Abstract
Background
Clostridium difficile infection (CDI) is public health threat and associated with significant mortality. However, there is a paucity of objectively derived CDI severity scoring systems to predict mortality.
Aims
To develop a novel CDI risk score to predict mortality entitled: Clostridium difficile Associated Risk of Death Score (CARDS).
Methods
We obtained data from the United States 2011 Nationwide Inpatient Sample (NIS) database. All CDI-associated hospitalizations were identified using discharge codes (ICD-9-CM, 008.45). Multivariate logistic regression was utilized to identify independent predictors of mortality. CARDS was calculated by assigning a numeric weight to each parameter based on their odds ratio in the final logistic model. Predictive properties of model discrimination were assessed using the c-statistic and validated in an independent sample using the 2010 NIS database.
Results
We identified 77,776 hospitalizations, yielding an estimate of 374,747 cases with an associated diagnosis of CDI in the United States, 8% of whom died in the hospital. The 8 severity score predictors were identified on multivariate analysis: age, cardiopulmonary disease, malignancy, diabetes, inflammatory bowel disease, acute renal failure, liver disease and ICU admission, with weights ranging from −1 (for diabetes) to 5 (for ICU admission). The overall risk score in the cohort ranged from 0 to 18. Mortality increased significantly as CARDS increased. CDI-associated mortality was 1.2% with a CARDS of 0 compared to 100% with CARDS of 18. The model performed equally well in our validation cohort.
Conclusion
CARDS is a promising simple severity score to predict mortality among those hospitalized with CDI.
Keywords: Clostridium difficile, C. difficile, epidemiology, severity score, CARDS
Introduction
Clostridium difficile infection (CDI) is the most common healthcare-associated infection in the United States.1 It is a major global public health threat 2–7 with mortality rates rapidly rising5 and the economic burden of CDI reported up to $4.8 billion annually in the United States alone.8–10
CDI therapies span the spectrum of efficacy and costs;11,12 however, CDI severity appears to be a key determinant of absolute and relative treatment efficacy.13 Expert-based classifications11 and to a lesser degree general comorbidity indexes (e.g. Horn Index) have been adopted into practice and CDI therapy studies.14 However, a robust, objectively derived CDI severity metric is lacking.15 Previously epidemiologic studies have either examined national patterns or derived clinical prediction tools from single institution cohorts.15 Beyond the methodological concerns of these tools, linked to a lack of weighting variables, validation and calibration, data generated from these small cohorts suffer from limitations in generalizability.15 Additionally, single institution studies often fail to capture less prevalent conditions that have established strong associations with CDI-associated mortality, such as inflammatory bowel disease.15–18
Large administrative databases offer a number of advantages including generalizability over a broad population and a large sample size facilitating adequate power to examine exposures and outcomes.19 However, most diseases studied in administrative databases are not risk adjusted for disease severity.20 Development and validation of CDI severity scores in administrative database will allow estimation of CDI trends in disease outcomes stratified by severity, and facilitate comparisons of severity across populations. Accordingly, a robust CDI severity metric will deepen our understanding of CDI population-based trends, and aid in determining if temporal changes in outcomes are uniform across all CDI or limited to those with milder disease, an unmet need.21
Accordingly, given the paucity of a robust severity scoring system to predict CDI-associated inhospital mortality, our study aimed to develop and validate an objectively derived severity score to predict CDI-associated mortality using a large administrative database.
