Abstract
Marijuana, behind only tobacco and alcohol, is the most popular recreational drug in America with prevalence rates of use rising over the past decade. A wide range of research has highlighted neurocognitive deficits associated with marijuana use, particularly when initiated during childhood or adolescence. Neuroimaging, describing alterations to brain structure and function, has begun to provide a picture of possible mechanisms associated with the deleterious effects of marijuana use. This chapter provides a neurodevelopmental framework from which recent data on brain structural and functional abnormalities associated with marijuana use is reviewed. Based on the current data, we provide aims for future studies to more clearly delineate the effects of marijuana on the developing brain and to define underlying mechanisms of the potential long-term negative consequences of marijuana use.
1. INTRODUCTION
Marijuana is the most commonly used illicit substance in the world and prevalence rates have been increasing over the past decade. Globally, 3–5% of the population above age 15 report current marijuana use, and in the United States 8.4% of the population above age 12 report past month use, with a peak in the 18–25-year age range in which nearly 20% of the population endorse current marijuana use (SAMHSA, 2014; UNODC, 2014). Socially and culturally, use of marijuana has become more widely accepted and viewed as less harmful over the past decade. For example, fewer than 40% of American high school seniors (17–18 years old) reported that they believe it is risky to use marijuana regularly (Johnston, O’Malley, Miech, Bachman, & Schulenberg, 2015), despite the fact that over 4 million Americans experience a marijuana use disorder each year (Center for Behavioral Health Statistics & Quality, 2015). With increased use, changing perceptions of the harmfulness of use, and the movement to decriminalize and even legalize use in numerous locales, it has become crucial to characterize the effects of marijuana on human brain development and function.
Varieties of marijuana (Cannabis sativa) contain over 70 identified cannabinoids, of which the primary psychoactive component is Δ9-tetrahydrocannabinol (THC; ElSohly & Slade, 2005). THC exerts its effects on the endogenous cannabinoid system primarily via cannabinoid-1 (CB1) receptors. CB1 receptors are distributed broadly throughout the brain with particular areas of concentration in prefrontal, cerebellar, temporal, and hippocampal regions (Burns et al., 2007; Glass, Faull, & Dragunow, 1997; cf. Fig. 1B). These brain regions rich in CB1 receptors are instrumental for a wide range of cognitive abilities including executive functioning, reward processing, and memory; thus, the potential effects of THC on brain functioning are equally broad. During acute administration, THC decreases learning and memory (Ranganathan & D’Souza, 2006), psychomotor performance and attention (Ramaekers, Kauert, Theunissen, Toennes, & Moeller, 2009), and reward responsiveness (van Hell et al., 2012), presumably through the alterations of the endocannabinoid system (Rubino et al., 2009). Recent evidence implicates a more complex interaction of THC with both glutamate and γ-aminobutyric acid (GABA) receptors, which may alter more fundamental functions, such as neural oscillatory activity (Raver & Keller, 2014; Rubino & Parolaro, 2016).
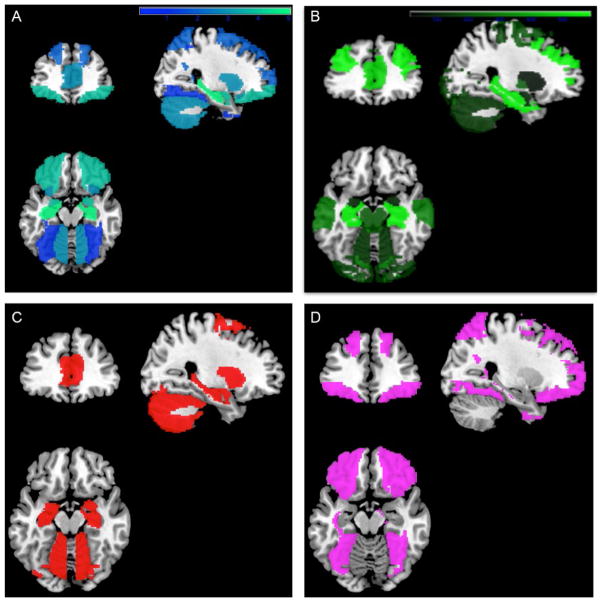
Fig. 1.
Weighted color maps. (A) Neuroanatomical alterations in marijuana users (blue (dark gray in the print version)–green (light gray in the print version)), relative to control subjects. (B) Brain map with regional distribution of cannabinoid receptor density [dark green (dark gray in the print version)–light green (light gray in the print version); range, 40–1680 density of receptor binding sites, measured via autoradiographic techniques (3)]. Lighter colors indicate evidence from more studies and greater density of receptors. (C) Binary map (red (gray in the print version)) illustrates overlap between (A) and (B), including regions high in cannabinoid receptors that also show neuroanatomical alterations. (D) Binary map (violet (light gray in the print version)) illustrates nonoverlap between (A) and (B), including areas that showed neuroanatomic alterations and are low in cannabinoid receptors. Used with permission from Lorenzetti, V., Solowij, N., & Yücel, M. (2016). The role of cannabinoids on neuroanatomical alterations in cannabis users. Biological Psychiatry, 79, e17–e31. doi:10.1016/j.biopsych.2015.11.013.
Animal models have demonstrated neurotoxic effects of THC (eg, cell shrinkage and damage to DNA structure) particularly in cortical areas with a high density of CB1 receptors (Chan, Hinds, Impey, & Storm, 1998). THC appears to block synaptic plasticity and neurogenesis, particularly in hippocampal regions, which can have an effect on brain volume (Mato et al., 2004). THC has also been shown to interfere with mesolimbic dopamine pathway functioning (Bossong et al., 2008; Kolb, Gorny, Limebeer, & Parker, 2006). THC activation of CB1 receptors located on GABAergic interneurons in the ventral tegmental area results in increased dopamine release in the nucleus accumbens. This dopamine activity is a common mechanism among drugs of abuse (Cass et al., 2014; Cheer, Wassum, Heien, Phillips, & Wightman, 2004; Gessa, Melis, Muntoni, & Diana, 1998) associated with feelings of reward, reinforcing effects (Kook & Weiss, 1992; Robbin & Everitt, 2002), and drug seeking behaviors (Aarts et al., 2010; Koepp et al., 1998; Urban et al., 2012). By contrast, continued exposure to drugs of abuse is associated with downregulation of dopamine receptors in brain reward systems (Koob & Le Moal, 2001; Koob & Volkow, 2010).
In addition, animal models have demonstrated that exposure to THC during adolescence compared to exposure during adulthood alters maturation of neural networks, particularly in the prefrontal cortex (PFC) and hippocampus (Renard et al., 2016; Rubino & Parolaro, 2016), and results in enduring cognitive deficits (DiNieri & Hurd, 2012; Quinn et al., 2007). For example, exposing rats to THC repeatedly during adolescence resulted in decreased spatial memory performance in adulthood, and this effect seems to be driven by changes in the hippocampus (Rubino et al., 2009). Specifically, rats exposed to THC during adolescence exhibited hippocampal deficits including fewer synaptic connections, less efficient connectivity, and reduced capacity for neuroplasticity (Rubino et al., 2009). In the PFC, Cass et al. (2014) reported that exposure to cannabinoid agonists during early and middle adolescence led to frequency-dependent disinhibition (ie, repeated CB1 receptor stimulation elicits PFC network disinhibition) in adulthood, which was apparently a result of downregulation of GABA transmission. Such modulation of the functioning of the PFC could decrease synaptic plasticity that is essential for the maturation of circuits underlying a broad range of functional domains from decision making to executive functioning (Selemon, 2013). Adolescent exposure to cannabinoid agonists, therefore, can have long lasting effects on the inhibitory and excitatory regulation of the PFC.
Direct downregulation of CB1 receptors may be a key mechanism of marijuana’s effects on brain function. Relatively large magnitude adaptations in CB1 receptor functioning are central to the development of tolerance to the behavioral effects of marijuana, but the rate of downregulation and desensitization varies widely by brain region, with PFC exhibiting faster downregulation and basal ganglia exhibiting slower downregulation (Sim-Selley, 2003). Interestingly, in studies utilizing positron emission tomography and FMPEP-d2, a radioligand for CB1 receptors, the adaptations in CB1 receptors in humans appear largely reversible. Chronic marijuana users exhibited a 20% decrease in cortical CB1 receptors compared to controls at baseline, but after a month of abstinence from marijuana, differences were no longer detectable between users and controls (Hirvonen et al., 2012). In animal models, there is an inverse relationship between CB1 receptor expression in cortical and striatal regions, such that striatal regions with more CB1 receptor expression receive input from cortical regions with less expression, and critically, the expression levels of CB1 receptors in these circuits decrease from adolescence to adulthood (Van Waes, Beverley, Siman, Tseng, & Steiner, 2012; Verdurand et al., 2011). Thus, exogenous influences of THC on these circuits could counteract typical maturational trajectories and yield changes in regions critical for decision making and addiction processes (Volkow & Baler, 2014; Volkow, Koob, & McLellan, 2016).
Studies in humans have demonstrated a number of neurocognitive differences between marijuana users and nonusing controls including impaired verbal learning and memory, attention, psychomotor function, and decision making (Broyd, van Hell, Beale, Yücel, & Solowij, 2016; Volkow, Swanson, et al., 2016). Evidence seems to suggest that some deficits in verbal learning and memory function may recover over time with abstinence (Schreiner & Dunn, 2012); persistent use, however, has been associated with poor long-term neurocognitive performance (Meier et al., 2012) and poorer health outcomes (ie, chronic bronchitis, increased risk of respiratory infections, and vascular conditions such as stroke or infarctions; Volkow, Baler, Compton, & Weiss, 2014). Taken together with animal models showing deficits in brain development when THC is administered during adolescence, it would seem that marijuana use, particularly during this critical developmental period, could negatively affect neurocognitive development. Indeed, individuals who begin using marijuana during adolescence exhibit lower IQs and seem to have less crystallized intelligence (ie, acquired knowledge through skills and experience; Jackson et al., 2016). At the same time, the direct effect of marijuana use on neurocognitive deteriorations during adolescence has not been shown to be significantly related to frequency of use, and may be attributable to genetic or familial factors that contribute to risk for both marijuana use initiation and low intellectual achievement (Jackson et al., 2016). Therefore, characterizing changes in brain structure and function related to marijuana use may help differentiate risk factors from consequences of marijuana use. Careful study design is necessary to overcome some of the inherent challenges in illustrating specific effects of marijuana, as marijuana use often cooccurs with alcohol and nicotine consumption, and heritable factors (eg, genetics and personality traits) likely contribute to brain differences independent of marijuana use (Hurd, Michaelides, Miller, & Jutras-Aswad, 2014).
