Abstract
Objectives
Clinical guidelines recommend norepinephrine as initial vasopressor of choice for septic shock, with dopamine suggested as an alternative vasopressor in patients at low risk for arrhythmias. We sought to determine practice patterns and outcomes associated with vasopressor selection in a large, population-based cohort of patients with septic shock that allows for assessment of outcomes in clinically important subgroups.
Design
We performed a retrospective cohort study to determine factors associated with choice of dopamine as compared with norepinephrine as initial vasopressor for patients with septic shock. We used propensity score matching to compare risk of hospital mortality based on initial vasopressor. We performed multiple sensitivity analyses using alternative methods to address confounding and hospital-level clustering. We investigated interaction between vasopressor selection and mortality in clinical subgroups based on arrhythmia and cardiovascular risk.
Setting
Enhanced administrative data (Premier, Inc) from 502 US hospitals during years 2010–2013.
Subjects
61,122 patients admitted with septic shock who received dopamine or norepinephrine as initial vasopressor during the first two days of hospitalization.
Interventions
None
Measurements and Main Results
Norepinephrine (77.6%) was the most frequently used initial vasopressor during septic shock. Dopamine was preferentially selected by cardiologists, in the Southern US, at non-teaching hospitals, for older patients with more cardiovascular comorbidities, and was used less frequently over time. Patients receiving dopamine experienced greater hospital mortality (propensity matched cohort: N=38,788, 25% vs 23.7%; OR 1.08, 95% CI 1.02–1.14). Sensitivity analyses showed similar results. Subgroup analyses showed no evidence for effect modification based on arrhythmia risk or underlying cardiovascular disease.
Conclusions
In a large population-based sample of patients with septic shock in the US, use of dopamine as initial vasopressor was associated with increased mortality among multiple clinical subgroups. Areas where use of dopamine as initial vasopressor are more common represent potential targets for quality improvement intervention.
Keywords: septic shock, vasopressor agents, norepinephrine, dopamine, clinical practice patterns
Introduction
Septic shock is an immediately life-threatening manifestation of infection characterized by loss of adequate end organ perfusion. Septic shock affects approximately 73 per 100,000 US adults(1) with a rising incidence; average case-fatality rates are 20–30%.(1–3) Approximately one-half of patients with sepsis and evidence of inadequate tissue perfusion have hemodynamic collapse unresponsive to adequate fluid resuscitation requiring the use of vasopressor medications.(3, 4) The vasopressors most frequently used initially to improve hemodynamics during septic shock, norepinephrine and dopamine, have differing physiological profiles. Depending on dose, dopamine has stronger beta1-receptor agonist properties and results in relatively increased cardiac chronotropic and inotropic response with increased cardiac output; norepinephrine has stronger alpha1-agonist effects with more potent peripheral vasoconstriction.(5)
Results of a randomized controlled trial including 1044 patients with septic shock in Europe showed an increase in new arrhythmias with the use of dopamine as compared with norepinephrine.(6) A subsequent meta-analysis of six trials including 1408 patients demonstrated increased mortality for dopamine as compared with norepinephrine.(7) As a result, norepinephrine emerged as the guideline-recommended first line vasopressor of choice during septic shock, with use of dopamine suggested as an alternative in selected patients with low risk of arrhythmias.(4) However, outcomes associated with vasopressor choice in subgroups of patients with septic shock, such as those with low risk of arrhythmia, are unclear.
Further, knowledge gaps exist regarding utilization patterns and patient outcomes associated with choice of vasopressors for septic shock in the United States (US). Improved understanding of demographics, clinical characteristics, hospital factors, and temporal trends associated with selection of initial vasopressor during septic shock may inform efforts to target implementation of current Guidelines. Comparing effectiveness of dopamine and norepinephrine in a large, population-based cohort may reveal important insights regarding real-world effectiveness and allow for the assessment of clinical outcomes among clinical subgroups of patients with septic shock in whom Guidelines continue to suggest dopamine (e.g. patients at low risk of arrhythmias). We explored practice patterns and patient outcomes associated with initial choice of vasopressor therapy in a large, population-based cohort of patients hospitalized with septic shock in the US between 2010–2013.
