Abstract
Honey bees (Apis mellifera) are the best studied model of aging among the social insects. As in other social insects, the reproductive queen far outlives her non-reproductive workers despite developing from the same genome in the same colony environment. Thus, the different social roles of the two female castes are critical for the profound phenotypic plasticity. In several special cases, such as the reproductive workers of Apis mellifera capensis, within-caste plasticity enables further studies of the fecundity – longevity syndrome in honey bees. At present, molecular evidence suggests that a reorganization of physiological control pathways may facilitate longevity of reproductive individuals. However, the social role and social environment of the different colony members are also very important and one of the key future questions is how much social facilitation versus internal regulation is responsible for the positive association between fecundity and longevity in honey bees.
Introduction
Among social insects, the Western Honey Bee Apis mellifera (L) has been studied in most detail due to its long affiliation with humans and contemporary ecological and economic importance. Honey bees live in large, perennial colonies headed by a single queen that produces all other colony members. Males (=drones) exclusively serve reproductive functions and are usually produced only in the mating season. The remaining colony members are female workers that fulfil all non-reproductive tasks in the colony, ranging from nursing and nest maintenance to food processing and foraging [1]. Workers exhibit a typical age-based behavioural progression from tasks in the nest centre to outside activities but remain behaviourally plastic and fulfil sets of different tasks at all ages [2]. Colony reproduction occurs via swarming when the mother queen departs her nest with a part of the existing workers to establish a new colony before the next generation queen has emerged to take over the existing nest [1]. Mating occurs at the beginning of the queen’s life with multiple males to give rise to genetically diverse colonies despite the strict monogyny of the species.
Female eggs are bi-potent and early larval nutrition triggers alternative queen or worker development via DNA methylation changes [3]. A queen develops 25% faster and grows about 25% heavier than typical workers [1]. The most significant distinction is the functional reduction of the reproductive tract in workers compared to queens: worker ovaries are usually less than 10% of the queen ovary in size and they lack the capability to mate and lay fertilized eggs. Similar to many other social hymenoptera, the workers of honey bees are restricted to laying haploid male eggs and do so only under exceptional circumstances [4]. Importantly, honey bee queens have been recorded to live for several years, much longer than the normally non-reproductive workers of their colony [5].
Long-lived, Reproductive Specialists
Although there are some costs of mating for queens [6], the reproductive role coincides with a longer life span. Honey bee queens mature and can lay up to 2000 eggs per day, which corresponds roughly to the body weight of the queen herself. The functional role of the queen is largely restricted to this reproductive function. The queen signals her fertility status with a set of glandular secretions, the queen pheromones [7] and workers may replace her if the pheromone profile is compromised. Studies indicate that queens can live several years but it is unclear how many actually do [5], especially in recent years of declining honey bee health. The development, maintenance, and physiological activity of the large queen ovary is either not costly or is overcompensated by other factors. Ovary grafts to increase ovary size of workers did not increase acute mortality but led to an earlier onset of foraging [8], which indicates that role-dependent mortality costs of large ovaries may exist. One compensatory factor that prolongs queen life may be the lack of foraging activity in honey bee queens. The metabolic rates during foraging flights are energetically much more demanding than the oxidative stress generated by egg laying [9,10]. Workers suffer the metabolic costs of foraging flights as well as predation risks. Queens only participate in flights for mating and in subsequent years for brief swarming events. In addition, queens are fed with royal jelly, a special diet with high protein content, throughout their life time [11]. In contrast, workers feed on pollen, which is difficult to digest [12] and requires repeated defecation flights, whereas the queen can stay protected in the nest.
Worker Life History Plasticity
The regular age-based division of labour of honey bee workers may have evolved in response to aging and mortality patterns [13]: Proximately, external mortality and internal physiological re-organization lead to higher mortality [14] and faster functional senescence [15] in foragers, although inside tasks, such as brood care, also have mortality costs [16]. Honey bee workers display a wide range of life expectancies, dependent on the rate of their behavioural ontogeny, which in turn is influenced by social environment, such as colony age composition [17] and size [18], and external environment, such as season [19,20]. Recent life expectancy estimates of honey bee workers can vary from 18 to 30 days in one experiment [21], but earlier studies report higher life expectancies, ranging from 20 to 50 days [9] or even 28 to 154 days across multiple seasons [20]. Low food reserves and other forms of stress can accelerate the behavioural progression towards foraging through physiological control circuits (see below), and pheromone signals emitted by brood [22] and the queen [23] also influence the onset of foraging. Despite intensive functional genomic studies that reveal large shifts in neuronal state [24], it is not quite clear how these factors interact. Under special circumstances, the transition from in-hive tasks to foraging can also be reversed [25], which results in physiological rejuvenation [26,27] and presumably an extension of life, although the latter has never been directly shown.
