SUMMARY
Although the adult mammalian spinal cord lacks intrinsic neurogenic capacity, glial cells can be reprogrammed in vivo to generate neurons after spinal cord injury (SCI). How this reprogramming process is molecularly regulated, however, is not clear. Through a series of in vivo screens, we show here that the p53-dependent pathway constitutes a critical checkpoint for SOX2-mediated reprogramming of resident glial cells in the adult mouse spinal cord. While it has no effect on the reprogramming efficiency, the p53 pathway promotes cell cycle exit of SOX2-induced adult neuroblasts (iANBs). As such, silencing of either p53 or p21 markedly boosts the overall production of iANBs. A neurotrophic milieu supported by BDNF and NOG can robustly enhance maturation of these iANBs into diverse but predominantly glutamatergic neurons. Together, these findings have uncovered critical molecular and cellular checkpoints that may be manipulated to boost neuron regeneration after SCI.
Keywords: in vivo reprogramming, p21, p53, SOX2, BDNF, noggin, spinal cord injury
Graphical Abstract
INTRODUCTION
Severe morbidity and mortality are commonly associated with spinal cord injury (SCI). Human patients who survive SCI frequently live with paralysis and extremely reduced quality of life and productivity. The financial and emotional burdens to patients and their caregivers are enormous. There is currently no effective cure. This is largely because SCI often results in a permanent loss of neurons and the disruption of neural circuits that are critical for normal motor, sensory, and autonomic function (Bradbury and McMahon, 2006; Rossignol et al., 2007; Thuret et al., 2006).
Unlike discrete regions of the adult brain in which new neurons are constantly born (Jessberger and Gage, 2014; Kempermann et al., 2015; Lim and Alvarez-Buylla, 2016), the adult mammalian spinal cord exhibits minimal regenerative capacity under physiological and pathological conditions (Chi et al., 2006; Horky et al., 2006; Horner et al., 2000; Shechter et al., 2007; Vessal et al., 2007; Yang et al., 2006). Proliferative cells in the intact adult spinal cord exclusively give rise to glial cells (Horner et al., 2000). In response to SCI, astrocytes, ependymal cells, NG2-glia, pericytes, fibroblasts, and microglia become activated, proliferate, migrate, and generate scar-forming glial cells surrounding the damaged regions (Fitch and Silver, 2008; Goritz et al., 2011; Horky et al., 2006; Johansson et al., 1999; Ohori et al., 2006; Sellers et al., 2009; Sofroniew, 2009). Injury-induced neurogenesis in the adult spinal cord is extremely rare and new neurons are not detectable in most cases (Horky et al., 2006; Johansson et al., 1999; Su et al., 2014). However, neurosphere-forming cells can be isolated from the adult spinal cord and show multi-lineage differentiation potential in vitro (Ohori et al., 2006; Shihabuddin et al., 2000), suggesting the fate of certain resident non-neuronal cells may be manipulated for neurogenesis in vivo. Indeed, growth factor treatment and ectopic expression of NEUROG2 can stimulate endogenous neural progenitors to produce neurons after SCI (Ohori et al., 2006). We and others also have shown that resident glial cells, such as astrocytes and NG2 glia, can be in vivo reprogrammed to neurons in the adult brain and spinal cord (Guo et al., 2014b; Heinrich et al., 2014; Islam et al., 2015; Liu et al., 2015; Niu et al., 2015; Niu et al., 2013; Su et al., 2014; Torper et al., 2015; Torper et al., 2013).
In contrast to a direct reprogramming process controlled by ectopic expression of NEUROD1 or other neurogenic factors (Guo et al., 2014b; Liu et al., 2015; Torper et al., 2015; Torper et al., 2013), SOX2-mediated in vivo reprogramming of brain astrocytes is a multistep process that passes through ASCL1+ progenitors and DCX+ neuroblasts before transitioning into mature neurons (Niu et al., 2015). Both ASCL1 and the orphan nuclear receptor TLX are critically involved in this process (Islam et al., 2015; Niu et al., 2015). Importantly, SOX2-induced adult neuroblasts (iANBs) can be similarly detected in the injured adult spinal cord, even though the total number of iANBs and iANB-derived mature neurons was low (Su et al., 2014). To understand the reprogramming process and improve the production of iANBs in the adult spinal cord, we modified several procedures and screened a series of transcription factors and signaling molecules. We found that the p53 pathway is a critical checkpoint that controls cell cycle exit of iANBs. Downregulation of this pathway leads to a significant increase of iANBs, which can mature into both excitatory and inhibitory neurons in the presence of neurotrophic factors in the injured adult spinal cord.