Materials and Methods
Patient sample
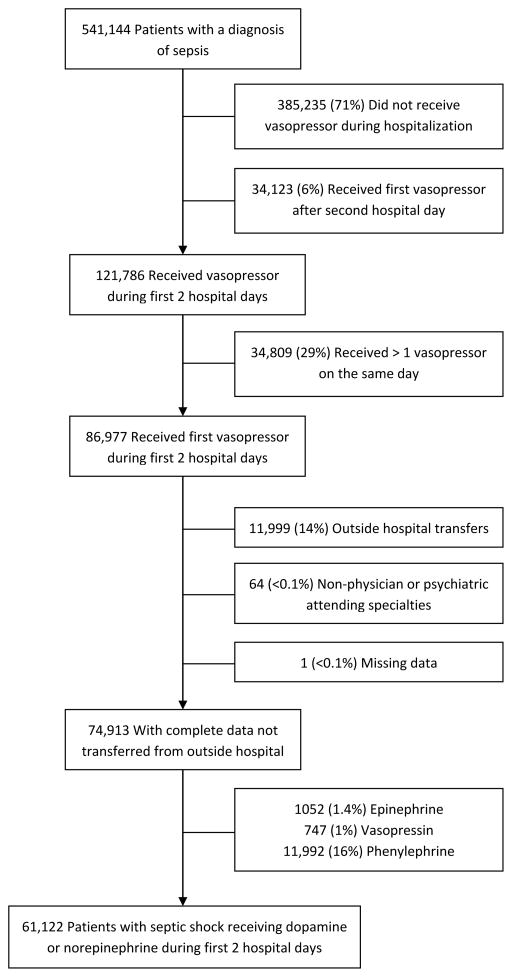
We performed a retrospective cohort study among patients with septic shock using the Premier (Premier, Charlotte, NC) enhanced administrative database. Premier data include standard hospital discharge files as well as date-stamped pharmacy, laboratory, and diagnostics billing information from over 500 hospitals across all geographic regions of the US, accounting for approximately 20% of US hospitalizations. We identified patients admitted with septic shock using previously validated methods including patients with a diagnosis of sepsis or septic shock (Supplemental Digital Content - Table 1) (8, 9) who received norepinephrine or dopamine during the first two days of hospitalization. We excluded patients who received more than one vasopressor on the initial day of vasopressor administration due to inability to distinguish the initial vasopressor choice. Patients transferred from another hospital were excluded since there was no documentation of initial vasopressor. We also excluded patients with missing covariates and patients with non-physician or psychiatric attending specialties. Because of our focus on norepinephrine and dopamine, we excluded patients who received less frequently used initial vasopressors phenylephrine (N=11,999, 14%), vasopressin (N=747, 1%), and epinephrine (N=1,052, 1.4%).
Vasopressor exposure
We identified vasopressor utilization from pharmacy billing files. We characterized initial vasopressor exposure as the first vasopressor initiated during the first two days of hospitalization in order to attenuate potential bias from unmeasured changes in clinical status or severity of illness between time of admission and receipt of each vasopressor.
Covariates
From the Premier database we identified year of hospitalization, patient demographics, hospital characteristics, attending physician specialty, location of admission, and use of intensive care. We characterized comorbid conditions (10–12), acute organ failures that were present on admission (9, 13), and site of infection (14) using diagnosis code algorithms (Supplemental Digital Content - Table 1).
Outcomes
We identified factors associated with choice of norepinephrine or dopamine as first vasopressor. We also compared hospital mortality between patients who received dopamine versus norepinephrine as initial vasopressor during septic shock.
Statistical Analysis
Full details of statistical analysis are included in the Supplemental Methods. We used SAS version 9.4 (Cary, NC) for all analyses, with an alpha threshold of 0.05. Study procedures were approved by the Boston University Medical Campus Institutional Review Board.
We report continuous variables using means and standard deviations or median and interquartile range depending on distribution, and categorical variables as percentages. We evaluated differences in baseline characteristics using standardized differences with a threshold of 0.2 (i.e. the characteristic of interest accounts for 1% of the variance in vasopressor use); conventional p-values may show clinically insignificant between-group statistical differences when sample size is large.(15) Using information from all measured covariates, we constructed a non-parsimonious logistic regression model to calculate the probability of receiving dopamine or norepinephrine. We used a propensity score-based approach to attenuate confounding by observed covariates.(16) To analyze the association between vasopressor choice and mortality we matched patients using a 1:N match structure based on nearest propensity score in a hierarchical 8-to-1 digit match, without replacement or incomplete matches. We then used conditional logistic regression to compare hospital mortality between patients who received dopamine or norepinephrine,(17) and Kaplan-Meier curves to visually demonstrate outcomes, censoring observation time at hospital discharge. Finally, we estimated the proportion of septic shock-related deaths attributable to initial vasopressor choice (population attributable fraction). We performed a number of sensitivity analyses to explore the robustness of our primary analysis (see Supplemental Methods for details).