Methods
Data Source and Study Population
The data source for our study was the United States 2011 Nationwide Inpatient Sample (NIS), the largest source of all-payer hospital discharge information in the United States. The NIS retrospectively contains all discharge data from a 20% stratified sample of community hospitals. The 2011 NIS captures data from 1,049 hospitals within 46 states, accounting for 8,023,590 hospital discharges.22 Each individual hospitalization is treated as its own entry and coded with a single primary diagnosis, up to 24 secondary diagnoses and as many as 15 procedure-associated codes derived from the International Classification of Disease, 9th Edition, clinical modification (ICD-9-CM). Our target population consisted of patients with a primary or secondary discharge diagnosis of CDI (ICD-9-CM 008.45, n=77,776, which estimates a total of 374,747 CDI hospitalizations in the United States). The ICD-9 code for CDI has been validated and is widely used to estimate healthcare burden due to this disease.23,24
Definition of Outcomes and Variables of Interest
The primary outcome of interest was in-hospital mortality among patients with CDI, which was directly obtained from the NIS dataset. Demographic variables of age, gender, race, insurance status, and median income quartile per zip code were ascertained from the NIS database. Missing data from age (n=9), gender (n=9), and mortality status (n=142) were excluded. “Native American” and “Other Race” were combined given the small numbers. To facilitate a practical, simple severity score, age was categorized into 4 strata: 18–40 years, 41–60 years, 61–80 years, 81–100 years. Comorbidity burden was examined utilizing the validated and widely used Charlson comorbidity index, which consists of 17 distinct conditions, with a higher composite score representing greater comorbidity.25 Previously validated coding algorithms for defining Charlson comorbidities using ICD-9 administrative data was utilized.26 Meaningful Charlson comorbidity categories were grouped to facilitate ease of use (Supplemental Material eTable 1). Briefly, the categories included: 1) cardiopulmonary disease (myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic obstructive pulmonary disease), 2) hepatic disease (mild, moderate or severe liver disease), 3) diabetes (diabetes with or without chronic complications), 4) malignancy (metastatic solid tumors and malignancy including lymphoma/leukemia but excluding benign skin cancer). In addition to these potential predictors and demographics, a number of other possible predictor variables were assessed a priori based on a literature review, including acute renal failure, inflammatory bowel disease, rheumatic arthritis, hemiplegia/paraplegia, peptic ulcer disease, and HIV/AIDS, with corresponding ICD-9 codes contained in Supplemental Material (eTable 1). Intensive care unit (ICU) admission is a significant predictor of mortality and accordingly a surrogate variable was synthesized as this data element is not embedded in the NIS dataset. An ICU admission was defined by identification of any of the following procedure codes: continuous invasive mechanical ventilation for 96 consecutive hours or more, or of unspecified duration (ICD-9, 96.72, 96.70), arterial catherization (38.91), systemic arterial pressure monitoring (89.61), or central venous pressure monitoring (89.62). Each of these procedures are traditionally performed only in high-monitoring care units, thus have high specificity as surrogates for ICU admission.
Statistical Analysis
Data were analyzed utilizing Stata 13.0 (StataCorp, College Station, TX). The Stata svy survey estimation command was used to account for the NIS stratified sampling technique for nationwide estimates. Continuous variables were summarized using means and standard deviations and compared using a t-test. Categorical variables were summarized using proportions and compared using the chi-square test. Using a priori predictors, a stepwise multivariate logistic regression with backward elimination was performed to identify independent predictors of mortality, with a p-value threshold of 0.05 for retention in the model. Next, using the significant predictors generated for the above logistic regression, a multivariate logistic regression model was developed. The risk score (CARDS) was constructed assigning weights for each individual predictive variable corresponding to the odds ratio, rounded to nearest whole number. The cumulative severity score was calculated by adding the weighted, rounded values for each independent predictor variable. The discriminative ability of the severity score was assessed using c-statistic and performance compared to age, gender, Charlson co-morbidity index, with and without ICU admission. The c-statistic ranges from 0.5 to 1.0, with 1.0 indicating perfect discrimination and 0.5 indicating no ability to discriminate.
Validation of the severity score
The severity score was validated in an independent cohort of all CDI hospitalizations using the 2010 NIS database (n=67,715, which estimates a total of 335,963 CDI hospitalizations in the United States). All variable manipulation and statistical tests were performed identical to the initial analysis. The performance of the CARDS score in the validation cohort was compared by assessing mortality associated with specific values of the severity score as well as comparing the overall performance of the model using a c-statistic.
IRB approval was not required at our institution as the NIS is a publically available de-identified database.
Results
Study Population
We identified 77,776 CDI hospitalizations in the NIS 2011 dataset, translating to an estimate of 374,747 CDI cases in the United States, of which 8% were associated with in-hospital mortality. The total study population had a mean age of 69 years (standard deviation (SD) 17 years) with 59% females (45,595) and 41% males (32,181). Whites were the most common race, comprising 68% of the cohort with Blacks and Hispanics comprising 12% and 7% respectively. As expected given the older age of our sample, Medicare, as captured within NIS, was the predominant insurance type accounting for 54,456 hospitalizations (70%). The mean Charlson comorbidity index was 2.31 (SD 2.22) and the mean length of stay 11.57 days (SD 14.48). Table 1 presents demographic and clinical data from patients in hospital with CDI stratified by in-hospital mortality. Patients with a CDI who died in hospital were older and had more comorbidities (mean Charlson comorbidity index 3.01 versus 2.25, p<0.001). Both groups had the majority of hospitalizations occur in females, Whites and with Medicare insurance. There were no significant differences among median income per zip code in either group (Table 1).
Table 1.