RESULTS
The p53 pathway restricts generation of spinal iANBs
In contrast to robust generation of iANBs and adult-born neurons in the striatum (Niu et al., 2013), SOX2-mediated reprogramming in the adult spinal cord was less efficient with only about 200 DCX+ cells detected surrounding the injected area at 4–8 weeks post-injection (wpi) of virus (Su et al., 2014). We then conducted a series of in vivo screens for factors that might potentiate SOX2 reprogramming ability. Most of these screens, including conditional deletion of Pten or ectopic expression of Pax6, Neurog2, Ascl1, Sox11, Tlx, Pou5f1, Olig2, Ptf1a, Sox1, Sox3, Brn2, NeuroD1, Fgf2, miR-9/9* or miR-124, failed to significantly enhance the appearance of iANBs.
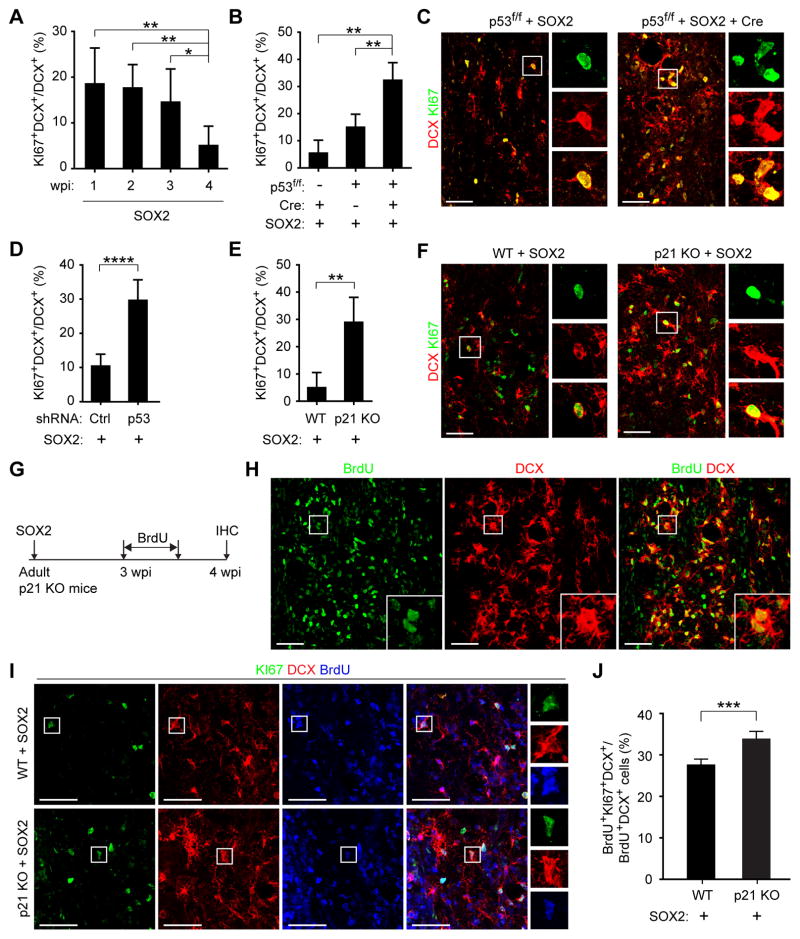
Conditional removal of p53, on the other hand, greatly enhanced the number of SOX2-induced iANBs with about 1,500 DCX+ cells detected at 5 or 6 wpi (Figure S1A, B). Very interestingly, the largest increase came from optimization of several procedures, such as improvements on SOX2 virus quality and injection methods. These optimizations led to the detection of approximately 10,000 DCX+ cells in the SOX2 alone injected spinal region at 4 wpi (Figure S1C). Even under this optimized condition, additional genetic deletion of p53 continued to result in a roughly 100% increase in the number of iANBs (Figure 1A, B). This increase was also observed when p53 expression was transiently downregulated by polymerase II- and human GFAP promoter-dependent expression of p53 shRNA (Figure 1C and S2A–D).
Figure 1. The p53 pathway impedes iANB production in vivo.
(A and B) Conditional deletion of p53 promotes SOX2-dependent iANBs in the adult spinal cord. iANBs were determined by DCX staining at 4 wpi (mean±s.d.; n=4 mice per group; *p=0.05 by Student’s t-test). Nuclei were counterstained with Hoechst 33342 (Hst). wpi, weeks post virus injection. Scale bar, 50 μm.