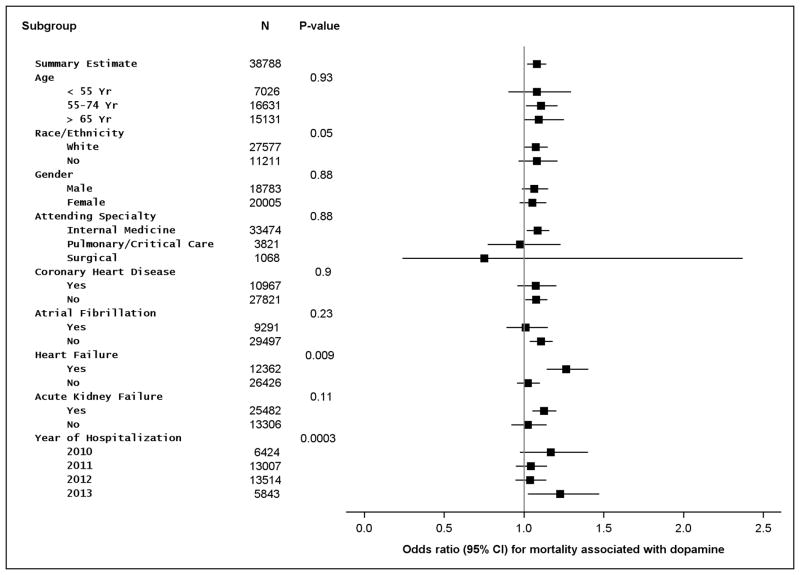
Differences in clinical subgroups were tested using tests of multiplicative interaction in conditional logistic regression analysis among our primary propensity score matched sample (Supplemental Methods). Surviving Sepsis Campaign Guidelines suggest use of dopamine as an alternative vasopressor among patients with septic shock and low-probability of arrhythmias or impaired systolic function.(4) We hypothesized that younger patients and those without a history of atrial fibrillation may have lower arrhythmia risk and, along with patients who have a history of heart failure, may experience better outcomes associated with use of dopamine during septic shock.
Results
Among 121,786 patients with sepsis present on admission who received a vasopressor at any time during hospitalization, we identified 61,122 eligible patients with septic shock who received dopamine or norepinephrine (Figure 1). Patients were on average 68 ± 15.3 years, 51.3% were female and 71.1% were white, with average hospital mortality of 24.4%.
Figure 1.
Flow chart detailing the number, percent, and reason for exclusion of patients from the analysis
A greater proportion of patients with septic shock received norepinephrine (77.6%) as compared with dopamine (22.4%) as initial vasopressor. Baseline clinical variables stratified by initial vasopressor choice are shown in Supplemental Digital Content - Table 2. In multivariable analysis, hospital or physician factors associated with use of dopamine included Southern US geographic location, non-teaching hospital status, and Cardiology provider specialty. Patient factors associated with use of dopamine included older age, a history of heart failure, valvular disease, coronary arterial disease, or renal impairment, and no history of atrial fibrillation, venous thromboembolic disease, or cancer. Patients receiving initial dopamine also had fewer acute organ failures, less need for mechanical ventilation at admission, and a lower incidence of sepsis resulting from pneumonia or gastrointestinal sources. Dopamine was used less frequently over time (Table 1).
Table 1.
Factors associated with choice of norepinephrine vs. dopamine as initial vasopressor.
