unit by use of continuous glucose monitoring: what level of measurement error is acceptable? Clin Chem. 2014;60:1500-1509. [DOI] [PubMed] [Google Scholar]
- 26. Van Herpe T, De Moor B, van den Berghe G, Mesotten D. Modeling of effect of glucose sensor errors on insulin dosage and glucose bolus computed by LOGIC-Insulin. Clin Chem. 2014;60:1510-1518. [DOI] [PubMed] [Google Scholar]
- 27. Strasma PJ, Finfer S, Flower O, et al. Use of an intravascular fluorescent continuous glucose sensor in ICU patients. J Diabetes Sci Technol. 2015;9:762-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van Herpe T, De Brabanter J, Beullens M, De Moor B, van den Berghe G. Glycemic penalty index for adequately assessing and comparing different blood glucose control algorithms. Crit Care. 2008;12:R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vogelzang M, van der Horst IC, Nijsten MW. Hyperglycaemic index as a tool to assess glucose control: a retrospective study. Crit Care. 2004;8:R122-R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Finfer S, Wernerman J, Preiser JC, et al. Consensus recommendations on measurement of blood glucose and reporting glycemic control in critically ill adults. Crit Care. 2013;17:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Penning S, Pretty C, Preiser JC, Shaw GM, Desaive T, Chase JG. Glucose control positively influences patient outcome: a retrospective study. J Crit Care. 2015;30:455-459. [DOI] [PubMed] [Google Scholar]
- 32. Mackenzie IM, Whitehouse T, Nightingale PG. The metrics of glycaemic control in critical care. Intensive Care Med. 2011;37:435-443. [DOI] [PubMed] [Google Scholar]
- 33. Krinsley JS, Preiser JC. Time in blood glucose range 70 to 140 mg/dl >80% is strongly associated with increased survival in non-diabetic critically ill adults. Crit Care. 2015;19:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abdelmalak BB, Knittel J, Abdelmalak JB, et al. Preoperative blood glucose concentrations and postoperative outcomes after elective non-cardiac surgery: an observational study. Br J Anaesth. 2014;112:79-88. [DOI] [PubMed] [Google Scholar]
- 35. Hsu CW, Sun SF, Lin SL, Huang HH, Wong KF. Moderate glucose control results in less negative nitrogen balances in medical intensive care unit patients: a randomized, controlled study. Crit Care. 2012;16:R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Waeschle RM, Brauer A, Hilgers R, et al. Hypoglycaemia and predisposing factors among clinical subgroups treated with intensive insulin therapy. Acta Anaesthesiol Scand. 2014;58:223-234. [DOI] [PubMed] [Google Scholar]
- 37. Egi M, Bellomo R, Stachowski E, et al. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med. 2008;36:2249-2255. [DOI] [PubMed] [Google Scholar]
- 38. Krinsley JS. Glycemic control, diabetic status, and mortality in a heterogeneous population of critically ill patients before and during the era of intensive glycemic management: six and one-half years experience at a university-affiliated community hospital. Semin Thorac Cardiovasc Surg. 2006;18:317-325. [DOI] [PubMed] [Google Scholar]
- 39. Falciglia M, Freyberg RW, Almenoff PL, D’Alessio DA, Render ML. Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med. 2009;37:3001-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lanspa MJ, Hirshberg EL, Phillips GD, Holmen J, Stoddard G, Orme J. Moderate glucose control is associated with increased mortality compared with tight glucose control in critically ill patients without diabetes. Chest. 2013;143:1226-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sechterberger MK, Bosman RJ, Oudemans-van Straaten HM, et al. The effect of diabetes mellitus on the association between measures of glycaemic control and ICU mortality: a retrospective cohort study. Crit Care. 2013;17:R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Egi M, Bellomo R, Stachowski E, et al. The interaction of chronic and acute glycemia with mortality in critically ill patients with diabetes. Crit Care Med. 2011;39:105-111. [DOI] [PubMed] [Google Scholar]
- 43. Plummer MP, Bellomo R, Cousins CE, et al. Dysglycaemia in the critically ill and the interaction of chronic and acute glycaemia with mortality. Intensive Care Med. 2014;40:973-980. [DOI] [PubMed] [Google Scholar]
- 44. van den Berghe G, Wilmer A, Milants I, et al. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes. 2006;55:3151-3159. [DOI] [PubMed] [Google Scholar]
- 45. Van Ackerbroeck S, Schepens T, Janssens K, et al. Incidence and predisposing factors for the development of disturbed glucose metabolism and DIabetes mellitus AFter Intensive Care admission: the DIAFIC study. Crit Care. 2015;19:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van den Berghe G, Wouters PJ, Kesteloot K, Hilleman DE. Analysis of healthcare resource utilization with intensive insulin therapy in critically ill patients. Crit Care Med. 2006;34:612-616. [DOI] [PubMed] [Google Scholar]
- 47. Krinsley JS, Jones RL. Cost analysis of intensive glycemic control in critically ill adult patients. Chest. 2006;129:644-650. [DOI] [PubMed] [Google Scholar]