In the current chapter, we will (1) describe a neurodevelopmental framework for understanding the effects of marijuana use on brain structure and function, (2) summarize the robust results and discuss recent findings from neuroimaging studies of the effects of marijuana use on brain structure and function, and (3) highlight remaining questions and areas of ongoing evaluation in understanding the effects of marijuana use on the human brain.
2. NEURODEVELOPMENTAL FRAMEWORK FOR UNDERSTANDING THE EFFECTS OF MARIJUANA ON BRAIN STRUCTURE AND FUNCTION
The human brain continues to mature throughout adolescence, a time during which dramatic changes occur in neurochemistry and anatomy including both cortical and hormonal alterations (Giedd et al., 1999; Gogtay et al., 2004; Luciana, 2013; Raznahan et al., 2011). Gray matter (GM) decreases following an inverted U-shaped developmental progression—peaking in childhood and decreasing through the early 20s (Giedd et al., 1999, 2009). The measures of GM (cortical thickness (CT), surface area (SA), and their product, cortical volume) follow different developmental trajectories with CT peaking between 2 and 4 years of age and then steadily declining through adulthood, while SA increases up to roughly age 12 before leveling off through age 30 (Amlien et al., 2014; Walhovd, Fjell, Giedd, Dale, & Brown, 2016). The trajectories of CT and SA yield the cortical GM volumes that decrease from around ages 10–12 through adulthood. Normally, age-related decreases in GM localized to the prefrontal and temporal cortices (Giedd et al., 1999; Gogtay et al., 2004) and in subcortical structures, including the striatum and thalamus (Sowell, Thompson, Holmes, Jernigan, & Toga, 1999; Toga, Thompson, & Sowell, 2006), are attributed to pruning of excessive neurons and to cortical myelination (Huttenlocher & Dabholkar, 1997; Shaw et al., 2008).
White matter (WM) volume increases linearly during adolescence through young adulthood (Giedd et al., 1999, 2009), yielding relatively stable total brain volumes after puberty. WM maturation is associated with greater structural connectivity between brain regions and, consequently, more efficient communication between frontal and subcortical regions (Sowell et al., 1999; Toga et al., 2006). In addition to increasing volume of WM, the quality of the microstructure continues to mature to more efficient and well-connected networks. Common measures from diffusion tensor imaging (DTI) used to infer WM microstructure include fractional anisotropy (FA), or directionally restricted diffusion of water, radial diffusivity (RD), or the perpendicular diffusion of water, and mean diffusivity (MD), or the overall magnitude of diffusion. Increasing FA is associated with more efficient and mature WM, while higher RD and MD are associated with less efficient WM. FA increases throughout adolescence and young adulthood with a relatively stable peak between 20 and 40 years, while RD and MD tend to reflect the opposite trends with decreases through adolescence and young adulthood with a relatively stable minimum between 18 and 40 years of age (Bava et al., 2010; Lebel et al., 2012). These general developmental trajectories form the basis for understanding and interpreting potential changes associated with marijuana use.
The peak incidence of marijuana use occurs in later adolescence and early adulthood, so, it is critical to approach questions of marijuana effects on brain structure and function from a developmental perspective. Casey, Oliveri, and Insel (2014) presented a neurodevelopmental framework that is useful for conceptualizing critical questions of potential alterations to development by outside factors, such as drugs of abuse. The first concept is developmental trajectories, which is largely described by the extant literature on adolescent brain development. Starting from these well-established developmental trajectories, summarized earlier, allows us to describe alterations in terms of delays, deviations, or regressions from normal trajectories.
The second concept is sensitive periods, or windows of development in which the brain may be uniquely malleable and perhaps susceptible to influences that could alter its trajectory (Casey et al., 2014). The changes occurring in the adolescent brain, characterized by plasticity and maturational changes, represent both opportunities and vulnerabilities that make this developmental period critical (Dahl, 2004; Fuhrmann, Knoll, & Blakemore, 2015; Luciana, 2013). This concept is relevant to the goal of identifying developmentally defined epochs of exposure to marijuana that yield stronger and perhaps more deleterious effects on developmental trajectories.
The third concept is dynamic interaction of systems (Casey et al., 2014). Comprehending the developmental cascades at play in determining the effects of marijuana use on brain structure and function requires sophisticated models of complex interactions. Making attempts to characterize the interaction of multiple brain systems maturing at different rates over adolescence and early adulthood will be critical for understanding how environmental events (eg, onset of marijuana use) may modulate the function or development of these systems. Furthermore, capturing synergism between genetics, behavioral traits, and experience is a vital and complex process. As we summarize results from imaging studies of the effects of marijuana use on the brain in humans, we will frequently refer to these concepts to help guide interpretations and to delineate questions and areas for future investigations.
3. STRUCTURAL CHANGES ASSOCIATED WITH MARIJUANA USE
3.1 Gray Matter
Neuroanatomic differences in GM between marijuana users and healthy controls have been reported in several specific regions, but there is very little evidence of global differences in intracranial volume or total GM volume (Lorenzetti, Solowij, Fornito, Ian Lubman, & Yucel, 2014). Many studies have reported smaller volumes in regions rich in CB1 receptors in marijuana users, and while several studies have summarized large portions of this literature (Batalla et al., 2013; Bhattacharyya, Atakan, Martin-Santos, Crippa, & McGuire, 2012; Lorenzetti et al., 2014; Lorenzetti, Solowij, & Yücel, 2016; Martín-Santos et al., 2010), we will summarize the most robust findings here while also highlighting recent reports (ie, since 2010; Table 1 and Fig. 1).
Table 1.
Recent Studies (2010–2015) Comparing Morphometric Brain Measures with Marijuana Users and Nonusers
| Authors | Key Findings | Groups | n | Age Range Mean (SD) [Range] |
Sex % Male |
|---|---|---|---|---|---|
| Ashtari et al. (2011) | MJ <CON volume in right and left hippocampus; In MJ smaller hippocampus volume =more use |
MJ abstinent 30+ days—mean 6 months |
14 | 19.3 (0.8) [18–20] | 100% |
| CON | 14 | 18.5 (1.4) [18–20] | 100% | ||
| Battistella et al. (2014) | RMJ <OMJ volume in the medial temporal cortex, temporal pole, parahippocampal gyrus, insula, and orbitofrontal cortex; Lower age of onset =smaller volumes RMJ >OMJ volume in cerebellum |
OMJ | 22 | 25 (2.8)a [19–29] | 100% |
| RMJ | 23 | 23 (2.2) [20–28] | 100% | ||
| Churchwell et al. (2010) | MJ <CON volume in right medial orbital PFC; Lower age of onset =smaller volumes |
MJ | 18 | 17.7 (0.9) [16–19] | 89% |
| CON | 18 | 17.2 (0.8) [16–19] | 67% | ||
| Cousijn et al. (2012) | MJ >CON volume in anterior cerebellum; Within MJ higher use =smaller volume in amygdala and hippocampus |
MJ | 33 | 21.3 (2.4) [18–25] | 64% |
| CON | 42 | 21.9 (2.4) [18–25] | 62% | ||
| Demirakca et al. (2011) | MJ <CON volume in right hippocampus; Within MJ higher THC in THC/CBD ratio =smaller volume |
MJ | 11 | NR [19–25]b | 100% |
| CON | 13 | NR [19–25]b | 100% | ||
| Filbey et al. (2014) | MJ =MJ only <CON volume in right middle OFC, and left superior OFC | MJ | 48 | 28.3 (8.3) [NR] | 69% |
| MJ only No alcohol use |
27 | 28.1 (8.9) [NR] | 63% | ||
| CON | 62 | 30.0 (7.4) [NR] | 63% | ||
| Filbey et al. (2015) | MJ early with more use>MJ late with more use thickness in anterior dorsolateral frontal cortex (moderator effect) | MJ early onset<age 16 | 20 | 32.5 (8.01) [21–50] | 55% |
| MJ late onset>age 16 | 22 | 30.3 (7.19) [21–47] | 73% | ||
| Gilman et al. (2014) | MJ >CON GM density in left nucleus accumbens, hypothalamus, sublenticular extended amygdala, and left amygdala; Significant shape differences in left nucleus accumbens and right amygdala |
MJ | 20 | 21.3 (1.9) [18–25] | 45% |
| CON | 20 | 20.7 (1.9) [18–25] | 45% | ||
| Jacobus et al. (2015) | MJ + ALC >CON thickness across frontal and parietal regions; MJ >CON (controlling for alcohol) thickness in left temporal and right entorhinal cortices |
MJ + ALC | 30 | Baseline: 18.2 (0.8) [16–19] Year 1.5: 19.6 (0.8) Year 3: 21.2 (0.7) |
63% |
| CON | 38 | Baseline: 17.7 (0.9) [16–19] Year 1.5: 19.1 (0.9) Year 3: 20.8 (1.0) |
76% | ||
| Lopez-Larson et al. (2011) | MJ <CON thickness in right caudal middle frontal, bilateral insula, and bilateral superior frontal regions MJ >CON thickness in the bilateral lingual, right superior temporal, right inferior parietal and left paracentral regions Within MJ lower age of onset =thicker right superior frontal gyrus |
MJ | 18 | 17.8 (1.0) [16–19] | 94.4% |
| CON | 18 | 17.3 (0.8) [16–19] | 66.6% | ||
| Lorenzetti et al. (2015) | MJ <CON volume in hippocampus and amygdala | MJ | 15 | 40 (9) [NR] | 100% |
| CON | 15 | 36 (10) [NR] | 100% | ||
| Medina et al. (2010) | MJ >CON volume in inferior posterior vermis (cerebellum) | MJ | 16 | 18.11 (0.74) [16–18] | 75% |
| CON | 16 | 18.01 (0.97) [16–18] | 63% | ||
| Schacht et al. (2012) | MJ <CON volume in hippocampus and left amygdala MJ <CON CB1 receptor genetic variation (rs2023239 G allele) =lower volume hippocampus (group × time interaction) |
MJ full | 94 | 24.2 (7.4) [NR] | 34% |
| MJ matched | 37 | 27.8 (8.7) [NR] | 37% | ||
| CON | 37 | 27.3 (7.9) [NR] | 37% | ||
| Weiland et al. (2015) | No volume or shape differences in subcortical structures examined (nucleus accumbens, amygdala, hippocampus, and cerebellum) | Adult MJ | 29 | 27.4 (7.1) [19–53] | 55% |
| Adult CON | 29 | 27.5 (6.8) [18–53] | 55% | ||
| Adol MJ | 50 | 16.7 (1.1) [14–18] | 82% | ||
| Adol CON | 50 | 16.8 (1.0) [14–18] | 72% | ||
| Yip et al. (2014) | MJ <CON volume in putamen Within MJ smaller putamen volume =higher probability of relapse |
MJ dependent | 20 | 26.7 (2.2) [NR] | 100% |
| CON | 20 | 29.2 (2.3) [NR] | 100% |
Notes: ALC, alcohol use group; CON, control group; MJ, marijuana use group; MJ+ ALC, marijuana and alcohol use group; NR, not reported; OMJ, occasional marijuana use group; RMJ, regular marijuana use group.