| Variable | Adjusted ORa (95% CI) |
|---|---|
| Age (5 year increment) | 0.96 (0.96 – 0.97) |
| Location | |
| South | Reference |
| Midwest | 1.02 (0.77 – 1.34) |
| Northeast | 1.39 (1.07 – 1.81) |
| West | 1.72 (1.33 – 2.22) |
| Teaching Hospital | 1.50 (1.20 – 1.86) |
| Provider Specialty | |
| Cardiology | Reference |
| Internal Medicine | 1.50 (1.28 – 1.76) |
| Pulmonary/CCM | 1.69 (1.41 – 2.02) |
| Surgery | 1.86 (1.54 – 2.26) |
| Healthcare point of entry | 1.07 (1.02 – 1.12) |
| Diabetes | 1.04 (1.00 – 1.07) |
| Heart Failure | 0.84 (0.81 – 0.88) |
| Coronary Artery Disease | 0.91 (0.87 – 0.95) |
| Atrial Fibrillation | 1.07 (1.03 – 1.11) |
| Valvular Heart Disease | 0.93 (0.88 – 0.98) |
| Venous Thromboembolic Disease | 1.11 (1.01 – 1.22) |
| Renal Impairment | 0.88 (0.84 – 0.91) |
| Cancer | 1.22 (1.17 – 1.29) |
| Acute respiratory failure | 1.11 (1.06 – 1.16) |
| Acute renal failure | 1.07 (1.03 – 1.11) |
| Acute hematologic failure | 1.11 (1.07 – 1.16) |
| Acute metabolic failure | 1.21 (1.16 – 1.26) |
| Inotrope | 0.99 (0.92 – 1.07) |
| Mechanical ventilation (day 1) | 1.30 (1.23 – 1.37) |
| Pneumonia | 1.05 (1.02 – 1.09) |
| Gastrointestinal Infection | 1.10 (1.04 – 1.16) |
| Year of Hospitalization | |
| 2010 | Reference |
| 2011 | 1.03 (0.97 – 1.10) |
| 2012 | 1.51 (1.41 – 1.63) |
| 2013 | 1.99 (1.83 – 2.18) |
Adjusted for other variables presented in the table as well as sex, race, comorbidities, presence of additional acute organ failures, and sources of infection.
OR = odds ratio, CI = confidence interval, CCM = critical care medicine.
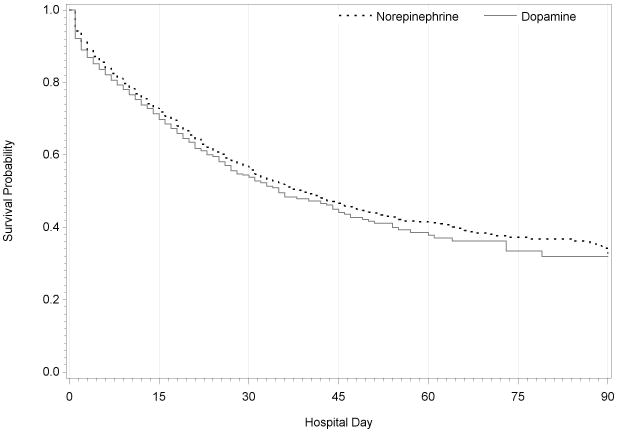
Prior to propensity-score matching, patients receiving norepinephrine as initial vasopressor had unadjusted in-hospital mortality of 24.5%, similar to patients receiving dopamine (23.9%, p=0.16). Among patients who received dopamine and norepinephrine, propensity score matching resulted in similar distribution of propensity scores (Supplemental Digital Content - Figure 1) and well-balanced measured covariates (Supplemental Digital Content - Table 2). After matching patients based on the propensity score, patients who received dopamine experienced greater hospital mortality as compared with patients receiving norepinephrine (N=38,788, 25% vs 23.7%; OR 1.08, 95% CI 1.02–1.14) (Figure 2). An estimated 1.7% of septic shock-related deaths may be attributable to use of dopamine as initial vasopressor. Additional sensitivity analyses showed similar results to the matched cohort, with initial dopamine use associated with increased hospital mortality among patients with septic shock (Table 2).
Figure 2.
Kapan-Meier curves for in-hospital mortality comparing patients with septic shock receiving norepinephrine (N = 15243) or dopamine (N = 5081) on the first day of hospitalization in a 1:3 propensity score matched sample (Wilcoxon p = 0.0001)
Table 2.
Sensitivity analyses
| Type of Analysis | N | Adjusted OR (95% CI) | p-value |
|---|---|---|---|
| Primary propensity-matched analysis | 38,788 | 1.08 (1.02–1.14) | 0.004 |
| GEEa adjusted by propensity score | 61,122 | 1.05 (0.99 –1.11) | 0.1 |
| Inverse probability of treatment weights | 61,122 | 1.07 (1.00 – 1.13) | 0.03 |
| Stratified by propensity score deciles | 38,788 | 1.06 (1.02 – 1.11) | 0.001 |
| Multivariable-adjusted GEEa | 61,122 | 1.00 (0.95 – 1.06) | 0.88 |
| Instrumental variable (per 10% incremental increase in hospital use of dopamine) | 61,122 | 1.05 (1.04 – 1.07) | < 0.001 |
GEE: generalized estimating equation logistic regression analysis with hospital clusters
Analyses examining whether clinical outcomes differed by subgroups with differing risk for arrhythmias showed effect estimates consistent with the main outcome: no subgroup showed improved outcomes with use of dopamine (Figure 3).