Summary of demographic data of hospitalized patients with Clostridium difficile infection who had fatal event compared to those who survived among the United States Nationwide Inpatient Sample*
| Demographic Characteristics | CDI-associated mortality cohort (n =6,168) | CDI-associated survivor cohort (n = 71,608) | p-value |
|---|---|---|---|
| Age categories - N (%) | p<0.001 | ||
| 18 – 40 years | 142 (2) | 5,353 (7) | |
| 41– 60 years | 793 (13) | 14,969 (21) | |
| 61 – 80 years | 2,736 (44) | 30,464 (43) | |
| 81 – 100 years | 2,497 (41) | 20,822 (29) | |
| Gender - N (%) | p< 0.001 | ||
| Female | 3,344 (54) | 42,251 (59) | |
| Male | 2,824 (46) | 29,357 (41) | |
| Race - N (%) | p<0.001 | ||
| White | 4,144 (67) | 48,894 (68) | |
| Black | 782 (13) | 8,415 (12) | |
| Hispanic | 487 (8) | 5,094 (7) | |
| Asian/Pacific Islander | 136 (2) | 1,144 (12) | |
| Other Race | 188 (3) | 1,966 (3) | |
| No Race Reported | 431 (7) | 6,095 (9) | |
| Mean Length of Stay | 15.35 (21.85) | 11.24 (13.62) | p<0.001 |
| Insurance Source - N (%) | p<0.001 | ||
| Medicare | 4,726 (77) | 49,730 (70) | |
| Medicaid | 418 (7) | 6,172 (9) | |
| Private | 788 (13) | 12,798 (17) | |
| Other | 219 (4) | 3,064 (4) | |
| Median income quartile per zip code - N (%) | p=0.316 | ||
| 1 | 1,550 (26) | 18,097 (26) | |
| 2 | 1,416 (23) | 16,718 (23) | |
| 3 | 1,572 (26) | 18,567 (26) | |
| 4 | 1,521 (25) | 16,898 (24) | |
| Charlson comorbidity index - mean (SE) | 3.01 (2.44) | 2.25 (2.19) | P<0.001 |
Unweighted United States 2011 NIS hospitalization data
Predictors of CDI-associated mortality
The multivariate analysis of predictors of CDI-associated in-hospital mortality is highlighted in Table 2. A progressive increase in age was associated with greater odds of CDI-associated mortality, particularly patients between 81–100 years (OR 4.12, 95% CI: 3.39–4.99) compared adults between 18–40 years. ICU admission and acute renal failure were among the strongest predictors of CDI-associated mortality with a five-fold (OR 5.29, 95% CI: 4.85–5.77) and threefold increase in mortality (OR 2.93, 95% CI: 2.76–3.13), respectively. Chronic conditions were also independent predictors of CDI-associated mortality including liver disease (OR 2.00, 95% CI: 1.78–2.25), malignancy (OR 1.89, 95% CI: 1.74–2.05), inflammatory bowel disease (OR 1.72, 95% CI: 1.49–1.99) and cardiopulmonary disease (OR 1.46, 95% CI: 1.38–1.56). Diabetes was associated with decreased odds of CDI-associated mortality in multivariate analysis (OR 0.83, 95% CI: 0.77–0.88).
Table 2.
Multivariate analysis of predictors of CDI-associated mortality.* Eight significant severity score predictors were identified. ICU admission, age and acute renal failure were strong predictors of mortality whereas liver disease, malignancy, inflammatory bowel disease and cardiopulmonary disease were moderate predictors of mortality. Diabetes had a modest protective impact on CDI-associated mortality.
| Characteristic | Odds ratio | 95% Confidence interval |
|---|---|---|
| Age | ||
| 18 – 40 years | Reference | |
| 41 – 60 years | 1.51 | 1.24–1.82 |
| 61 – 80 years | 2.51 | 2.06–3.06 |
| 81 – 100 years | 4.12 | 3.39–4.99 |
| ICU admission | ||
| No | Reference | |
| Yes | 5.29 | 4.85–5.77 |
| Acute renal failure | ||
| No | Reference | |
| Yes | 2.93 | 2.76–3.13 |
| Liver disease | ||
| No | Reference | |
| Yes | 2.00 | 1.78 – 2.25 |
| Malignancy (with or without metastatic disease) | ||
| No | Reference | |
| Yes | 1.89 | 1.74 – 2.05 |
| Inflammatory bowel disease | ||
| No | Reference | |
| Yes | 1.72 | 1.49 – 1.99 |
| Cardiopulmonary disease | ||
| No | Reference | |
| Yes | 1.46 | 1.38–1.56 |
| Diabetes (with or without complications) | ||
| No | Reference | |
| Yes | 0.83 | 0.77 – 0.88 |
Survey weighted United States 2011 NIS hospitalization data
Development and Performance of CARDS
Table 3 presents the weights of the individual components of the severity score. Patients between 81–100 years, 61–80 years, 41–60 years, and 18–40 years received 4 points, 3 points, 2 points and 0 points, respectively. Patients admitted to the ICU/critical care ward received 5 points, and those with acute renal failure received 3 points. Patients with liver disease (mild, moderate or severe), inflammatory bowel disease (Crohn’s disease or ulcerative colitis), or malignancy with or without metastatic disease received 2 points for presence of any of these three conditions (maximum 6 points). Patients also received a maximum of 1 point if they have any of cardiopulmonary diseases previously defined. Patients with diabetes with or without chronic complications receive −1 points, subtracting a point from their cumulative CARDS. Therefore, the possible CARDS ranged from −1 to 19, with a maximum total CARDS of 18 in our dataset (Figure 1). Mortality increased significantly as CARDS increased. This ranged from 1.2% CDI-associated mortality with a CARDS of 0 to 100% CDI-associated mortality with CARDS of 18 (Table 4). CARDS performed well in terms of model discrimination compared to existing models. CARDS had a c-statistic of 0.77 whereas a standard model of age, gender, Charlson comorbidity index, and ICU admission only had a c-statistic of 0.73. CARDS also outperformed a model of age, gender, Charlson comorbidity index alone, which had a c-statistic of 0.63 (Supplemental Material eFigure 1).