Median (SD).
No means provided but groups were reported as equal.
Smaller hippocampal volumes in marijuana users relative to healthy controls have been one of the most consistently reported findings (Ashtari et al., 2011; Demirakca et al., 2011; Lorenzetti et al., 2015; Matochik, Eldreth, Cadet, & Bolla, 2005; Schacht, Hutchison, & Filbey, 2012; Yucel et al., 2008). These studies reported cross-sectional comparisons of marijuana users and nonusing controls with varying levels of marijuana use history, and many studies examining the effect of dosage or lifetime exposure show an inverse relationship between use and hippocampal volume, with more use resulting in smaller volumes (Ashtari et al., 2011; Matochik et al., 2005; Yucel et al., 2008). In a sample that did not exhibit group differences between marijuana users and controls, higher lifetime marijuana use within the marijuana-using group was associated with smaller hippocampal volumes (Cousijn et al., 2012). Thus, dose and duration appear critical for effects of marijuana on hippocampal volume.
Other factors may also contribute to smaller hippocampal volumes in marijuana users. For example, in a small sample of young adults, higher levels of THC relative to cannabidiol (CBD) in a hair sample, reflecting use over the past 4–5 months, was associated with smaller hippocampal volumes (Demirakca et al., 2011). Furthermore, an examination of genetic variants of a CB1 receptor gene (ie, rs2023239) provided evidence that genes may interact with marijuana use, as carriers of the G allele who used marijuana exhibited smaller hippocampal volumes compared with healthy controls carrying the G allele, as well as compared with marijuana-using carriers of the A allele (Schacht et al., 2012). Identifying a genetic component that may interact with marijuana use is an important step in differentiating risk factors for marijuana use from outcomes of THC toxicity following marijuana use.
In addition to effects on hippocampal volume, several studies have reported consequences of marijuana use on the amygdala and striatum, including increased GM density (likely reflecting denser dendritic branching; Gilman et al., 2014; Matochik et al., 2005), and decreased volumes (Lorenzetti et al., 2015; Schacht et al., 2012; Yip et al., 2014). In other structures, associations between marijuana use and brain volumes are less well delineated. For example, in marijuana users compared to controls, one study reported a slight increase in accumbens volume (Gilman et al., 2014), another reported greater GM density in the left nucleus accumbens extending to subcallosal cortex, hypothalamus, and amygdala (Gilman et al., 2014), and marijuana use has also been associated with smaller and thinner insular cortex (Battistella et al., 2014; Lopez-Larson et al., 2011).
The cerebellum, noted as a key target of marijuana exposure in imaging studies, is a region rich in CB1 receptors. Two studies that included adolescents and young adults reported larger cerebellar volumes among marijuana users compared to controls (Cousijn et al., 2012; Medina, Nagel, & Tapert, 2010), while a study in a much older sample with a longer history of marijuana use reported smaller cerebellar volumes compared to controls (Solowij et al., 2011). Additionally, in a large study that recruited a sample of adult marijuana users and controls and a sample of adolescent marijuana users and controls, no significant differences in volume were found in subcortical structures (eg, amygdala, hippocampus, and cerebellum) in either sample (Weiland et al., 2015). Weiland et al. (2015) highlight that their marijuana users and controls were matched on alcohol use severity using the Alcohol Use Disorders Identification Test (AUDIT); however, characterizing marijuana use groups based on the past 2 months (for adults) and past 3 months (for adolescents) of use limited their ability to examine effects of cumulative lifetime use or age of onset.
The morphometry of PFC is also affected by marijuana use (Fig. 1). Specifically, marijuana use has been associated with smaller volumes in the orbitofrontal cortex (OFC; Battistella et al., 2014; Churchwell, Lopez-Larson, & Yurgelun-Todd, 2010; Filbey et al., 2014) and with thinner cortices in caudal middle frontal regions (Lopez-Larson et al., 2011). Interestingly, age of onset was negatively correlated with thickness in two studies (Filbey, McQueeny, DeWitt, & Mishra, 2015; Lopez-Larson et al., 2011). In both, earlier onset of marijuana use (ie, before age 16) was associated with thicker PFC. Lopez-Larson et al. (2011) examined participants while they were still adolescents (ie, 16–19 years old) and reported a negative correlation between age of onset of regular marijuana use and the superior frontal gyrus, whereas Filbey et al. (2015) collected data on older participants (ie, 21–50 years old) and reported that those who initiated marijuana use before age 16 exhibited thicker anterior dorsolateral PFC. Taken together, these findings demonstrate that early marijuana use onset interacts with cortical development during adolescence, likely leading to the disruption of typical development (eg, pruning and plasticity).
As mentioned, the majority of currently published data on the effects of marijuana use on GM is cross-sectional; very few studies have examined changes over time using longitudinal methods. One study utilizing a single MRI acquisition at age 12 to predict substance use outcomes, at age 16 reported that smaller OFC (but not amygdala, hippocampus, or anterior cingulate cortex) volumes were associated with a greater probability of initiation of marijuana use by age 16 (Cheetham et al., 2012). The authors controlled for other substance use and also examined whether smaller OFC volumes predicted the onset of other substance use, and reported that OFC volumes were uniquely associated with the onset of marijuana use. Thus, regional volume in the OFC appears to be specifically related to THC use, and could be a potential risk marker for initiating and maintaining marijuana use.
Some of the only studies to date that have utilized longitudinal MRI data to examine the effects of marijuana use have come out of our group (Tapert and colleagues). For example, we recently examined CT over 3 years of late adolescence (18–21 years old) in a sample of heavy marijuana and alcohol users compared to controls (Jacobus et al., 2015). Substance users exhibited thicker cortices in all four lobes of the brain that persisted over the 3-year period, which might reflect a reduction in expected developmental pruning. Thickness measures trended downward in both groups, as expected, but the substance-using sample exhibited a shallower decline compared to controls (eg, in the paracentral lobule). Furthermore, when controlling for alcohol use, lifetime marijuana use was positively associated with CT while age of onset of marijuana use was negatively associated with CT in the temporal lobe, indicating that some of the observed differences were likely specific to marijuana use. Another recent study, examining 16–18-year-old participants over 18 months described similar attenuation of CT declines (ie, shallower slope of change) in marijuana users compared to controls (Epstein & Kumra, 2015a). These longitudinal data largely support reports from cross-sectional studies, and underscore the concept that changes in cortical morphometry are dynamic and influenced by preexisting vulnerabilities as well as by different levels of neurotoxicity or attenuated neuroplasticity related to dose, duration, and age of onset of marijuana use.
3.2 White Matter
A majority of studies examining WM integrity in cross-sectional samples of adolescent and adult marijuana users have reported decreased FA and increased MD and RD indicating less efficient or less mature WM microstructure in users compared to controls in widely dispersed brain regions (Table 2). For example, central WM regions such as the genu, rostrum, and splenium of the corpus callosum (Arnone et al., 2008; Gruber, Dahlgren, Sagar, Gönenç, & Lukas, 2014; Gruber, Silveri, Dahlgren, & Yurgelun-Todd, 2011), as well as the superior longitudinal fasciculus and arcuate fasciculus (Ashtari, Cervellione, Cottone, Ardekani, & Kumra, 2009; Bava et al., 2009; Thatcher, Pajtek, Chung, Terwilliger, & Clark, 2010) show higher FA in controls compared to marijuana users. Some studies report increased FA (ie, purportedly more efficient or mature WM) among users in regions such as the forceps minor (Filbey et al., 2014) and the internal capsule and superior longitudinal fasciculus (Bava et al., 2009), though such results are tentative since DTI findings can be affected by important covariates such as age of onset of marijuana use (Gruber et al., 2014, 2011) and sex (Thatcher et al., 2010).
Table 2.