Figure 3.
Subgroup analyses in the 1:3 matched cohorts comparing mortality in dopamine vs. norepinephrine as initial vasopressor in septic shock. CI = confidence interval.
Discussion
We characterized the practice patterns of initial vasopressor choice and explored the ‘real world’ effectiveness of norepinephrine as compared with dopamine in a representative cohort of more than 60,000 patients hospitalized with septic shock in the US. We found high rates of norepinephrine use as initial vasopressor, three-fold more common than dopamine, with increasing use of norepinephrine over time. Dopamine was more likely to be selected by cardiologists, in the Southern US, at non-teaching hospitals, for older patients with more cardiovascular comorbidities and fewer acute organ failures. After adjusting for baseline differences, our results showed an association between use of dopamine and increased mortality during septic shock as compared with norepinephrine. We did not find evidence for a mortality benefit of dopamine in any subgroup studied, including patients who may be at lower risk of arrhythmia. Based on our results, hospital case-fatality may be reduced by approximately 1.7% if clinicians currently using dopamine switched to initial use of norepinephrine during septic shock.
The preferential use of norepinephrine for septic shock in the US is in accordance with clinical practice guidelines and similar to findings observed in a European multicenter cohort study where norepinephrine use was four-fold more common than dopamine.(18) Our findings of increased mortality associated with dopamine use are also consistent with those from a meta-analysis of clinical trials by De Backer et al,(7) which showed similar effect estimates to our study. Our results expand upon efficacy trials by demonstrating increased effectiveness of norepinephrine over dopamine during septic shock in a large (N=61,122) population-based, real-world sample.
Evidence supporting the current Surviving Sepsis Campaign suggestion for dopamine as an alternative initial vasopressor in patients with “low risk of tachyarrhythmias and absolute or relative bradycardia” is low (grade 2C).(4) Our findings provide novel evidence that does not support use of dopamine among a subgroup of patients with low risk for arrhythmias. We found no strong evidence for effect modification by clinical subgroups with different arrhythmia risks. If replicated, our findings suggesting norepinephrine as the initial vasopressor of choice among all patients with septic shock may potentially be used to simplify the message of future Guidelines.
Increasing use of norepinephrine for patients with septic shock provides evidence for uptake of clinical practice guidelines. However, we also identify areas in which dopamine use is more likely, providing potential targets for focused education, implementation training, and quality improvement interventions. Our findings add novel evidence showing differential management strategies between clinical specialties for patients with septic shock. Cardiologists were the least likely to select norepinephrine as initial vasopressor, despite the probability that patients with cardiac disease may have higher risk of arrhythmias, supporting recent calls by the American Heart Association for further training of cardiologists in non-cardiac critical care.(19) In addition we identified teaching hospitals as almost 50% more likely to use norepinephrine as initial vasopressor during septic shock, suggesting slower uptake of guidelines at non-teaching hospitals. Large regional variations in sepsis care between countries have previously been described,(1) but variation in sepsis-specific practice patterns within the US were unclear. Our findings of lower use of norepinephrine in the Southern US is especially intriguing in light of previous research showing that sepsis mortality is greatest in the Southern states.(20) Further studies are needed to assess whether targeting improvement efforts to areas of lower evidence uptake reduces mortality.
Our study had limitations warranting discussion. As with all observational studies, unmeasured confounding cannot be excluded. For example, we were unable to directly measure correlation between vasopressor choice and other guideline-recommended therapy such as earlier antibiotics or volume resuscitation. However, we obtained similar results performing multiple sensitivity analyses to address measured confounding, included hospitals as clusters to account for correlated care practices within a hospital, and used an instrumental variable analysis meant to attenuate unmeasured confounders. Sensitivity analyses using traditional multivariable-adjusted regression did not show a statistically significant mortality difference between vasopressors, but standard multivariable models are more likely to produce biased estimates than propensity score-based models upon which our primary analyses were based, and inclusion of multiple covariates may have diminished power to detect a statistically significant difference.(16) Our results should not be generalized to patients who received multiple simultaneous vasopressors, who were excluded from our analysis. Additionally, we were unable to assess differences in outcomes by vasopressor dosage, duration of use, or assess long-term outcomes.