Table 3.
Summary of Clostridium difficile Associated Risk of Death Score (CARDS) to predict mortality among hospitalized patients with Clostridium difficile infection. Points are summated for each CARDS predictor that a hospitalized patient with Clostridium diffcile infection posses, in turn, yielding a CARDS total associated with a mortality rate. The CARDS total range is between −1 to 19.
| Predictors | Points |
|---|---|
| Critical care/ICU admission | 5 |
| Age | |
| 18 – 40 years | 0 |
| 41 – 60 years | 2 |
| 61 – 80 years | 3 |
| 81 – 100 years | 4 |
| Renal failure (acute) | 3 |
| Diabetes | −1 |
| Serious comorbidities | |
| Cardiopulmonary disease | 1 |
| Liver disease | 2 |
| Inflammatory bowel disease | 2 |
| Malignancy | 2 |
| CARDS Total | −1 to 19 |
Figure 1.
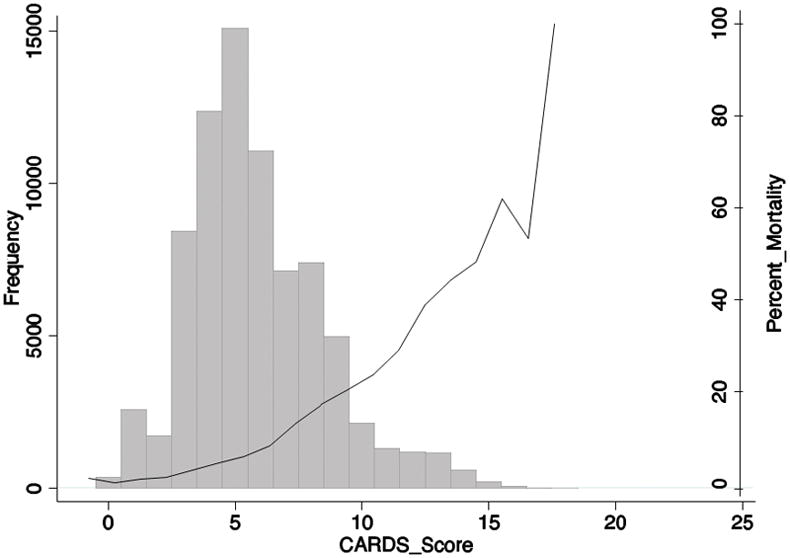
CARDS distribution and percent mortality by score among patients hospitalized with Clostridium difficile infection. Among the United States 2011 Nationwide Inpatient Sample, CARDS totals formed a normal distribution, and the possible CARDS total ranged from −1 to 19, with a maximum total CARDS of 18 in the dataset. Clostridium difficile infection-associated mortality increased significantly as CARDS total increased.
Table 4.
CARDS total and predicted CDI-associated mortality rate. Mortality increased significantly as CARDS total increased. Clostridium difficile infection-associated mortality was 1.2% with a CARDS of 0 compared to 100% with CARDS of 18 among the United States 2011 Nationwide Inpatient Sample (NIS). A similar prediction was demonstrated in an external validation cohort, United States NIS 2010 dataset.
| CARDS Total | Mortality (NIS 2011) | Mortality (NIS 2010) |
|---|---|---|
| 0 | 1.15% | 0.33% |
| 5 | 4.4% | 4.5% |
| 10 | 20.8% | 23.3% |
| 15 | 48.1% | 49.7% |
| 18 | 100.00% | 100.0% |
Validation of CARDS
The performance of CARDS was validated in an independent sample of all CDI hospitalizations from the 2010 NIS dataset. Similar to the derivation cohort, the validation cohort had a maximum total CARDS was 18, with mortality increasing as CARDS increased. This ranged from 0.3% CDI-associated mortality with a CARDS of 0 to 100% CDI-associated mortality with CARDS of 18 (Table 4). Importantly, the severity scoring system had a comparable performance with a c-statistic of 0.77.