Recent Studies (2009–2015) Comparing White Matter Integrity Measures Between Marijuana Users and Nonusers
| Authors | Key Findings | Groups | n | Age Range Mean (SD) [Range] |
Sex % Male |
|---|---|---|---|---|---|
| Ashtari et al. (2009) | MJ <CON FA in arcuate fasciculus MJ <CON RD in arcuate fasciculus |
MJ abstinent 30+ days |
14 | 19.3 (0.8) [18–21] | 100% |
| CON | 14 | 18.5 (1.4) [17–21] | 100% | ||
| Bava et al. (2009) | MJ + ALC <CON FA in left superior longitudinal fasciculus, left postcentral gyrus, bilateral crus cerebri, and inferior frontal and temporal tracts MJ + ALC >CON FA in right occipital, internal capsule, and superior longitudinal fasciculus |
MJ + ALC | 36 | 17.9 (0.9) [16–19] | 72% |
| CON | 36 | 17.8 (0.8) [16–19] | 72% | ||
| Bava et al. (2013) | MJ + ALC <CON FA in right splenium, right prefrontal thalamic fibers, and right corona radiate at baseline and 18 months MJ + ALC >CON MD & RD in superior longitudinal fasciculus, right posterior thalamic radiations, right prefrontal thalamic fibers, right superior temporal gyrus, right inferior longitudinal fasciculus, and left posterior corona radiate at baseline and 18 months |
MJ + ALC | 41 | Time 1: 18.4 (1.2) [16–21] Time 2: 19.8 (1.1) [17–22] |
73% |
| CON | 51 | Time 1: 17.9 (1.1) [16–21] Time 2: 19.3 (1.1) [17–22] |
63% | ||
| Becker et al. (2015) | MJ >CON FA in genu, forceps minor; MJ <CON growth in FA in superior longitudinal fasciculus, left superior frontal gyrus, left corticospinal tract, and right anterior thalamic radiation Within MJ higher use =reduced longitudinal growth in FA |
MJ | 23 | Time 1: 19.5 (0.7) [18–20] Time 2 + 2.2 (0.5) |
70% |
| CON | 23 | Time 1: 19.2 (2.3) [15–23] Time 2: +2.2 (0.5) years |
70% | ||
| Epstein and Kumra (2015b)a | MJ <CON FA in inferior longitudinal fasciculus over 18 months More use over 18 months =lower FA |
MJ | 19 | Baseline: 16.6 (1.5) Year 1.5: +1.5 years |
58% |
| CON | 29 | Baseline: 16.5 (2.2) Year 1.5: +1.5 years |
45% | ||
| Filbey et al. (2014) | MJ >CON FA in forceps minor MJ <CON RD in forceps minor |
MJ | 48 | 28.3 (8.3) [NR] | 69% |
| MJ exc marijuana use only | 27 | 28.1 (8.9) [NR] | 63% | ||
| CON | 62 | 30.0 (7.4) [NR] | 63% | ||
| Gruber et al. (2014) | MJ <CON FA in genu and left internal capsule MJ <CON MD in genu Within MJ earlier age onset =lower FA |
MJ | 25 | 23.1 (3.5) [NR] | 72.0% |
| CON | 18 | 23.2 (5.9) [NR] | 38.9% | ||
| Gruber et al. (2011) | MJ <CON FA in left frontal regions Within MJ earlier age onset =lower FA |
MJ | 15 | 25 (8.7) [NR] | 93.3% |
| CON | 15 | 25.2 (8.4) | 93.3% | ||
| Jacobus, McQueeny, et al. (2009) | MJ + ALC <CON FA in corona radiate and superior longitudinal fasciculus ALC <CON FA in corona radiata, inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, cerebellar peduncle, and superior longitudinal fasciculus MJ + ALC >ALC FA in corona radiata, inferior fronto-occipital fasciculus, cerebellar peduncle, and superior longitudinal fasciculus |
MJ + ALC | 14 | 18.2 (0.7) [16–19] | 79% |
| ALC | 14 | 18.1 (0.7) [16–19] | 86% | ||
| CON | 14 | 17.3 (0.8) [16–19] | 86% | ||
| Jacobus, Thayer, et al. (2013) | Within MJ + ALC lower FA in fornix and superior corona radiata at time 1 =higher substance use and risk taking at time 2 | MJ + ALC | 47 | Time 1: 18.0 (0.9) [16–19] Time 2: 19.5 (0.9) |
59% |
| CON | 49 | Time 1: 17.6 (0.8) [16–19] Time 2: 19.0 (0.9) |
73% | ||
| Jacobus, Squeglia, Infante, et al. (2013) | MJ decreased FA from time 1 to time 2 (12 clusters) MJ decreased and ALC increased FA from time 1 to time 2 (6 clusters) |
MJ initiators | 8 | Time 1: 18.2 (0.7) [17–19] Time 2: 21.2 (NR) [20–22] |
NR |
| ALC initiators | 8 | Time 1: 17.5 (0.8) [17–19] Time 2: 20.6 (NR) [20–23] |
NR | ||
| Jacobus, Squeglia, Bava, et al. (2013) | MJ + ALC =ALC <CON FA in 14 clusters ALC <MJ + ALC <CON FA in uncinate fasciculus MJ + ALC =MJ =decrease FA from baseline to year 3 in 12 clusters |
MJ + ALC | 21 | Baseline: 17.9 (NR) [16–19] Year 1.5: 19.4 (NR) [17–20] Year 3: 20.9 (NR) [19–21] |
62% |
| ALC | 17 | Baseline: 17.9 (NR) [16–19] Year 1.5: 19.3 (NR) [17–20] Year 3: 20.9 (NR) [19–22] |
64% | ||
| CON | 16 | Baseline: 17.9 (NR) [16–19] Year 1.5: 19.4 (NR) [17–20] Year 3: 20.9 (NR) [19–22] |
50% | ||
| Zalesky et al. (2012) | MJ <CON WM connectivity in the right fimbria of the hippocampus (fornix), splenium of the corpus callosum, and commissural fibers Within MJ lower age of onset =higher RD | MJ | 59 | 33.4 (10.9) [NR] | 47% |
| CON | 33 | 31.5 (12.0) [NR] | 42% |
Notes: ALC, alcohol use group; CON, control group; FA, fractional anisotropy; MJ, marijuana use group; MJ+ALC, marijuana and alcohol use group; NR, not reported; RD, radial diffusivity; MD, mean diffusivity; WM, white matter.
An early-onset schizophrenia group was reported in this study, but is not summarized here.
In order to elucidate some of complexities from the many cross-sectional studies on marijuana’s effects on WM, our lab reported on a series of longitudinal studies of late adolescence. We found that over an 18-month follow-up period of continued use, heavy marijuana and alcohol use resulted in systematically lower FA and higher MD, specifically in the superior longitudinal fasciculus, right posterior thalamic radiations, right prefrontal thalamic fibers, right superior temporal gyrus, right inferior longitudinal fasciculus, and left posterior corona radiata (Bava, Jacobus, Thayer, & Tapert, 2013). Marijuana use did not independently predict these effects, but drinking predicted decreased FA and increased MD over the follow-up period. Another recent study reported that 16–18-year-old marijuana users exhibited decreasing FA values in the inferior longitudinal fasciculus over 18 months compared to healthy controls (Epstein & Kumra, 2015b).
In a separate longitudinal study including three groups of adolescents (heavy drinkers, heavy drinkers and heavy marijuana users, and controls) examined over 3 years (ie, 18–21), we found that controls exhibited greater FA than both user groups across the majority of regions examined and that both user groups exhibited lower FA values at each time point over the 3 years (Jacobus, Squeglia, Bava, & Tapert, 2013). The substance-using groups differed in only one region, the right uncinate fasciculus, where the heavy drinking and marijuana-using group exhibited higher FA than the heavy drinking only group. We further examined a group of adolescents who reported little substance use at baseline (16–18 years old), but then initiated either alcohol or marijuana by the 3-year follow-up assessment (19–22 years old) to try to parse out premorbid WM differences from those related to substance use (Jacobus, Squeglia, Infante, Bava, & Tapert, 2013). In this small study (n=8 alcohol initiators and n=8 alcohol and marijuana initiators), we found decreasing FA in the alcohol and marijuana group, but not in the alcohol-only group across 20 different brain regions. This study, while preliminary, represents one of the only studies to date that has examined WM microstructure prior to and after the initiation of alcohol and marijuana use in adolescents and highlights a number of regions (ie, splenium of the corpus callosum, forceps minor, fronto-occipital fasciculus, superior corona radiata, posterior corona radiata, corticospinal tract, internal capsule, uncinated fasciculus, and superior longitudinal fasciculus) of interest for future studies.
Another longitudinal examination of WM over 2 years of late adolescence/early adulthood (ie, 19–21 years old) conducted by Becker, Collins, Lim, Muetzel, and Luciana (2015) compared heavy marijuana users who concurrently used alcohol, with controls who had no marijuana and limited alcohol use (Becker et al., 2015). Their group reported higher FA and lower RD in the controls compared to substance users over 2 years in the superior longitudinal fasciculus and corticospinal tract. Marijuana users also exhibited higher FA, particularly in the right genu of the corpus callosum. Interestingly, the authors found that higher levels of marijuana use reported in the past 12 months at follow-up was negatively associated with FA in the superior longitudinal fasciculus and corticospinal tract. While the marijuana-using group also reported significantly more alcohol use than the control group, alcohol use was unrelated to the observed FA changes over the 2 years. These results are consistent with our smaller study examining the onset of marijuana and alcohol use, in that marijuana and alcohol, but not alcohol alone, were associated with greater WM deficits.
The WM alterations associated with marijuana use are likely attributable to effects on pruning and myelination processes occurring during adolescence and early adulthood (Lebel et al., 2012) and could underlie functional alterations later in life. For example, Filbey et al. (2014) examined a sample of adult marijuana users and found they exhibited smaller OFC volumes, but greater functional connectivity in the OFC. Another group reported the same increased OFC functional connectivity in a sample of 14–20 years old adolescent and young adult marijuana users (Lopez-Larson, Rogowska, & Yurgelun-Todd, 2015). Similar results have been reported in resting state data (Orr et al., 2013) and in functional connectivity underlying cognitive control tasks (Harding et al., 2012). The authors postulate that the increased functional connectivity coupled with decreased volume and structural connectivity in WM tracts (ie, decreased FA), may indicate a compensatory mechanism or “neural scaffolding” effect, wherein neurodevelopmental trajectories are modulated by exposure to marijuana and result in long-term functional alterations (Filbey et al., 2014). To explore the functional consequences of structural differences related to marijuana use further, we will briefly review findings related to functional brain imaging studies.