- 48. Sadhu AR, Ang AC, Ingram-Drake LA, Martinez DS, Hsueh WA, Ettner SL. Economic benefits of intensive insulin therapy in critically Ill patients: the targeted insulin therapy to improve hospital outcomes (TRIUMPH) project. Diabetes Care. 2008;31:1556-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van Hooijdonk RT, Steuten LM, Kip MM, et al. Health economic evaluation of a strict glucose control guideline implemented using point-of-care testing in three intensive care units in the Netherlands. Appl Health Econ Health Policy. 2015;13:399-407. [DOI] [PubMed] [Google Scholar]
- 50. Krinsley JS. Is glycemic control of the critically ill cost-effective? Hosp Pract. 2014;42:53-58. [DOI] [PubMed] [Google Scholar]
- 51. Aragon D. Evaluation of nursing work effort and perceptions about blood glucose testing in tight glycemic control. Am J Crit Care. 2006;15:370-377. [PubMed] [Google Scholar]
- 52. Rood E, Bosman RJ, van der Spoel JI, Taylor P, Zandstra DF. Use of a computerized guideline for glucose regulation in the intensive care unit improved both guideline adherence and glucose regulation. J Am Med Inform Assoc. 2005;12:172-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Evans A, Le Compte A, Tan CS, et al. Stochastic targeted (STAR) glycemic control: design, safety, and performance. J Diabetes Sci Technol. 2012;6:102-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Van Herpe T, Mesotten D, Wouters PJ, et al. LOGIC-insulin algorithm-guided versus nurse-directed blood glucose control during critical illness: the LOGIC-1 single-center, randomized, controlled clinical trial. Diabetes Care. 2013;36:188-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pachler C, Plank J, Weinhandl H, et al. Tight glycaemic control by an automated algorithm with time-variant sampling in medical ICU patients. Intensive Care Med. 2008;34:1224-1230. [DOI] [PubMed] [Google Scholar]
- 56. Vogelzang M, Zijlstra F, Nijsten MW. Design and implementation of GRIP: a computerized glucose control system at a surgical intensive care unit. BMC Med Inform Decis Mak. 2005;5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pielmeier U, Andreassen S, Juliussen B, Chase JG, Nielsen BS, Haure P. The Glucosafe system for tight glycemic control in critical care: a pilot evaluation study. J Crit Care. 2010;25:97-104. [DOI] [PubMed] [Google Scholar]
- 58. Boyd JC, Bruns DE. Performance requirements for glucose assays in intensive care units. Clin Chem. 2014;60:1463-1465. [DOI] [PubMed] [Google Scholar]
- 59. Arnal JM, Garnero A, Novonti D, et al. Feasibility study on full closed-loop control ventilation (IntelliVent-ASV) in ICU patients with acute respiratory failure: a prospective observational comparative study. Crit Care. 2013;17:R196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weller P, Roudsari A, Carson E. Clinical decision support systems. In: Feng DD, ed. Biomedical Information Technology. Amsterdam, Netherlands: Elsevier; 2008:375-391. [Google Scholar]
- 61. Christiansen JS, Svendsen PA, Deckert T. Insulin treatment and state of control before, during, and after connection to a glucose controlled insulin infusion system (Biostator). Horm Metab Res Suppl. 1979;8: 131-134. [PubMed] [Google Scholar]
- 62. Thabit H, Tauschmann M, Allen JM, et al. Home Use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015;373:2129-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kropff J, Del FS, Place J, et al. 2 month evening and night closed-loop glucose control in patients with type 1 diabetes under free-living conditions: a randomised crossover trial. Lancet Diabetes Endocrinol. 2015;3:939-947. [DOI] [PubMed] [Google Scholar]
- 64. Nimri R, Muller I, Atlas E, et al. MD-Logic overnight control for 6 weeks of home use in patients with type 1 diabetes: randomized crossover trial. Diabetes Care. 2014;37:3025-3032. [DOI] [PubMed] [Google Scholar]
- 65. Yatabe T, Yamazaki R, Kitagawa H, et al. The evaluation of the ability of closed-loop glycemic control device to maintain the blood glucose concentration in intensive care unit patients. Crit Care Med. 2011;39:575-578. [DOI] [PubMed] [Google Scholar]
- 66. Leelarathna L, English SW, Thabit H, et al. Feasibility of fully automated closed-loop glucose control using continuous subcutaneous glucose measurements in critical illness: a randomized controlled trial. Crit Care. 2013;17:R159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Okabayashi T, Shima Y, Sumiyoshi T, et al. Intensive versus intermediate glucose control in surgical intensive care unit patients. Diabetes Care. 2014;37:1516-1524. [DOI] [PubMed] [Google Scholar]
- 68. Bequette BW. Analysis of algorithms for intensive care unit blood glucose control. J Diabetes Sci Technol. 2007;1:813-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cinotti R, Ichai C, Orban JC, et al. Effects of tight computerized glucose control on neurological outcome in severely brain injured patients: a multicenter sub-group analysis of the randomized-controlled open-label CGAO-REA study. Crit Care. 2014;18:498. [DOI] [PMC free article] [PubMed] [Google Scholar]