Discussion
Clostridium difficile infection is a major public health threat with significant associated mortality, supported by this study, which reports an 8% CDI-associated mortality rate among hospitalized patients in the United States. Despite the impact of CDI, there is an absence of a robust CDI severity scoring system. Accordingly, we developed the Clostridium difficile Associated Risk of Death Score (CARDS), an objectively derived severity score to predict CDI-associated mortality.
Our study highlights that several predictive risk factors and the CARDS model displays good discriminative ability, which was validated in an independent CDI cohort. Age has been identified has a risk factor of initial CDI development and CDI-associated mortality.11 A recent systematic review27 highlights advanced age as an independent predictor of CDI-associated mortality with estimates ranging from risk ratio (RR) 1.41 (95% CI: 1.1–1.81)28 in those between 75–84 years to as high as OR 6.5 (95% CI: 1.7–24.3) in those ≥75 years.29 Interestingly, data from the 2007 NIS dataset supports the progressive increase odds of CDI-associated death with increasing age, although estimates were modest particularly at the most advanced ages. Stewart and Hollenbeak30 reported ORs of 2.45 (95% CI: 2.31–2.61) and 1.81 (1.71–1.92) for CDI-associated mortality in patients over 70 years and between 51–70 years, respectively, compared to their young adult reference. In our study, ICU admission was also a strong independent predictor of CDI-associated mortality (OR 5.23, 95% CI: 4.79–5.72). This is in keeping with data from a small, observational cohort that suggests septic shock, ward-to-ICU transfer, and increased APACHE scores as significant independent predictors of 30-day mortality.31 A number of chronic comorbidities are important predictors of CDI-associated mortality. Inflammatory bowel disease, malignancy and liver disease were all independently identified to increase the odds of CDI-associated death in our model. Our results are consistent with emerging literature that highlights the deleterious consequences of these comorbidities when linked to CDI.17,32,33
There is a paucity of data examining the relationship between diabetes mellitus and CDI. In a small study, diabetes mellitus has been identified as a risk factor for recurrence of CDI34; however, to our knowledge, our study is the first to demonstrate that diabetes was associated with decreased odds of CDI-associated mortality (OR 0.83, 95% CI: 0.77–0.88). Interestingly, this association become stronger on multivariate analysis compared to uncontrolled, univariate analysis, which does not support the hypothesis that the diabetes effect is likely because of adjustment for other co-morbidity and may not be specific to diabetes. We offer two possible explanations for this relationship. First, Viladominu and colleagues35 demonstrated that CDI-infected mice treated with pioglitazone, a common anti-diabetic medication, ameliorated colitis through the peroxisome proliferator-activation receptor pathway. If a similar inverse association between thiazolidinedione use and severity of CDI exists in humans, this reduction in severity in users may explain the inverse association observed between diabetes and mortality in CDI patients. This hypothesis merits further evaluation in cohorts with data on medication use. Second, diabetic patients are likely to have had more statin use, which has been shown to have a dose-response protective effect on mortality in patients with CDI.36 Overall, as the the relationship between diabetes mellitus and CDI has been reported to be protective37 and associated with mortality38, further studies exploring the mechanisms behind this are warranted.
For clinicians, CARDS may hold promise to stratify severity among similarly presenting CDI cases, guiding which patients may benefit from early expensive treatments such as fidaxomicin or vancomycin, closer follow-up, or both. This is particularly important given the CDI outcomes among IBD patients39–42 and the rise of community acquired CDI.43 Additionally, CARDS may be useful in CDI therapy clinical trials, in order, to better account for severity driving outcomes. A recent systematic review of CDI complication prediction scores concluded current tools are ‘suboptimal’ and of ‘debatable utility’ given methodological limitations including a lack of validation, performance and issues rooted in a small sample size.15 Although there may be other factors that drive disease severity that are not captured in administrative databases, CARDS may have clinical utility serving as a framework over which other established laboratory and medication factors may be overlaid to leverage a more comprehensive CDI severity metric, given its validation, robust c-statistic and derivations from 71,357 CDI hospitalizations.