Reproductive Workers
Although worker sterility is the rule, workers can activate their ovaries to produce offspring. This usually happens whenever the queen is lost and cannot be replaced (Bourke 1988). Reproductive workers experience lower mortality rates [21], although they do not to live the degree of queens, and functional genomic studies suggest that the differences between normal and reproductive workers are much smaller than between workers and queens [28]. Rarely worker reproduction can be observed in the presence of the queen, which is known as the ‘anarchic syndrome’ [29]. In the extreme, laying workers can turn into pseudoqueens [30]. These pseudoqueens do no longer engage in worker tasks, but instead they behave and are treated like a queen [30]. However, their reproductive success is limited because they usually only reproduce up to ten days [31].
The pseudoqueen phenotype is most extreme in the Cape honey bee, A. m. capensis, where laying workers can develop into individuals living up to five months whereas foraging workers have a normal lifespan of a few weeks only [31]. These pseudoqueens can even act as social parasites by invading foreign colonies, killing the resident queen, and establishing themselves as reproductives [32]. In the first days after invasion there is a high risk of being killed by the host workers but once established, the host workers treat these workers as a fully functional queen with no phenotypic indications of senescence [31]. Due to an incomplete meiosis, workers of A. m. capensis produce diploid female offspring [= thelytoky, 33,34]. This trait has been repeatedly suggested to be controlled by a single recessive locus [35–38]. More recently the transcription factor gemini was suggested to be the controlling gene [36]. But although gemini controls ovary activation and queen pheromone production (Jarosch et al. submitted) it does not cause thelytoky [39, Aumer et al. submitted]. The simple mode of inheritance based on a single gene with two allelic forms, one for fecundity and one for sterility, in combination with the evolution of social parasitism can be used as an exceptional test system for studying the trade-off between fecundity and longevity by comparing individuals of the same caste with the same physiological constrains: the ones short-lived sterile altruistic workers, the others long-lived highly fecund social parasites. Both longevity comparisons and reproduction manipulations by using queen pheromones that can inhibit pseudoqueen reproduction at various stages [40] can experimentally be implemented.
Physiological Regulation of Longevity
The alternative developmental trajectories of queen- and worker-destined larvae are proximally caused by large scale gene expression differences [41]. In addition, the faster queen development is fuelled by higher mitochondrial activity [42], which may provide a cellular mechanism for the longevity of queens: In the light of similar mass-specific metabolic rates [43], more mitochondria may make queens less susceptible to the toxic by-products of the oxidative phosphorylation pathway because a higher mitochondrial redundancy reduces oxidative stress and makes cells more resistant to it. This reduced stress hypothesis is compatible with findings that the upregulation of classic antioxidant defence genes seems not to be responsible for the increased life expectancy of honey bee queens [44]. However, in honey bees the egg yolk protein vitellogenin serves an important antioxidant function [45] and might be partly responsible for the concomitant longevity and reproductive specialization in queens [46,47].
In most insects, juvenile hormone triggers reproductive activation and expression of egg yolk precursor proteins [48], but this regulatory link is reversed in honey bees where juvenile hormone and vitellogenin have a mutually suppressive relationship that regulates social behaviour and life history [49,50]. As a key reproductive protein, vitellogenin is much more expressed in queens, where it may exert its protective effect against oxidative stress [47] in addition to serving its role in provisioning the maturing eggs. Interestingly, the relation between nutrition and insulin-like signalling, a major regulator of life expectancy throughout animals [51], might also be inversed in honey bees [47]. The abundant food supply that honey bee queens experience during development and adulthood may not lead to a down-regulation of longevity-assurance genes via JH and insulin-like signalling. Thus, molecular studies suggest some inversions in the regulatory circuits that control life history and aging in most other animal species in a conserved fashion [52]. The coincidence of these physiological differences and the unusual, positive association between reproduction and longevity supports the general hypothesis that internal, physiological mechanisms support the unique, long-lived stem cell function of honey bee queens and presumably other social insect reproductives [53].