4. FUNCTIONAL MRI OBSERVATIONS IN MARIJUANA USE
Several recent reviews have summarized the consequences of marijuana use on neurocognitive functioning in adolescence (Jackson et al., 2016) and adulthood (Auer, Vittinghoff, Yaffe, et al., 2016; Broyd et al., 2016), and have highlighted deficits in verbal memory, processing speed, and executive functioning (Volkow, Swanson, et al., 2016). Presumably functional MRI studies reflect the brain changes underlying the long-term neurocognitive deficits; however, the connection between structural brain changes and functional manifestations has not been clearly defined. For example, our lab has described fMRI findings from spatial working memory (Padula, Schweinsburg, & Tapert, 2007; Schweinsburg et al., 2008, 2005; Schweinsburg, Schweinsburg, Medina, et al., 2010), verbal encoding (Schweinsburg, McQueeny, Nagel, Eyler, & Tapert, 2010), and inhibitory control tasks (Tapert et al., 2007), and in each case marijuana users and controls demonstrated similar task performance, but the underlying brain activation patterns differed significantly. In our studies, marijuana users’ relative to controls’ brain function was characterized by increased activation in parietal, superior temporal, hippocampal, and posterior cingulate regions during working memory demands, and increased parietal and frontal activation during response inhibition.
Other studies in young adults have corroborated the differential activation depicted in our adolescent samples, including a study that reported hypoactivity in frontal and temporal cortices, and relative hyperactivity in the parahippocampal region during a learning task (Nestor, Roberts, Garavan, & Hester, 2008), and a study that reported greater activation in marijuana users compared to controls in frontal and temporal regions during a visuospatial working memory task (Smith, Longo, Fried, Hogan, & Cameron, 2010). In each of these cases, marijuana users and controls performed similarly on the tasks, but exhibited differential brain activation reflecting potential compensatory responses by the marijuana-using groups (Filbey et al., 2015; Schweinsburg, McQueeny, et al., 2010).
Age of onset and amount of marijuana consumed appear to modulate fMRI results. Earlier onset and more intense marijuana use during adolescence was linked to less brain activation (ie, BOLD response), and users who began using marijuana in late adolescence showed notably higher brain activation compared to earlier onset users (Jacobus, Bava, Cohen-Zion, Mahmood, & Tapert, 2009). A recent study examining performance on a Stroop task in adults showed that marijuana users performed poorly on the task compared to controls, and also exhibited a different pattern of activation in the cingulate compared to controls (Sagar et al., 2015). Specifically, the subset of marijuana smokers with an onset of regular use prior to age 16 exhibited significant activation in the anterior cingulate, which was not evident in the late onset marijuana users or controls (Sagar et al., 2015). Thus, the weakened capacity of marijuana users to rely on brain regions typically required for task execution (eg, executive functioning and working memory) appears to result in the recruitment of additional brain regions associated with attentional or verbal processing that are not typically utilized.
Marijuana use appears to affect the mesolimbic dopamine system, a target of many, if not all other drugs of abuse (Filbey & DeWitt, 2012). Tasks requiring brain reward systems revealed a similar constellation of results; namely, that marijuana users and controls did not differ in their performance of the tasks, but exhibited differential activation of reward pathways, including increased activation of regions of the basal ganglia (ie, caudate, putamen, and nucleus accumbens) and the insula (Hester, Nestor, & Garavan, 2009; van Hell et al., 2010) during reward anticipation. Additionally, marijuana users exhibited decreased activation in the insula, anterior cingulate, and putamen during loss trials in a monetary incentive delay task (Nestor, Hester, & Garavan, 2010; Wesley, Hanlon, & Porrino, 2011). Thus, marijuana users may be more sensitive to rewards and less sensitive to negative feedback or losses.
In summary, functional MRI studies reveal relatively intact performance that is accomplished with differential activation patterns in marijuana users compared to controls. The data support a hypothesis of expanding neural recruitment that entails compensatory activation in brain regions not typically utilized to accomplish the task. Future studies with more difficult neurocognitive tasks and over a larger range of ages may provide additional evidence and clarify underlying functional changes resulting from marijuana use.
5. REMAINING QUESTIONS AND FUTURE DIRECTIONS
The literature on brain structure and function is beginning to elucidate the specific and primarily detrimental effects of marijuana use, particularly when initiated while the brain is still maturing during early to mid-adolescence. Data indicates that adolescence is indeed a sensitive period in development in which marijuana use can result in long-term negative effects. Additional research is needed to clarify the developmental trajectories of neurobiological systems including the endocannabinoid system and related neuronal systems, and to more clearly characterize the effects of marijuana use on these systems. Complex interactions between the development of the endocannabinoid system with other factors including genetics (Schacht et al., 2012), age of use onset (Gruber et al., 2014), and behavioral traits (Wesley et al., 2011) will be crucial to untangle in future studies (Hurd et al., 2014). In order to obtain data capable of addressing some of these issues, a number of methodological and conceptual advancements must also be considered in the coming years.
One such advancement is a clearer depiction of the mechanisms of action of marijuana’s effects on the developing brain. Much of the neurotoxic effects of marijuana are associated with THC acting on CB1 receptors, which in turn affects a number of other neurotransmitter systems and neurodevelopmental processes (eg, synaptic plasticity and neurogenesis in the hippocampus; Lubman, Cheetham, & Yücel, 2015). THC is only one of more than 70 cannabinoids in marijuana and not all of these cannabinoids have neurotoxic effects. In fact CBD may even counteract some of the negative effects of THC (Bhattacharyya et al., 2010; Demirakca et al., 2011). Additional studies are needed to parse out the potentially countervailing effects of different cannabinoids.
In order to better understand the complex effects of marijuana, researchers also need to improve assessment techniques. The majority of marijuana use data come from self-report and are quantified with crude measures of “days of use,” “number of joints,” or “grams” consumed. While it is inherently difficult to quantify an unregulated substance, it is clear that potency of marijuana has been steadily increasing and different methods of administration inevitably lead to different active doses (ElSohly et al., 2016). Therefore, developing better and more standardized assessment techniques is essential to addressing the complexities of THC doses and potency (Lorenzetti et al., 2016). It is possible, for example, to utilize hair samples that will provide an index of use over 3–5 months and also provide a basic ratio of THC and CBD (Demirakca et al., 2011). As toxicology tests of urine and other bodily fluids continue to improve (Hartman et al., 2015, 2016), researchers will need to update current methods and incorporate additional techniques to address some of these fundamental questions of pharmacokinetics and pharmacodynamics of marijuana.
Lastly, researchers must collect longitudinal data that can disentangle precursors of risk from consequences of marijuana use. Nearly, all of the existing imaging literature on the effects of marijuana on neurodevelopment is cross-sectional in nature and cannot address questions of cause and effect. Approaching these questions from a neurodevelopmental perspective requires understanding of (1) typical trajectories, (2) sensitive periods, and (3) complex interactions at multiple levels (Casey et al., 2014). None of these aims can be sufficiently addressed solely with cross-sectional data. Fortunately, funding organizations have recently prioritized large scale, longitudinal studies, which will provide data to address some of the remaining questions. For example, in the United States, the National Institutes of Health funded the National Consortium on Alcohol and Neuro-Development in Adolescence (http://www.ncanda.org) to examine the effects of alcohol and other drugs on neurodevelopmental trajectories in a large sample (n=831) of adolescents including annual neuroimaging and neurocognitive assessments (Brown et al., 2015; Pfefferbaum et al., 2015); the European Union has funded the IMAGEN project (http://www.imagen-europe.com), which examines 2000 adolescents from age 14 to 19 with multiple imaging sessions and neurocognitive assessments (Schumann et al., 2010). Further, the National Institutes of Health has recently approved funding for the most ambitious project to date, the Adolescent Brain Cognitive Development Study (http://www.abcdstudy.org), which will follow 10,000 adolescents starting from ages 9 to 10 for at least 10 years, including imaging and neurocognitive assessment every 2 years and annual assessments of substance use and a multitude of other measures related to development. With the coming influx of longitudinal data, researchers will be able to address many of the gaps in understanding the effects of marijuana and other drugs on brain development and functioning.
6. CONCLUSIONS
Mounting evidence suggests that marijuana use negatively impacts brain structure and function. While there is potential recovery from some of the negative effects of prolonged use, long-term deleterious effects are present and more likely with early age of onset and protracted use. Data from both animal and human models highlight the particularly sensitive period of adolescence for adverse effects of marijuana through the modulation of the neurodevelopmental trajectories. The mechanisms that underlie such modulations are not fully understood and likely result from multiple levels of complex interactions including onset, dose, and duration of marijuana use as well as neurobiological factors including genetic risk. Prolonged marijuana use could result in persistent changes to brain structure and function that underlie the adverse cognitive outcomes associated with heavy use. Future prospective studies with enhanced assessment of marijuana use coupled with MRI assessment prior to and following marijuana use initiation will provide additional clarification of these complex effects of marijuana use on brain structure and function.