For epidemiologists, given that CARDS is grounded in a national database, it offers utility in adjustment for disease severity in research using administrative data, enabling meaningful comparisons between populations and over time. Additionally, this severity score helps highlight an unmet need, if improvements in mortality are only identified in specific severity strata, for example those with mild disease. Such effects have been seen in secular trends for other diseases such as inflammatory bowel disease where the development of severity score using administrative data allowed one to define that reduction in colectomy rates were seen only in those with mild or moderate severity of hospitalizations with no differences in rates over the past decade in those with the most severe disease. Lastly, CARDS may seamlessly be adapted into a quality improvement metric for which public health officials and hospital administrators can monitor the burden of CDI in individual hospitals. If severity scores are persistently elevated it may signal to stakeholders that selected NAP-1/027 testing might be required or other exploration should be undertaken.
This study does have a few limitations. The absence of clinical and treatment data, such as the use of antibiotics, thiazolidinedione or proton pump inhibitor medications are salient limitations. Also, the United States NIS is a discharge abstract database whereby repeat admissions for recurrent CDI cannot be differentiated. Individual-level clinical data are not traditionally available in large administrative databases that capture the national burden of disease. The absence of laboratory investigations (e.g. white blood cell count) that have been previously described as associated with mortality, is a relevant limitation; however, this data is not commonly available in large administrative database.23 Efforts were made to generate a surrogate marker for elevated creatinine, another salient laboratory parameter, in the model by using the acute renal failure diagnostic code. Additionally, physiological parameters and laboratory investigations change rapidly, so it is difficult to ascertain which is the relevant measurement, in turn reducing the consistency of scoring among observers. While we modeled for the presence of several co-morbidities, it is possible that the severity of such co-morbid illnesses could additionally influence outcome and we were not able to completely adjust for this. We were unable to directly ascertain ICU-level care and used surrogates of mechanical ventilation or hemodynamic monitoring. We acknowledge that this assumption may underestimate the number of C difficile patients care for in an ICU-setting. However, given the wide variability between institutions in the threshold for ICU-care, we elected to choose markers that would retain high specificity for this covariate. We were also unable to control or delineate fatal adverse events related to CDI-specific treatments. Methodologically, non-linear relationships may exist and merit exploration in future studies. Despite the absence of clinical, laboratory and treatment data, given the widespread use and familiarity of diagnosis and procedure codes, CARDS offers a simple, robust and practical tool. Future studies building upon CARDS should aim to include laboratory investigations and microbial markers such as ribotype though such markers as the NAP-1/027 ribotype have not been consistently associated with more severe disease.
In conclusion, to our knowledge, CARDS is the first objectively derived severity score to predict CDI-associated mortality using a national administrative database. Although validation in other cohorts is needed, this simple severity improves generalizability and is a promising tool for epidemiologists and clinicians.
Supplementary Material
Acknowledgments
Source of funding: Zain Kassam was supported by a Harvard University Frank Knox Memorial Fellowship. Ananthakrishnan is supported in part by a grant from the National Institutes of Health (K23 DK097142).
Guarantor of the article: Ashwin N. Ananthakrishnan MBBS, MPH
Specific author contributions: ZK: study design, data collection, statistical analysis, interpretation of results, writing the paper; CCF: study design, data collection, statistical analysis, interpretation of results; MS: study design, interpretation of results, critical revisions to manuscript; EA: study design, interpretation of results, critical revisions to manuscript; GK: study design, interpretation of results, critical revisions to manuscript; GN: study design, interpretation of results, critical revisions to manuscript; AA: study design, interpretation of results, critical revisions to manuscript. All authors approved the final version of this manuscript.
Financial support: ZK was supported by a Harvard Frank Knox Memorial Fellowship during the course of this work. No other relevant financial support relevant to this manuscript.
Potential competing interests: None relevant to this manuscript.