Social Evolution of Individual Life Expectancy
Advanced social evolution in honey bees has shifted the focus of selection from the individual to the colony level and many traits can be understood as colony-level adaptations [54]. Colony resource allocation toward individuals influences their life expectancy but may not be selected to maximize the lifespan of all colony members [46]. Under specific circumstances, colony members even sacrifice their life in defence of their colony [55]. Such sacrificial behaviour is only observed by non-reproductive individuals of social insects and may be regarded as equivalent to the regular deaths of somatic cells of any multicellular organism in defence against environmental insults [56] or pathogens [57]. Just like somatic cells, the non-reproductive workers are disposable for the larger unit of selection, in contrast to the reproductive individuals.
The honey bee queen has been compared to the gonad of the colony super-organism [58] but it may be more analogous to a universal stem cell because the queen produces not only the next generation of sexual males and females, but also the non-reproductive workers to grow and replace the somatic portion of the colony. In multicellular organisms stem cells are particularly protected [59] and by analogy, we would also expect mechanisms that particularly protect the longevity of the honey bee queen. Such adaptations lie at the centre of the positive association between fecundity and longevity and their study may shed light on the evolution of aging more generally. The initial key question in our quest for understanding the high fecundity/longevity syndrome of social insect queens is how much of it can be attributed to reorganization of internal physiological mechanisms and how much is due to social facilitation comprised of external factors, such as behavioural interactions and environment [52].
As described above, the control circuits connecting reproduction with the major hormone and growth regulatory processes seem to have been fundamentally altered in honey bee social evolution [52, 60]. This rewiring may provide a proximate explanation for the positive relation between fertility and longevity and may be reflected more widely in age-related molecular changes, as recently described in the ant Cardiocondyla obscurior [61]. However, these intrinsic adaptations have presumably been enabled by the social environment during social evolution. Honey bee workers feed queens with glandular secretions that provide a direct resource transfer to the queen. Workers also protect the queen by physical defence and grooming behaviour. In addition, the society reduces mortality of its members by building a physical structure that provides a safe, homeostatic environment, which explains in part the difference in mortality of pre-foraging and foraging workers [14]. Although we do not exactly know which specific factors are important, these combined social effects enable the longevity of a few select individuals externally, in addition to internal changes but studies of the relative importance of this social facilitation of queen longevity are still needed in honey bees.
Conclusions
Due to the phenotypic plasticity and genotypic diversity, honey bees provide several interesting experimental opportunities to pursue the study of the relation between fecundity and longevity within and between castes (Figure 1). Molecular mechanisms of longevity regulation may be reversed or preserved [62]. In particular, the pseudoqueen phenomenon of A. m. capensis provides an exceptional test system to investigate the fecundity/longevity relation in honey bees by comparing long-lived reproductive workers with short-lived sterile workers. At present, it appears that a combination of social facilitation and internal re-organization of physiological pathways is responsible for the reversal of the concurrence of the high reproductive rate and exceptional longevity in honey bee queens but more studies are needed.
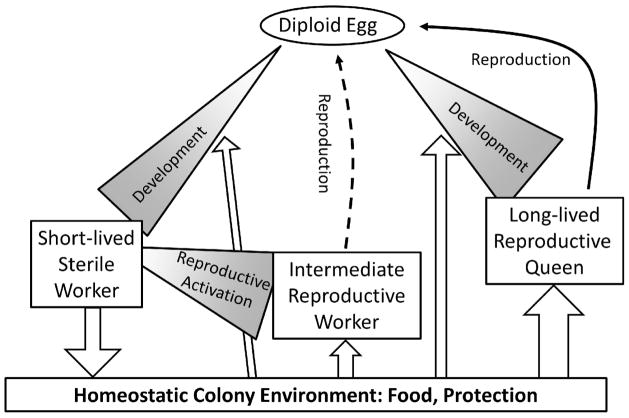
Figure 1.
Depending on resources, development of a fertilized egg leads to a short-lived, sterile worker or a long-lived reproductive queen phenotype. In the absence of reproductive inhibition from a queen, adult workers can become reproductively active which have an intermediate life expectancy, although only workers of the Cape honey bee can produce diploid eggs. White arrows indicate benefits, such as behavioural interactions, resource transfers, or protection, that may explain a proximate “social facilitation” of the longevity of reproductive individuals.