Conclusions
We have identified a ‘real world’ mortality benefit associated with use of norepinephrine when compared to dopamine in a large, population-based study of patients with septic shock in the US. We did not find any clinical subgroup in which dopamine appeared to improve mortality outcomes; a finding that does not support current Surviving Sepsis Campaign Guideline suggestions that dopamine may be used when arrhythmia risk is low. In addition, our results demonstrate specific areas in which dopamine as initial vasopressor during septic shock is more common, where performance improvement interventions may be most efficiently targeted.
Supplementary Material
Supplemental Figure 1: Distribution of propensity scores by initial vasopressor in the 1:3 propensity score matched cohort
Supplemental Table 1: International Classification of Disease-9th Edition-Clinical Modification (ICD-9) codes used to identify comorbid conditions, acute organ failures, and site of infection. ICD-9 = International Classification of Disease-9th Edition-Clinical Modification
Supplemental Table 2: Baseline characteristics of patients with septic shock by initial vasopressor choice in the full and 1:3 propensity matched cohort
Acknowledgments
Sources of support: NIH NHLBI K01HL116768
References
- 1.Walkey AJ, Wiener RS, Lindenauer PK. Utilization patterns and outcomes associated with central venous catheter in septic shock: a population-based study. Critical care medicine. 2013;41(6):1450–1457. doi: 10.1097/CCM.0b013e31827caa89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. Jama. 2014;311(13):1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 3.Investigators P. Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. The New England journal of medicine. 2014;370(18):1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive care medicine. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marik PE, Mohedin M. The contrasting effects of dopamine and norepinephrine on systemic and splanchnic oxygen utilization in hyperdynamic sepsis. Jama. 1994;272(17):1354–1357. [PubMed] [Google Scholar]
- 6.De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. The New England journal of medicine. 2010;362(9):779–789. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
- 7.De Backer D, Aldecoa C, Njimi H, et al. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis*. Critical care medicine. 2012;40(3):725–730. doi: 10.1097/CCM.0b013e31823778ee. [DOI] [PubMed] [Google Scholar]
- 8.Iwashyna TJ, Odden A, Rohde J, et al. Identifying patients with severe sepsis using administrative claims: patient-level validation of the angus implementation of the international consensus conference definition of severe sepsis. Medical care. 2014;52(6):e39–43. doi: 10.1097/MLR.0b013e318268ac86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. The New England journal of medicine. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 10.Birman-Deych E, Waterman AD, Yan Y, et al. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Medical care. 2005;43(5):480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 11.Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Medical care. 2004;42(8):801–809. doi: 10.1097/01.mlr.0000132391.59713.0d. [DOI] [PubMed] [Google Scholar]
- 12.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 13.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical care medicine. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Leligdowicz A, Dodek PM, Norena M, et al. Association between source of infection and hospital mortality in patients who have septic shock. American journal of respiratory and critical care medicine. 2014;189(10):1204–1213. doi: 10.1164/rccm.201310-1875OC. [DOI] [PubMed] [Google Scholar]
- 15.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, N.J: L. Erlbaum Associates; 1988. [Google Scholar]
- 16.Rosenbaum PR, Rubin DB. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 17.Parsons L. Performing a 1:N Case-Control Match on Propensity Score. Proceedings of the 29th SAS Users Group; 2004; Montreal. 2004. [Google Scholar]
- 18.Sakr Y, Reinhart K, Vincent JL, et al. Does dopamine administration in shock influence outcome? Results of the Sepsis Occurrence in Acutely Ill Patients (SOAP) Study. Critical care medicine. 2006;34(3):589–597. doi: 10.1097/01.CCM.0000201896.45809.E3. [DOI] [PubMed] [Google Scholar]
- 19.Morrow DA, Fang JC, Fintel DJ, et al. Evolution of critical care cardiology: transformation of the cardiovascular intensive care unit and the emerging need for new medical staffing and training models: a scientific statement from the American Heart Association. Circulation. 2012;126(11):1408–1428. doi: 10.1161/CIR.0b013e31826890b0. [DOI] [PubMed] [Google Scholar]
- 20.Wang HE, Devereaux RS, Yealy DM, et al. National variation in United States sepsis mortality: a descriptive study. International journal of health geographics. 2010;9:9. doi: 10.1186/1476-072X-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Distribution of propensity scores by initial vasopressor in the 1:3 propensity score matched cohort
Supplemental Table 1: International Classification of Disease-9th Edition-Clinical Modification (ICD-9) codes used to identify comorbid conditions, acute organ failures, and site of infection. ICD-9 = International Classification of Disease-9th Edition-Clinical Modification
Supplemental Table 2: Baseline characteristics of patients with septic shock by initial vasopressor choice in the full and 1:3 propensity matched cohort