Footnotes
Author contributions: Z Kassam: study concept and design, data collection, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content
C Fabersunne: data collection, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content
Alm, Smith, Kaplan, Nguyen: data collection, analysis and interpretation of data, critical revision of the manuscript for important intellectual content
A Ananthakrishnan: study concept and design, data collection, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, study supervision
References
- 1.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zilberberg M, Shorr A, Kollef M. Increase in adult Clostridium difficile-related hospitalizations and case-fatality rate, United States, 2000–2005. Emerg Infect Dis. 2008;14(6):929–931. doi: 10.3201/eid1406.071447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gravel D, Miller M, Simor A, et al. Health Care Associated Clostridium difficile Infection in Adults Admitted to Acute Care Hospitals in Canada: A Canadian Nosocomial Infection Surveillance Program Study. Clin Infect Dis. 2009;48(5):568–576. doi: 10.1086/596703. [DOI] [PubMed] [Google Scholar]
- 4.Bauer MP, Notermans DW, van Benthem BHB, et al. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011;377(9759):63–73. doi: 10.1016/S0140-6736(10)61266-4. [DOI] [PubMed] [Google Scholar]
- 5.Wiegand PN, Nathwani D, Wilcox MH, Stephens J, Shelbaya A, Haider S. Clinical and economic burden of Clostridium difficile infection in Europe: A systematic review of healthcare-facility-acquired infection. J Hosp Infect. 2012;81(1):1–14. doi: 10.1016/j.jhin.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Simango C, Uladi S. Detection of Clostridium difficile diarrhoea in Harare, Zimbabwe. Trans R Soc Trop Med Hyg. 2014;108(6):354–357. doi: 10.1093/trstmh/tru042. [DOI] [PubMed] [Google Scholar]
- 7.Collins D, Hawkey PM, Riley TV. Epidemiology of Clostridium difficile infection in Asia. Antimicrob Resist Infect Control. 2013;2(1):21. doi: 10.1186/2047-2994-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGlone S, Bailey R, Zimmer S. The economic burden of Clostridium difficile. Clin Microbiol Infect. 2012;18(3):282–289. doi: 10.1111/j.1469-0691.2011.03571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubberke E, Olsen M. Burden of clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(suppl 2):S88–S92. doi: 10.1093/cid/cis335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vonberg R, Reichardt C, Behnke M. Cost of nosocomial Clostridium difficile-associated diarrhoea. J Hosp Infect. 2008;70:15–20. doi: 10.1016/j.jhin.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478–498. doi: 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]
- 12.Fischer M, Cook G, Rogers N, et al. Rescue therapy with fecal microbiota transplantation in hospitalized patients with severe and severe-complicated Clostridium difficile infection. American College of Gastroenterology Annual Scientific Meeting; 2014; Oct 17–22; Philadelphia, PA. p. 935. [Google Scholar]
- 13.Zar F, Bakkanagari SR, Moorthi KMLST, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–307. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 14.Hu MY, Katchar K, Kyne L, et al. Prospective derivation and validation of a clinical prediction rule for recurrent Clostridium difficile infection. Gastroenterology. 2009;136(4):1206–1214. doi: 10.1053/j.gastro.2008.12.038. [DOI] [PubMed] [Google Scholar]
- 15.Abou Chakra CN, Pepin J, Valiquette L. Prediction tools for unfavourable outcomes in Clostridium difficile infection: A systematic review. PLoS One. 2012;7(1):e30258. doi: 10.1371/journal.pone.0030258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen GC, Kaplan GG, Harris ML, Brant SR. A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103:1443–1450. doi: 10.1111/j.1572-0241.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 17.Ananthakrishnan aN, McGinley EL, Binion DG. Excess hospitalisation burden associated with Clostridium difficile in patients with inflammatory bowel disease. Gut. 2008;57:205–210. doi: 10.1136/gut.2007.128231. [DOI] [PubMed] [Google Scholar]
- 18.Negrón ME, Barkema HW, Rioux K, et al. Clostridium Difficile Infection Worsens the Prognosis of Ulcerative Colitis. Can J Gastroenterol Hepatol. 2014;28(7):373–380. doi: 10.1155/2014/914303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ananthakrishnan AN, McGinley EL, Binion DG, Saeian K. A novel risk score to stratify severity of Crohn’s disease hospitalizations. Am J Gastroenterol. 2010;105(8):1799–1807. doi: 10.1038/ajg.2010.105. [DOI] [PubMed] [Google Scholar]
- 20.Molodecky NA, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Challenges associated with identifying the environmental determinants of the inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17(8):1792–1799. doi: 10.1002/ibd.21511. [DOI] [PubMed] [Google Scholar]
- 21.Ananthakrishnan AN, McGinley EL, Saeian K, Binion DG. Temporal trends in disease outcomes related to clostridium difficile infection in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17(4):976–983. doi: 10.1002/ibd.21457. [DOI] [PubMed] [Google Scholar]