Highlights.
Long-lived, reproductive honey bee queens outlive usually non-reproductive workers.
Worker longevity in honey bees is primarily driven by age-dependent division of labour.
Physiology and social facilitation contribute to longevity of honey bee queens
Honey bees are excellent for studying the relation between fecundity and longevity.
Acknowledgments
We would like to express our gratitude to Judith Korb for organizing this volume and the invitation to write this chapter. O.R. was financially supported by the National Institutes of Health (NIA # R21AG046837) and the US Army Research Office (W911NF-15-2-0045). D.A. and R.F.A.M. are part of the Research Unit 2281 ‘Sociality and the reversal of the fecundity-longevity trade-off’ funded by the Deutsche Forschungsgemeinschaft (project MO373/33-1 to R.F.A.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Winston ML. The Biology of the Honey Bee. Cambridge, Massachusetts: Harvard University Press; 1987. [Google Scholar]
- 2.Beshers SN, Fewell JH. Models of division of labor in social insects. Annual Review of Entomology. 2001;46:413–440. doi: 10.1146/annurev.ento.46.1.413. [DOI] [PubMed] [Google Scholar]
- 3.Kucharski R, Maleszka J, Foret S, Maleszka R. Nutritional control of reproductive status in honeybees via DNA methylation. Science. 2008;319:1827–1830. doi: 10.1126/science.1153069. [DOI] [PubMed] [Google Scholar]
- 4.Bourke AFG. Worker reproduction in the higher eusocial hymenoptera. Quarterly Review of Biology. 1988;63:291–311. [Google Scholar]
- 5.Page RE, Peng Y-SC. Aging and development in social insects with emphasis on the honey bee, Apis mellifera L. Experimental Gerontology. 2001;36:695–711. doi: 10.1016/s0531-5565(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 6.Hayworth MK, Johnson NG, Wilhelm ME, Gove RP, Metheny JD, Rueppell O. Added weights lead to reduced flight behavior and mating success in polyandrous honey bee queens (Apis mellifera) Ethology. 2009;115:698–706. [Google Scholar]
- 7.Le Conte Y, Hefetz A. Primer pheromones in social hymenoptera. Annual Review of Entomology. 2008;53:523–542. doi: 10.1146/annurev.ento.52.110405.091434. [DOI] [PubMed] [Google Scholar]
- 8**.Wang Y, Kaftanoglu O, Siegel AJ, Page RE, Amdam GV. Surgically increased ovarian mass in the honey bee confirms link between reproductive physiology and worker behavior. Journal of Insect Physiology. 2010;56:1816–1824. doi: 10.1016/j.jinsphys.2010.07.013. Transplanting exta ovaries into workers, this study shows that these ovaries can survive and it elucidates the behavioral and life history consequences of an experimentally enlarged ovary for non-reproductive honey bee workers. [DOI] [PubMed] [Google Scholar]
- 9**.Neukirch A. Dependence of the life-span of the honeybee (Apis mellifica) upon flight performance and energy-consumption. Journal of Comparative Physiology. 1982;146:35–40. A classic, carefully designed and executed study of aging in honey bee workers demonstrating that their life expectancy depends on behavior, specifically foraging acitivity. [Google Scholar]
- 10.Williams JB, Roberts SP, Elekonich MM. Age and natural metabolically-intensive behavior affect oxidative stress and antioxidant mechanisms. Experimental Gerontology. 2008;43:538–549. doi: 10.1016/j.exger.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 11*.Buttstedt A, Moritz RFA, Erler S. Origin and function of the major royal jelly proteins of the honeybee (Apis mellifera) as members of the yellow gene family. Biological Reviews. 2014;89:255–269. doi: 10.1111/brv.12052. This review highlights the importance of nutritional signals for female caste differentiation, focusing on the major royal jelly proteins. Examining existing studies, the case is made for a more general role in life history regulation. [DOI] [PubMed] [Google Scholar]
- 12.Crailsheim K. The protein balance of the honey bee worker. Apidologie. 1990;21:417–429. [Google Scholar]
- 13.Woyciechowski M, Kozlowski J. Division of labor by division of risk according to worker life expectancy in the honey bee (Apis mellifera L.) Apidologie. 1998;29:191–205. [Google Scholar]