References
- Aarts E, Roelofs A, Franke B, Rijpkema M, Fernandez G, Helmich RC, Cools R. Striatal dopamine mediates the interface between motivational and cognitive control in humans: Evidence from genetic imaging. Neuropsychopharmacology. 2010;35(9):1943–1951. doi: 10.1038/npp.2010.68. http://dx.doi.org/10.1038/npp.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlien IK, Fjell AM, Tamnes CK, Grydeland H, Krogsrud SK, Chaplin TA, … Walhovd KB. Organizing principles of human cortical development—Thickness and area from 4 to 30 years: Insights from comparative primate neuroanatomy. Cerebral Cortex. 2014;26(1):257–267. doi: 10.1093/cercor/bhu214. http://dx.doi.org/10.1093/cercor/bhu214. [DOI] [PubMed] [Google Scholar]
- Arnone D, Barrick TR, Chengappa S, Mackay CE, Clark CA, Abou-Saleh MT. Corpus callosum damage in heavy marijuana use: Preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. NeuroImage. 2008;41(3):1067–1074. doi: 10.1016/j.neuroimage.2008.02.064. http://dx.doi.org/10.1016/j.neuroimage.2008.02.064. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Avants B, Cyckowski L, Cervellione KL, Roofeh D, Cook P, … Kumra S. Medial temporal structures and memory functions in adolescents with heavy cannabis use. Journal of Psychiatric Research. 2011;45(8):1055–1066. doi: 10.1016/j.jpsychires.2011.01.004. http://dx.doi.org/10.1016/j.jpsychires.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari M, Cervellione K, Cottone J, Ardekani BA, Kumra S. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. Journal of Psychiatric Research. 2009;43(3):189–204. doi: 10.1016/j.jpsychires.2008.12.002. http://dx.doi.org/10.1016/j.jpsychires.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer R, Vittinghoff E, Yaffe K, et al. Association between lifetime marijuana use and cognitive function in middle age: The coronary artery risk development in young adults (cardia) study. JAMA Internal Medicine. 2016;176(3):352–361. doi: 10.1001/jamainternmed.2015.7841. http://dx.doi.org/10.1001/jamainternmed.2015.7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batalla A, Bhattacharyya S, Yücel M, Fusar-Poli P, Crippa JA, Nogué S, … Martin-Santos R. Structural and functional imaging studies in chronic cannabis users: A systematic review of adolescent and adult findings. PloS One. 2013;8(2):e55821. doi: 10.1371/journal.pone.0055821. http://dx.doi.org/10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistella G, Fornari E, Annoni JM, Chtioui H, Dao K, Fabritius M, … Giroud C. Long-term effects of cannabis on brain structure. Neuropsychopharmacology. 2014;39(9):2041–2048. doi: 10.1038/npp.2014.67. http://dx.doi.org/10.1038/npp.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Research: Neuroimaging. 2009;173(3):228–237. doi: 10.1016/j.pscychresns.2009.04.005. http://dx.doi.org/10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Thayer RE, Tapert SF. Longitudinal changes in white matter integrity among adolescent substance users. Alcoholism, Clinical and Experimental Research. 2013;37:E181–E189. doi: 10.1111/j.1530-0277.2012.01920.x. http://dx.doi.org/10.1111/j.1530-0277.2012.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Research. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. http://dx.doi.org/10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MP, Collins PF, Lim KO, Muetzel RL, Luciana M. Longitudinal changes in white matter microstructure after heavy cannabis use. Developmental Cognitive Neuroscience. 2015;16:23–35. doi: 10.1016/j.dcn.2015.10.004. http://dx.doi.org/10.1016/j.dcn.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Atakan Z, Martin-Santos R, Crippa JA, McGuire PK. Neural mechanisms for the cannabinoid modulation of cognition and affect in man: A critical review of neuroimaging studies. Current Pharmaceutical Design. 2012;18(32):5045–5054. doi: 10.2174/138161212802884636. http://dx.doi.org/10.2174/138161212802884636. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, … McGuire PK. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35(3):764–774. doi: 10.1038/npp.2009.184. http://dx.doi.org/10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossong MG, van Berckel BNM, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, … Kahn RS. [Delta]9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology. 2008;34(3):759–766. doi: 10.1038/npp.2008.138. http://dx.doi.org/10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- Brown SA, Brumback T, Tomlinson K, Cummins K, Thompson WK, Nagel BJ, … Tapert SF. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): A multisite study of adolescent development and substance use. Journal of Studies on Alcohol and Drugs. 2015;76(6):895–908. doi: 10.15288/jsad.2015.76.895. http://dx.doi.org/10.15288/jsad.2015.76.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd SJ, van Hell HH, Beale C, Yücel M, Solowij N. Acute and chronic effects of cannabinoids on human cognition: A systematic review. Biological Psychiatry. 2016;79(7):557–567. doi: 10.1016/j.biopsych.2015.12.002. http://dx.doi.org/10.1016/j.biopsych.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Burns HD, Van Laere K, Sanabria-Bohórquez S, Hamill TG, Bormans G, Eng WS, … Hargreaves RJ. [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(23):9800–9805. doi: 10.1073/pnas.0703472104. http://dx.doi.org/10.1073/pnas.0703472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Oliveri ME, Insel T. A neurodevelopmental perspective on the research domain criteria (RDoC) framework. Biological Psychiatry. 2014;76(5):350–353. doi: 10.1016/j.biopsych.2014.01.006. http://dx.doi.org/10.1016/j.biopsych.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Cass DK, Flores-Barrera E, Thomases DR, Vital WF, Caballero A, Tseng KY. CB1 cannabinoid receptor stimulation during adolescence impairs the maturation of GABA function in the adult rat prefrontal cortex. Molecular Psychiatry. 2014;19(5):536–543. doi: 10.1038/mp.2014.14. http://dx.doi.org/10.1038/mp.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health. 2015 Retrieved from, http://www.samhsa.gov/data/
- Chan GCK, Hinds TR, Impey S, Storm DR. Hippocampal neurotoxicity of Δ9-tetrahydrocannabinol. The Journal of Neuroscience. 1998;18(14):5322–5332. doi: 10.1523/JNEUROSCI.18-14-05322.1998. Retrieved from, http://www.jneurosci.org/content/18/14/5322.full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien MLAV, Phillips PEM, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. The Journal of Neuroscience. 2004;24(18):4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. http://dx.doi.org/10.1523/jneurosci.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Whittle S, Simmons JG, Yücel M, Lubman DI. Orbitofrontal volumes in early adolescence predict initiation of cannabis use: A 4-year longitudinal and prospective study. Biological Psychiatry. 2012;71(8):684–692. doi: 10.1016/j.biopsych.2011.10.029. http://dx.doi.org/10.1016/j.biopsych.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Churchwell JC, Lopez-Larson M, Yurgelun-Todd DA. Altered frontal cortical volume and decision making in adolescent cannabis users. Frontiers in Psychology. 2010;1:225. doi: 10.3389/fpsyg.2010.00225. http://dx.doi.org/10.3389/fpsyg.2010.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Grey matter alterations associated with cannabis use: Results of a VBM study in heavy cannabis users and healthy controls. NeuroImage. 2012;59(4):3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. http://dx.doi.org/10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: A period of vulnerabilities and opportunities. Annals of the New York Academy of Sciences. 2004;1021(1):1–22. doi: 10.1196/annals.1308.001. http://dx.doi.org/10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Demirakca T, Sartorius A, Ende G, Meyer N, Welzel H, Skopp G, … Hermann D. Diminished gray matter in the hippocampus of cannabis users: Possible protective effects of cannabidiol. Drug and Alcohol Dependence. 2011;114(2–3):242–245. doi: 10.1016/j.drugalcdep.2010.09.020. http://dx.doi.org/10.1016/j.drugalcdep.2010.09.020. [DOI] [PubMed] [Google Scholar]
- DiNieri JA, Hurd YL. Rat models of prenatal and adolescent cannabis exposure. In: Kobeissy HF, editor. Psychiatric disorders: Methods and protocols. Totowa, NJ: Humana Press; 2012. pp. 231–242. [DOI] [PubMed] [Google Scholar]
- ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in cannabis potency over the last two decades (1995–2014)—Analysis of current data in the United States. Biological Psychiatry. 2016;79(7):613–619. doi: 10.1016/j.biopsych.2016.01.004. http://dx.doi.org/10.1016/j.biopsych.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSohly MA, Slade D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sciences. 2005;78(5):539–548. doi: 10.1016/j.lfs.2005.09.011. http://dx.doi.org/10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Epstein KA, Kumra S. Altered cortical maturation in adolescent cannabis users with and without schizophrenia. Schizophrenia Research. 2015a;162(1–3):143–152. doi: 10.1016/j.schres.2014.11.029. http://dx.doi.org/10.1016/j.schres.2014.11.029. [DOI] [PubMed] [Google Scholar]
- Epstein KA, Kumra S. White matter fractional anisotropy over two time points in early onset schizophrenia and adolescent cannabis use disorder: A naturalistic diffusion tensor imaging study. Psychiatry Research: Neuroimaging. 2015b;232(1):34–41. doi: 10.1016/j.pscychresns.2014.10.010. http://dx.doi.org/10.1016/j.pscychresns.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, Segall J. Long-term effects of marijuana use on the brain. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(47):16913–16918. doi: 10.1073/pnas.1415297111. http://dx.doi.org/10.1073/pnas.1415297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, DeWitt SJ. Cannabis cue-elicited craving and the reward neurocircuitry. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2012;38(1):30–35. doi: 10.1016/j.pnpbp.2011.11.001. http://dx.doi.org/10.1016/j.pnpbp.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, McQueeny T, DeWitt SJ, Mishra V. Preliminary findings demonstrating latent effects of early adolescent marijuana use onset on cortical architecture. Developmental Cognitive Neuroscience. 2015;16:16–22. doi: 10.1016/j.dcn.2015.10.001. http://dx.doi.org/10.1016/j.dcn.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann D, Knoll LJ, Blakemore SJ. Adolescence as a sensitive period of brain development. Trends in Cognitive Sciences. 2015;19(10):558–566. doi: 10.1016/j.tics.2015.07.008. http://dx.doi.org/10.1016/j.tics.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Gessa G, Melis M, Muntoni A, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. European Journal of Pharmacology. 1998;341(1):39–44. doi: 10.1016/s0014-2999(97)01442-8. http://dx.doi.org/10.1016/S0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, … Rapoport JL. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. http://dx.doi.org/10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(5):465–470. doi: 10.1097/CHI.0b013e31819f2715. http://dx.doi.org/10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Kuster JK, Lee S, Lee MJ, Kim BW, Makris N, … Breiter HC. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. The Journal of Neuroscience. 2014;34(16):5529–5538. doi: 10.1523/JNEUROSCI.4745-13.2014. http://dx.doi.org/10.1523/jneurosci.4745-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, Faull RLM, Dragunow M. Cannabinoid receptors in the human brain: A detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77(2):299–318. doi: 10.1016/s0306-4522(96)00428-9. http://dx.doi.org/10.1016/S0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, … Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. http://dx.doi.org/10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gönenç A, Lukas SE. Worth the wait: Effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacology. 2014;231(8):1455–1465. doi: 10.1007/s00213-013-3326-z. http://dx.doi.org/10.1007/s00213-013-3326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Silveri MM, Dahlgren MK, Yurgelun-Todd D. Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Experimental and Clinical Psychopharmacology. 2011;19(3):231–242. doi: 10.1037/a0023034. http://dx.doi.org/10.1037/a0023034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding IH, Solowij N, Harrison BJ, Takagi M, Lorenzetti V, Lubman DI, … Yucel M. Functional connectivity in brain networks underlying cognitive control in chronic cannabis users. Neuropsychopharmacology. 2012;37(8):1923–1933. doi: 10.1038/npp.2012.39. http://dx.doi.org/10.1038/npp.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman RL, Brown TL, Milavetz G, Spurgin A, Gorelick DA, Gaffney G, Huestis MA. Controlled vaporized cannabis, with and without alcohol: Subjective effects and oral fluid-blood cannabinoid relationships. Drug Testing and Analysis. 2015 doi: 10.1002/dta.1839. http://dx.doi.org/10.1002/dta.1839. (online early view) [DOI] [PMC free article] [PubMed]
- Hartman RL, Brown TL, Milavetz G, Spurgin A, Gorelick DA, Gaffney GR, Huestis MA. Effect of blood collection time on measured Δ9-tetrahydrocan-nabinol concentrations: Implications for driving interpretation and drug policy. Clinical Chemistry. 2016;62(2):367–377. doi: 10.1373/clinchem.2015.248492. http://dx.doi.org/10.1373/clinchem.2015.248492. [DOI] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34(11):2450–2458. doi: 10.1038/npp.2009.67. http://dx.doi.org/10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, … Innis RB. Reversible and regionally selective downregulation of brain cannabinoid CB(1) receptors in chronic daily cannabis smokers. Molecular Psychiatry. 2012;17(6):642–649. doi: 10.1038/mp.2011.82. http://dx.doi.org/10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL, Michaelides M, Miller ML, Jutras-Aswad D. Trajectory of adolescent cannabis use on addiction vulnerability. Neuropharmacology. 2014;76(Pt. B):416–424. doi: 10.1016/j.neuropharm.2013.07.028. http://dx.doi.org/10.1016/j.neuropharm.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. The Journal of Comparative Neurology. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. http://dx.doi.org/10.1002/(SICI)1096-9861(19971020)387:2<167::AID-CNE1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Jackson NJ, Isen JD, Khoddam R, Irons D, Tuvblad C, Iacono WG, … Baker LA. Impact of adolescent marijuana use on intelligence: Results from two longitudinal twin studies. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(5):E500–E508. doi: 10.1073/pnas.1516648113. http://dx.doi.org/10.1073/pnas.1516648113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Bava S, Cohen-Zion M, Mahmood O, Tapert SF. Functional consequences of marijuana use in adolescents. Pharmacology, Biochemistry, and Behavior. 2009;92(4):559–565. doi: 10.1016/j.pbb.2009.04.001. http://dx.doi.org/10.1016/j.pbb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, Tapert SF. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicology and Teratology. 2009;31(6):349–355. doi: 10.1016/j.ntt.2009.07.006. http://dx.doi.org/10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Bava S, Tapert SF. White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: A 3-year investigation. Psychiatry Research: Neuroimaging. 2013a;214(3):374–381. doi: 10.1016/j.pscychresns.2013.07.014. http://dx.doi.org/10.1016/j.pscychresns.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Infante MA, Bava S, Tapert SF. White matter integrity pre- and post marijuana and alcohol initiation in adolescence. Brain Sciences. 2013b;3(1):396–414. doi: 10.3390/brainsci3010396. http://dx.doi.org/10.3390/brainsci3010396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Meruelo AD, Castro N, Brumback T, Giedd JN, Tapert SF. Cortical thickness in adolescent marijuana and alcohol users: A three-year prospective study from adolescence to young adulthood. Developmental Cognitive Neuroscience. 2015;16:101–109. doi: 10.1016/j.dcn.2015.04.006. http://dx.doi.org/10.1016/j.dcn.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Thayer RE, Trim RS, Bava S, Frank LR, Tapert SF. White matter integrity, substance use, and risk taking in adolescence. Psychology of Addictive Behaviors. 2013;27(2):431–442. doi: 10.1037/a0028235. http://dx.doi.org/10.1037/a0028235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the future national survey results on drug use: 2014 Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan; 2015. [Google Scholar]
- Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, … Grasby PM. Evidence for striatal dopamine release during a video game. Nature. 1998;393(6682):266–268. doi: 10.1038/30498. http://dx.doi.org/10.1038/30498. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Limebeer CL, Parker LA. Chronic treatment with Δ-9-tetrahydrocannabinol alters the structure of neurons in the nucleus accumbens shell and medial prefrontal cortex of rats. Synapse. 2006;60(6):429–436. doi: 10.1002/syn.20313. http://dx.doi.org/10.1002/syn.20313. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. http://dx.doi.org/10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kook GF, Weiss F. Neuropharmacology of cocaine and ethanol dependence. Recent Developments in Alcoholism. 1992;10:201–233. doi: 10.1007/978-1-4899-1648-8_11. [DOI] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60(1):340–352. doi: 10.1016/j.neuroimage.2011.11.094. http://dx.doi.org/10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, Terry J, Yurgelun-Todd D. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behavioural Brain Research. 2011;220(1):164–172. doi: 10.1016/j.bbr.2011.02.001. http://dx.doi.org/10.1016/j.bbr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson MP, Rogowska J, Yurgelun-Todd D. Aberrant orbitofrontal connectivity in marijuana smoking adolescents. Developmental Cognitive Neuroscience. 2015;16:54–62. doi: 10.1016/j.dcn.2015.08.002. http://dx.doi.org/10.1016/j.dcn.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti V, Solowij N, Fornito A, Ian Lubman D, Yucel M. The association between regular cannabis exposure and alterations of human brain morphology: An updated review of the literature. Current Pharmaceutical Design. 2014;20(13):2138–2167. doi: 10.2174/13816128113199990435. http://dx.doi.org/10.2174/13816128113199990435. [DOI] [PubMed] [Google Scholar]
- Lorenzetti V, Solowij N, Whittle S, Fornito A, Lubman DI, Pantelis C, Yücel M. Gross morphological brain changes with chronic, heavy cannabis use. The British Journal of Psychiatry. 2015;206(1):77–78. doi: 10.1192/bjp.bp.114.151407. http://dx.doi.org/10.1192/bjp.bp.114.151407. [DOI] [PubMed] [Google Scholar]
- Lorenzetti V, Solowij N, Yücel M. The role of cannabinoids on neuroanatomical alterations in cannabis users. Biological Psychiatry. 2016;79(7):e17–e31. doi: 10.1016/j.biopsych.2015.11.013. http://dx.doi.org/10.1016/j.biopsych.2015.11.013. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Cheetham A, Yücel M. Cannabis and adolescent brain development. Pharmacology & Therapeutics. 2015;148:1–16. doi: 10.1016/j.pharmthera.2014.11.009. http://dx.doi.org/10.1016/j.pharmthera.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Luciana M. Adolescent brain development in normality and psychopathology. Development and Psychopathology. 2013;25(25th Anniversary Special Issue 4 Pt. 2):1325–1345. doi: 10.1017/S0954579413000643. http://dx.doi.org/10.1017/S0954579413000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Santos R, Fagundo AB, Crippa JA, Atakan Z, Bhattacharyya S, Allen P, … McGuire P. Neuroimaging in cannabis use: A systematic review of the literature. Psychological Medicine. 2010;40(3):383–398. doi: 10.1017/S0033291709990729. http://dx.doi.org/10.1017/S0033291709990729. [DOI] [PubMed] [Google Scholar]
- Mato S, Chevaleyre V, Robbe D, Pazos A, Castillo PE, Manzoni OJ. A single in-vivo exposure to [Delta]9THC blocks endocannabinoid-mediated synaptic plasticity. Nature Neuroscience. 2004;7(6):585–586. doi: 10.1038/nn1251. http://dx.doi.org/10.1038/nn1251. [DOI] [PubMed] [Google Scholar]
- Matochik JA, Eldreth DA, Cadet JL, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug and Alcohol Dependence. 2005;77(1):23–30. doi: 10.1016/j.drugalcdep.2004.06.011. http://dx.doi.org/10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Tapert SF. Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Research. 2010;182(2):152–159. doi: 10.1016/j.pscychresns.2009.12.004. http://dx.doi.org/10.1016/j.pscychresns.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RSE, … Moffitt TE. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(40):E2657–E2664. doi: 10.1073/pnas.1206820109. http://dx.doi.org/10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor L, Hester R, Garavan H. Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. NeuroImage. 2010;49(1):1133–1143. doi: 10.1016/j.neuroimage.2009.07.022. http://dx.doi.org/10.1016/j.neuroimage.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor L, Roberts G, Garavan H, Hester R. Deficits in learning and memory: Parahippocampal hyperactivity and frontocortical hypoactivity in cannabis users. NeuroImage. 2008;40(3):1328–1339. doi: 10.1016/j.neuroimage.2007.12.059. http://dx.doi.org/10.1016/j.neuroimage.2007.12.059. [DOI] [PubMed] [Google Scholar]
- Orr C, Morioka R, Behan B, Datwani S, Doucet M, Ivanovic J, … Garavan H. Altered resting-state connectivity in adolescent cannabis users. The American Journal of Drug and Alcohol Abuse. 2013;39(6):372–381. doi: 10.3109/00952990.2013.848213. http://dx.doi.org/10.3109/00952990.2013.848213. [DOI] [PubMed] [Google Scholar]
- Padula C, Schweinsburg A, Tapert S. Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychology of Addictive Behaviors. 2007;21(4):478–487. doi: 10.1037/0893-164X.21.4.478. http://dx.doi.org/10.1037/0893-164X.21.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Pohl KM, Lane B, Chu W, Kwon D, … Sullivan EV. Adolescent development of cortical and white matter structure in the NCANDA sample: Role of sex, ethnicity, puberty, and alcohol drinking. Cerebral Cortex. 2015 doi: 10.1093/cercor/bhv205. http://dx.doi.org/10.1093/cercor/bhv205. (online early view) [DOI] [PMC free article] [PubMed]
- Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N, … McGregor IS. Adolescent rats find repeated [Delta]9-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology. 2007;33(5):1113–1126. doi: 10.1038/sj.npp.1301475. http://dx.doi.org/10.1038/sj.npp.1301475. [DOI] [PubMed] [Google Scholar]
- Ramaekers J, Kauert G, Theunissen E, Toennes S, Moeller M. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. Journal of Psychopharmacology. 2009;23(3):266–277. doi: 10.1177/0269881108092393. http://dx.doi.org/10.1177/0269881108092393. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, D’Souza DC. The acute effects of cannabinoids on memory in humans: A review. Psychopharmacology. 2006;188(4):425–444. doi: 10.1007/s00213-006-0508-y. http://dx.doi.org/10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- Raver SM, Keller A. Permanent suppression of cortical oscillations in mice after adolescent exposure to cannabinoids: Receptor mechanisms. Neuropharmacology. 2014;86:161–173. doi: 10.1016/j.neuropharm.2014.07.006. http://dx.doi.org/10.1016/j.neuropharm.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, … Giedd JN. How does your cortex grow? The Journal of Neuroscience. 2011;31(19):7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. http://dx.doi.org/10.1523/jneurosci.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard J, Vitalis T, Rame M, Krebs MO, Lenkei Z, Le Pen G, Jay TM. Chronic cannabinoid exposure during adolescence leads to long-term structural and functional changes in the prefrontal cortex. European Neuropsychopharmacology. 2016;26(1):55–64. doi: 10.1016/j.euroneuro.2015.11.005. http://dx.doi.org/10.1016/j.euroneuro.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Robbin TW, Everitt BJ. Limbic-striatal memory system and drug addiction. Neurobiology of Learning and Memory. 2002;78(3):625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. The impact of exposure to cannabinoids in adolescence: Insights from animal models. Biological Psychiatry. 2016;79(7):578–585. doi: 10.1016/j.biopsych.2015.07.024. http://dx.doi.org/10.1016/j.biopsych.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, Guidi S, Capurro V, Viganò D, … Parolaro D. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19(8):763–772. doi: 10.1002/hipo.20554. http://dx.doi.org/10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- Sagar KA, Dahlgren MK, Gönenç A, Racine MT, Dreman MW, Gruber SA. The impact of initiation: Early onset marijuana smokers demonstrate altered Stroop performance and brain activation. Developmental Cognitive Neuroscience. 2015;16:84–92. doi: 10.1016/j.dcn.2015.03.003. http://dx.doi.org/10.1016/j.dcn.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2013 national survey on drug use and health: Summary of national findings. Rockville, MD: US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration; 2014. [Google Scholar]
- Schacht JP, Hutchison KE, Filbey FM. Associations between cannabinoid receptor-1 (CNR1) variation and hippocampus and amygdala volumes in heavy cannabis users. Neuropsychopharmacology. 2012;37(11):2368–2376. doi: 10.1038/npp.2012.92. http://dx.doi.org/10.1038/npp.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner A, Dunn M. Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: A meta-analysis. Experimental and Clinical Psychopharmacology. 2012;20(5):420–429. doi: 10.1037/a0029117. http://dx.doi.org/10.1037/a0029117. [DOI] [PubMed] [Google Scholar]
- Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Buchel C, … Struve M. The IMAGEN study: Reinforcement-related behaviour in normal brain function and psychopathology. Molecular Psychiatry. 2010;15(12):1128–1139. doi: 10.1038/mp.2010.4. http://dx.doi.org/10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, McQueeny T, Nagel BJ, Eyler LT, Tapert SF. A preliminary study of functional magnetic resonance imaging response during verbal encoding among adolescent binge drinkers. Alcohol. 2010;44(1):111–117. doi: 10.1016/j.alcohol.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Research: Neuroimaging. 2008;163(1):40–51. doi: 10.1016/j.pscychresns.2007.04.018. http://dx.doi.org/10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug and Alcohol Dependence. 2005;79(2):201–210. doi: 10.1016/j.drugalcdep.2005.01.009. http://dx.doi.org/10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Medina KL, McQueeny T, Brown SA, Tapert SF. The influence of recency of use on fMRI response during spatial working memory in adolescent marijuana users. Journal of Psychoactive Drugs. 2010;42(3):401–412. doi: 10.1080/02791072.2010.10400703. http://dx.doi.org/10.1080/02791072.2010.10400703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD. A role for synaptic plasticity in the adolescent development of executive function. Translational Psychiatry. 2013;3:e238. doi: 10.1038/tp.2013.7. http://dx.doi.org/10.1038/tp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, … Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. The Journal of Neuroscience. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. http://dx.doi.org/10.1523/jneurosci.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Critical Reviews in Neurobiology. 2003;15(2):30. doi: 10.1615/critrevneurobiol.v15.i2.10. http://dx.doi.org/10.1615/CritRevNeurobiol.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- Smith A, Longo C, Fried P, Hogan M, Cameron I. Effects of marijuana on visuospatial working memory: An fMRI study in young adults. Psychopharmacology. 2010;210(3):429–438. doi: 10.1007/s00213-010-1841-8. http://dx.doi.org/10.1007/s00213-010-1841-8. [DOI] [PubMed] [Google Scholar]
- Solowij N, Yücel M, Respondek C, Whittle S, Lindsay E, Pantelis C, Lubman DI. Cerebellar white-matter changes in cannabis users with and without schizophrenia. Psychological Medicine. 2011;41(11):2349–2359. doi: 10.1017/S003329171100050X. http://dx.doi.org/10.1017/S003329171100050X. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Tapert S, Schweinsburg A, Drummond SA, Paulus M, Brown S, Yang T, Frank L. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194(2):173–183. doi: 10.1007/s00213-007-0823-y. http://dx.doi.org/10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher DL, Pajtek S, Chung T, Terwilliger RA, Clark DB. Gender differences in the relationship between white matter organization and adolescent substance use disorders. Drug and Alcohol Dependence. 2010;110(1–2):55–61. doi: 10.1016/j.drugalcdep.2010.02.004. http://dx.doi.org/10.1016/j.drugalcdep.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends in Neurosciences. 2006;29(3):148–159. doi: 10.1016/j.tins.2006.01.007. http://dx.doi.org/10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC. World drug report 2014 (Sales No. E.14.XI.7) Vienna, Austria: United Nations Office on Drugs and Crime; 2014. Retrieved from, http://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf. [Google Scholar]
- Urban NB, Slifstein M, Meda S, Xu X, Ayoub R, Medina O, … Abi-Dargham A. Imaging human reward processing with positron emission tomography and functional magnetic resonance imaging. Psychopharmacology. 2012;221(1):67–77. doi: 10.1007/s00213-011-2543-6. http://dx.doi.org/10.1007/s00213-011-2543-6. [DOI] [PubMed] [Google Scholar]
- van Hell HH, Jager G, Bossong MG, Brouwer A, Jansma JM, Zuurman L, … Ramsey NF. Involvement of the endocannabinoid system in reward processing in the human brain. Psychopharmacology. 2012;219(4):981–990. doi: 10.1007/s00213-011-2428-8. http://dx.doi.org/10.1007/s00213-011-2428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hell HH, Vink M, Ossewaarde L, Jager G, Kahn RS, Ramsey NF. Chronic effects of cannabis use on the human reward system: An fMRI study. European Neuropsychopharmacology. 2010;20(3):153–163. doi: 10.1016/j.euroneuro.2009.11.010. http://dx.doi.org/10.1016/j.euroneuro.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Van Waes V, Beverley J, Siman H, Tseng KY, Steiner H. CB1 cannabinoid receptor expression in the striatum: Association with corticostriatal circuits and developmental regulation. Frontiers in Pharmacology. 2012:3. doi: 10.3389/fphar.2012.00021. http://dx.doi.org/10.3389/fphar.2012.00021. [DOI] [PMC free article] [PubMed]
- Verdurand M, Nguyen V, Stark D, Zahra D, Gregoire MC, Greguric I, Zavitsanou K. Comparison of cannabinoid CB(1) receptor binding in adolescent and adult rats: A positron emission tomography study using [(18)F]MK-9470. International Journal of Molecular Imaging. 2011;2011:548123. doi: 10.1155/2011/548123. http://dx.doi.org/10.1155/2011/548123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Baler RD. Addiction science: Uncovering neurobiological complexity. Neuropharmacology. 2014;76(Pt. B):235–249. doi: 10.1016/j.neuropharm.2013.05.007. http://dx.doi.org/10.1016/j.neuropharm.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. New England Journal of Medicine. 2014;370(23):2219–2227. doi: 10.1056/NEJMra1402309. http://dx.doi.org/10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT. Neurobiologic advances from the brain disease model of addiction. New England Journal of Medicine. 2016;374(4):363–371. doi: 10.1056/NEJMra1511480. http://dx.doi.org/10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, … Baler R. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: A review. JAMA Psychiatry. 2016;73(3):292–297. doi: 10.1001/jamapsychiatry.2015.3278. http://dx.doi.org/10.1001/jamapsychiatry.2015.3278. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Giedd J, Dale AM, Brown TT. Through thick and thin: A need to reconcile contradictory results on trajectories in human cortical development. Cerebral Cortex. 2016 doi: 10.1093/cercor/bhv301. http://dx.doi.org/10.1093/cercor/bhv301. (online early access) [DOI] [PMC free article] [PubMed]
- Weiland BJ, Thayer RE, Depue BE, Sabbineni A, Bryan AD, Hutchison KE. Daily marijuana use is not associated with brain morphometric measures in adolescents or adults. The Journal of Neuroscience. 2015;35(4):1505–1512. doi: 10.1523/JNEUROSCI.2946-14.2015. http://dx.doi.org/10.1523/jneurosci.2946-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley MJ, Hanlon CA, Porrino LJ. Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Research: Neuroimaging. 2011;191(1):51–59. doi: 10.1016/j.pscychresns.2010.10.002. http://dx.doi.org/10.1016/j.pscychresns.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip SW, DeVito EE, Kober H, Worhunsky PD, Carroll KM, Potenza MN. Pretreatment measures of brain structure and reward-processing brain function in cannabis dependence: An exploratory study of relationships with abstinence during behavioral treatment. Drug and Alcohol Dependence. 2014;140:33–41. doi: 10.1016/j.drugalcdep.2014.03.031. http://dx.doi.org/10.1016/j.drugalcdep.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, Lubman DI. Regional brain abnormalities associated with long-term heavy cannabis use. Archives of General Psychiatry. 2008;65(6):694–701. doi: 10.1001/archpsyc.65.6.694. http://dx.doi.org/10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Solowij N, Yücel M, Lubman DI, Takagi M, Harding IH, … Seal M. Effect of long-term cannabis use on axonal fibre connectivity. Brain. 2012;135(7):2245–2255. doi: 10.1093/brain/aws136. http://dx.doi.org/10.3109/10826084.2010.482443. [DOI] [PubMed] [Google Scholar]