- 22.HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality; Rockville, MD: [Google Scholar]
- 23.Dubberke ER, Reske KA, McDonald LC, Fraser VJ. ICD-9 codes and surveillane for Clostridium difficile-associated disease. Emerg Infect Dis. 2006;12(10):1576–9. doi: 10.3201/eid1210.060016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubberke ER, Butler AM, Nyazee HA, et al. The impact of ICD-9-CM code rank order on the estimated prevelane of Clostridium difficile infections. Clin Infect Dis. 2011;53(1):20–5. doi: 10.1093/cid/cir246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;(40):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed]
- 26.Quan H, Sundararajan V, Halfon P, Fong a. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 27.Abou Chakra CN, Pepin J, Sirard S, Valiquette L. Risk factors for recurrence, complications and mortality in Clostridium difficile infection: A systematic review. PLoS One. 2014;9(6):e107420. doi: 10.1371/journal.pone.0098400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inns T, Gorton R, Berrington a, et al. Effect of ribotype on all-cause mortality following Clostridium difficile infection. J Hosp Infect. 2013;84(3):235–241. doi: 10.1016/j.jhin.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Lamontagne F, Labbé A-C, Haeck O, et al. Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Ann Surg. 2007;245(2):267–272. doi: 10.1097/01.sla.0000236628.79550.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart DB, Hollenbeak CS. Clostridium difficile Colitis: Factors Associated with Outcome and Assessment of Mortality at a National Level. J Gastrointest Surg. 2011;15:1548–1555. doi: 10.1007/s11605-011-1615-6. [DOI] [PubMed] [Google Scholar]
- 31.Kenneally C, Rosini JM, Skrupky LP, et al. Analysis of 30-day mortality for Clostridium difficile-associated disease in the ICU setting. Chest. 2007;132:418–424. doi: 10.1378/chest.07-0202. [DOI] [PubMed] [Google Scholar]
- 32.Kim ES, Kim YJ, Park CW, Cho KB, Jang BK, Chung WJ, Hwang JSPK. Response failure to the treatment of Clostridium difficile infection and its impact on 30-day mortality. Hepatogastroenterology. 2013;60(123):543–548. doi: 10.5754/hge12730. [DOI] [PubMed] [Google Scholar]
- 33.Bajaj JS, Ananthakrishnan AN, Hafeezullah M, et al. Clostridium difficile is associated with poor outcomes in patients with cirrhosis: A national and tertiary center perspective. Am J Gastroenterol. 2010;105(April 2009):106–113. doi: 10.1038/ajg.2009.615. [DOI] [PubMed] [Google Scholar]
- 34.Shakov R, Salazar RS, Kagunye SK, Baddoura WJ, DeBari V. Diabetes mellitus as a risk factor for recurrence of Clostridium difficile infection in the acute care hospital setting. Am J Infect Control. 2011;39(3):194–198. doi: 10.1016/j.ajic.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Viladomiu M, Hontecillas R, Pedragosa M, et al. Modeling the Role of Peroxisome Proliferator-Activated Receptor and MicroRNA-146 in Mucosal Immune Responses to Clostridium difficile. PLoS One. 2012;7(10):e47525. doi: 10.1371/journal.pone.0047525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park SW, Choi AR, Lee HJ, et al. The effects of statins on the clinical outcomes of Clostridium difficile infection in hospitalized patients. Ailment Pharmacol Ther. 2013;38(6):619–27. doi: 10.1111/apt.12439. [DOI] [PubMed] [Google Scholar]
- 37.Stewart DB, Hollenbeak CS. Clostridium difficile colitis: factors associated with outcome and assessment of morality at a national level. J Gastrointest Surg. 2011;15(9):1548–55. doi: 10.1007/s11605-011-1615-6. [DOI] [PubMed] [Google Scholar]
- 38.Honda H, Yamazaki A, Sato Y, Dubberke ER. Incidence and mortality associated with Clostridium difficile infection at a Japanese tertiary care center. Anaerobe. 2014;25:5–10. doi: 10.1016/j.anaerobe.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Murthy S, Steinhart H, Tinmouth J, et al. Impact of Clostridium difficile colitis on 5-year health outcomes in patients with ulcerative colitis. Aliment Pharmacol Ther. 2012;36(11):1032–39. doi: 10.1111/apt.12073. [DOI] [PubMed] [Google Scholar]
- 40.Ananthakrishnan A, Guzman-Perez R, Gainer V, et al. Predictors of severe outcomes associated with Clostridium difficile infection in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:789–95. doi: 10.1111/j.1365-2036.2012.05022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rampton D, Shanahan F. Increased health burden associated with Clostridium difficile diarrhea in inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34(3):394–5. doi: 10.1111/j.1365-2036.2011.04712.x. [DOI] [PubMed] [Google Scholar]
- 42.Goodhand J, Alazawi W, Rampton D, et al. Systematic review: Clostridium difficile and inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33(4):428–41. doi: 10.1111/j.1365-2036.2010.04548.x. [DOI] [PubMed] [Google Scholar]
- 43.Khanna S, Pardi D, Aronson S, et al. Outcomes in community-acquired Clostridium difficile infection. Aliment Pharmacol Ther. 2012;35(2):613–8. doi: 10.1111/j.1365-2036.2011.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