- 14.Rueppell O, Bachelier C, Fondrk MK, Page RE., Jr Regulation of life history determines lifespan of worker honey bees (Apis mellifera L.) Exp Gerontol. 2007;42:1020–1032. doi: 10.1016/j.exger.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behrends A, Scheiner R, Baker N, Amdam GV. Cognitive aging is linked to social role in honey bees (Apis mellifera) Experimental Gerontology. 2007;42:1146–1153. doi: 10.1016/j.exger.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amdam GV, Rueppell O, Fondrk MK, Page RE, Nelson CM. The nurse’s load: Early-life exposure to brood-rearing affects behavior and lifespan in honey bees (Apis mellifera) Experimental Gerontology. 2009;44:467–471. doi: 10.1016/j.exger.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rueppell O, Linford R, Gardner P, Coleman J, Fine K. Aging and demographic plasticity in response to experimental age structures in honeybees (Apis mellifera L) Behav Ecol Sociobiol. 2008;62:1621–1631. doi: 10.1007/s00265-008-0591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rueppell O, Kaftanouglu O, Page RE. Honey bee (Apis mellifera) workers live longer in small than in large colonies. Experimental Gerontology. 2009;44:447–452. doi: 10.1016/j.exger.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuda H, Sekiguchi K. Seasonal change of the honeybee worker longevity in Sapporo, north Japan, with notes on some factors affecting the life-span. Jpn J Ecol. 1966;16:206–212. [Google Scholar]
- 20.Sakagami SF, Fukuda H. Life tables for worker honeybees. Research in Population Ecology. 1968;10:127–139. [Google Scholar]
- 21*.Dixon L, Kuster R, Rueppell O. Reproduction, social behavior, and aging trajectories in honeybee workers. Age (Dordr) 2014;36:89–101. doi: 10.1007/s11357-013-9546-7. This experimental study investigates the aging implications of reproductive activation in workers, along with other social behaviors. The results suggest a strong survival advantage of reproductively active workers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maisonnasse A, Lenoir J-C, Beslay D, Crauser D, Le Conte Y. E-β-ocimene, a volatile brood pheromone involved in social regulation in the honey bee colony (Apis mellifera) PLoS One. 2010;5:e13531. doi: 10.1371/journal.pone.0013531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pankiw T, Huang ZY, Winston ML, Robinson GE. Queen mandibular gland pheromone influences worker honey bee (Apis mellifera L.) foraging ontogeny and juvenile hormone titers. Journal of Insect Physiology. 1998;44:685–692. doi: 10.1016/s0022-1910(98)00040-7. [DOI] [PubMed] [Google Scholar]
- 24.Zayed A, Robinson GE. Understanding the relationship between brain gene expression and social behavior: lessons from the honey bee. Annual Review of Genetics. 2012;46:591–615. doi: 10.1146/annurev-genet-110711-155517. [DOI] [PubMed] [Google Scholar]
- 25.Robinson GE, Page RE, Strambi C, Strambi A. Colony integration in honey bees - mechanisms of behavioral reversion. Ethology. 1992;90:336–348. [Google Scholar]
- 26.Amdam GV, Aase ALTO, Seehuus SC, Fondrk MK, Norberg K, Hartfelder K. Social reversal of immunosenescence in honey bee workers. Experimental Gerontology. 2005;40:939–947. doi: 10.1016/j.exger.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27*.Baker N, Wolschin F, Amdam GV. Age-related learning deficits can be reversible in honeybees Apis mellifera. Experimental Gerontology. 2012;47:764–772. doi: 10.1016/j.exger.2012.05.011. This study demonstrates that senescence is not age- but state-dependent in honey bee workers. Foragers that age quickly can be manipulated into reverting back to in-hive nurse bees and their learning improves, which is associated with proteomic changes. [DOI] [PubMed] [Google Scholar]
- 28*.Grozinger CM, Fan YL, Hoover SER, Winston ML. Genome-wide analysis reveals differences in brain gene expression patterns associated with caste and reproductive status in honey bees (Apis mellifera) Molecular Ecology. 2007;16:4837–4848. doi: 10.1111/j.1365-294X.2007.03545.x. In a microarray comparision the brain gene expression of honey bee queens exhibits much stronger differences to reproductive and non-reproductive workers than the differences between the two types of workers. This study indicates that adult social roles are not influential enough to overcome the consequences of the alternative developmental trajectories of the two female castes. [DOI] [PubMed] [Google Scholar]
- 29.Barron AB, Oldroyd BP, Ratnieks FLW. Worker reproduction in honey-bees (Apis) and the anarchic syndrome: a review. Behavioral Ecology and Sociobiology. 2001;50:199–208. [Google Scholar]
- 30.Sakagami SF. The False-Queen: Fourth Adjustive Response in Dequeened Honeybee Colonies. Behaviour. 1958;13:280–296. [Google Scholar]
- 31.Velthuis HHW, Ruttner F, Crewe RM. Differentiation in reproductive physiology and behaviour during the development of laying workers. In: Engels W, editor. Social Insects. Springer; 1990. pp. 231–243. [Google Scholar]
- 32.Neumann P, Moritz RFA. The Cape honeybee phenomenon: the sympatric evolution of a social parasite in real time? Behavioral Ecology and Sociobiology. 2002;52:271–281. [Google Scholar]
- 33.Onions GW. South African “fertile-worker bees”. South African Agricultural Journal. 1912;1:720–728. [Google Scholar]
- 34.Verma S, Ruttner F. Cytological Analysis of the Thelytokous Parthenogenesis in the Cape Honeybee (Apis mellifera capensis Escholtz) Apidologie. 1983;14:41–57. [Google Scholar]
- 35.Ruttner F, editor. Taxonomy and Biogeography of Honeybees. Berlin: Springer; 1988. [Google Scholar]
- 36*.Jarosch A, Stolle E, Crewe RM, Moritz RFA. Alternative splicing of a single transcription factor drives selfish reproductive behavior in honeybee workers (Apis mellifera) Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15282–15287. doi: 10.1073/pnas.1109343108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lattorff HMG, Moritz RFA, Crewe RM, Solignac M. Control of reproductive dominance by the thelytoky gene in honeybees. Biology Letters. 2007;3:292–295. doi: 10.1098/rsbl.2007.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lattorff HMG, Moritz RFA, Fuchs S. A single locus determines thelytokous parthenogenesis of laying honeybee workers (Apis mellifera capensis) Heredity. 2005;94:533–537. doi: 10.1038/sj.hdy.6800654. [DOI] [PubMed] [Google Scholar]
- 39.Chapman NC, Beekman M, Allsopp MH, Rinderer TE, Lim J, Oxley PR, Oldroyd BP. Inheritance of thelytoky in the honey bee Apis mellifera capensis. Heredity. 2015;114:584–592. doi: 10.1038/hdy.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dietemann V, Pflugfelder J, Hartel S, Neumann P, Crewe RM. Social parasitism by honeybee workers (Apis mellifera capensis Esch.): evidence for pheromonal resistance to host queen’s signals. Behavioral Ecology and Sociobiology. 2006;60:785–793. [Google Scholar]
- 41.Evans JD, Wheeler DE. Differential gene expression between developing queens and workers in the honey bee, Apis mellifera. Proceedings of the National Academy of Sciences, USA. 1999;96:5575–5580. doi: 10.1073/pnas.96.10.5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corona M, Estrada E, Zurita M. Differential expression of mitocondrial genes between queens and workers during caste determination in the honeybee Apis mellifera. Journal of Experimental Biology. 1999;202:929–938. doi: 10.1242/jeb.202.8.929. [DOI] [PubMed] [Google Scholar]
- 43.Fahrenholz L, Lamprecht I, Schricker B. Calorimetric investigations of the different castes of honey bees, Apis mellifera carnica. Journal of Comparative Physiology B. 1992;162:119–130. [Google Scholar]
- 44.Corona M, Hughes KA, Weaver DB, Robinson GE. Gene expression patterns associated with queen honey bee longevity. Mechanisms of Ageing and Development. 2005;126:1230–1238. doi: 10.1016/j.mad.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 45**.Seehuus SC, Norberg K, Gimsa U, Krekling T, Amdam GV. Reproductive protein protects sterile honey bee workers from oxidative stress. Proc Nat Acad Sci USA. 2006;103:962–967. doi: 10.1073/pnas.0502681103. This study made the seminar contribution to demonstrate that vitellogenin has important functions to prevent premature aging in addition to its role in reproduction. Therefore, it provided an initial mechanism for the reversal of the fecundity/longevity trade-off. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amdam GV, Omholt SW. The regulatory anatomy of honeybee lifespan. Journal of Theoretical Biology. 2002;216:209–228. doi: 10.1006/jtbi.2002.2545. [DOI] [PubMed] [Google Scholar]
- 47.Corona M, Velarde RA, Remolina S, Moran-Lauter A, Wang Y, Hughes KA, Robinson GE. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7128–7133. doi: 10.1073/pnas.0701909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flatt T, Tu MP, Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. Bioessays. 2005;27:999–1010. doi: 10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- 49.Amdam GV, Omholt SW. The hive bee to forager transition in honeybee colonies: the double repressor hypothesis. Journal of Theoretical Biology. 2003;223:451–464. doi: 10.1016/s0022-5193(03)00121-8. [DOI] [PubMed] [Google Scholar]
- 50.Amdam GV, Nilsen KA, Norberg K, Fondrk MK, Hartfelder K. Variation in endocrine signaling underlies variation in social life history. American Naturalist. 2007;170:37–46. doi: 10.1086/518183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- 52**.Rodrigues MA, Flatt T. Endocrine uncoupling of the trade-off between reproduction and somatic maintenance in eusocial insects. Current Opinion in Insect Science. doi: 10.1016/j.cois.2016.04.013. in press. A review that is dedicated to the endocrine aspects of the longevity/high fertility syndrome in social insects and thus explains the findings in more detail, drawing heavily on studies of honey bees. [DOI] [PubMed] [Google Scholar]
- 53**.Rueppell O, Königseder F, Heinze J, Schrempf A. Intrinsic survival advantage of social insect queens depends on reproductive activation. Journal of Evolutionary Biology. 2015;28:2349–2354. doi: 10.1111/jeb.12749. A simple study that nevertheless sheds light on the seminal question how much of the long-lived queen phenotype is intrinsic versus caused by social facilitation. This study used ants, but related studies may be performed in honey bees. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moritz R, Southwick EE. Bees as superorganisms: an evolutionary reality. Springer Science & Business Media; 1992. [Google Scholar]
- 54.Shorter JR, Rueppell O. A review on self-destructive defense behaviors in social insects. Insectes Sociaux. 2012;59:1–10. [Google Scholar]
- 56.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: Signaling for suicide and survival. Journal of Cellular Physiology. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 57.Cohen JJ, Duke RC, Fadok VA, Sellins KS. Apoptosis and programmed cell death in immunity. Annual Review of Immunology. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- 58.Goudie F, Oldroyd BP. Thelytoky in the honey bee. Apidologie. 2014;45:306–326. [Google Scholar]
- 59.Orciani M, Gorbi S, Benedetti M, Di Benedetto G, Mattioli-Belmonte M, Regoli F, Di Primio R. Oxidative stress defense in human-skin-derived mesenchymal stem cells versus human keratinocytes: Different mechanisms of protection and cell selection. Free Radical Biology and Medicine. 2010;49:830–838. doi: 10.1016/j.freeradbiomed.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 60.Flatt T, Amdam GV, Kirkwood TB, Omholt SW. Life-history evolution and the polyphenic regulation of somatic maintenance and survival. The Quarterly Review of Biology. 2013;88:185–218. doi: 10.1086/671484. [DOI] [PubMed] [Google Scholar]
- 61**.von Wyschetzki K, Rueppell O, Oettler J, Heinze J. Transcriptomic signatures mirror the lack of the fecundity/longevity trade-off in ant queens. Molecular Biology and Evolution. 2015;32:3173–3185. doi: 10.1093/molbev/msv186. A comprehensive transcriptomics study that emphasizes the importance of reproduction and differences in age-related gene expression changes in the ant model Cardiocondyla obscurior. The results suggest candidate molecular mechanisms for the reversed fecundity/longevity trade-off in social insects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rascón B, Amdam GV, Hubbard BP, Sinclair DA. The lifespan extension effects of resveratrol are conserved in the honey bee and may be driven by a mechanism related to caloric restriction. Aging. 2012;4:499–508. doi: 10.18632/aging.100474. [DOI] [PMC free article] [PubMed] [Google Scholar]