(C) shRNA-mediated knockdown of p53 expression enhances spinal iANBs. Cells were quantified at 4 wpi (mean±s.d.; n=6 mice per group; *p=0.027 by Student’s t-test). A shRNA against firefly luciferase was used as a control (Ctrl).
(D and E) The number of iANBs is increased in p21 null spinal cords. DCX+ iANBs were analyzed at 4 wpi (mean±s.d.; n=4 mice per group; *p=0.042 by Student’s t-test). Scale bar, 50 μm.
See also Figures S1 and S2.
p21 is a major downstream target of p53. Its expression in astrocytes is also dependent on p53 (Figure S2E). To examine the role of p21 in the reprogramming process, we injected SOX2-expressing virus into the spinal cord of adult p21 knockout (KO) mice. When examined at 4 wpi and compared to the wild type controls, the number of DCX+ cells was nearly doubled in the p21 KO mice (Figure 1D, E). Together, these results demonstrated a critical role of the p53-p21 pathway in controlling the SOX2-mediated generation of iANBs.
The p53 pathway promotes cell cycle exit of spinal iANBs
In response to stresses or insults, p53 activation induces cell cycle arrest or apoptosis to safeguard cells from undergoing uncontrolled proliferation (Lane and Levine, 2010). We examined iANB apoptosis by staining for activated caspase 3 and did not find a significant difference in the spinal cord with or without p53 downregulation by shRNA or in p21 KO mice when compared to wild type controls (Figure S3A, B). To further examine cell apoptosis, we performed Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling (TUNEL) assays. A few TUNEL+ cells were detected in spinal cords injected with the lentivirus expressing SOX2 and the control shRNA or shRNA-p53, although none of them were DCX+ (Figure S3C, D). A small but significant increase of TUNEL+ cells was observed in the group with shRNA-p53 only at 3 wpi but not at 4 wpi, suggesting that down-regulation of p53 did not enhance cell survival.
During adult neurogenesis, DCX+ neuroblasts consist of both early proliferating and later non-proliferating cells in the endogenous neurogenic niches (Lugert et al., 2012). A fraction of iANBs was also shown to be proliferative in the adult brain. We examined iANB proliferation in the adult spinal cord by staining for KI67, a nuclear protein present in all phases of the cell cycle but absent in quiescent cells (Scholzen and Gerdes, 2000). A time-course analysis showed that proliferation of SOX2-dependent iANBs was significantly decreased from 20% at 1 wpi to 5% at 4 wpi in the wild type spinal cord (Figure 2A). When p53 was conditionally deleted by Cre expression or downregulated through shRNAs, a roughly 3-fold increase in proliferating iANBs was observed when compared to controls at 4 wpi (Figures 2B–D).
Figure 2. The p53 pathway promotes cell cycle exit of iANBs.
(A) The ratio of proliferating iANBs decreases with time (mean±s.d.; n=5 mice per group; *p=0.03 and **p<0.01 by Student’s t-test).
(B) Conditional p53 deletion leads to increased ratio of proliferating iANBs. Cells were quantified in the virus-injected spinal regions at 4 wpi (mean+s.d.; n=4 mice per group; **p<0.01 by Student’s t-test).
(C) Immunohistochemistry showing influence of p53 status on iANB proliferation at 4 wpi. Scale bar, 50 μm.
(D) Knockdown of p53 expression increases ratio of proliferating iANBs when examined at 4 wpi (mean±s.d.; n=4–6 mice per group; ****p<0.0001 by Student’s t-test; Ctrl, control shRNA).
(E) The ratio of proliferating iANBs is enhanced in p21 KO mice when examined at 4 wpi (mean±s.d.; n=4 mice per group; **p=0.004 by Student’s t-test).
(F) Confocal images of proliferating iANBs in wild type (WT) or p21 KO mice. Scale bar, 50 μm.
(G) An experimental scheme to analyze cell proliferation and cell cycle exit. IHC, immunohistochemistry.
(H) Confocal images of iANBs traced by BrdU-incorporation in p21 KO mice. Scale bar, 50 μm.
(I) Confocal images of proliferating BrdU-traced iANBs at 4 wpi. Scale bar, 50 μm.
(J) More iANBs remain in cell cycle in the spinal cord of adult p21 KO mice (mean±s.d.; n=4 mice per group; ***p=0.001 by Student’s t-test).
See also Figure S3.
Similarly, immunohistochemistry analysis showed that p21 deletion resulted in a dramatic increase of proliferating iANBs: from 5% in wild type mice to nearly 30% in p21 KO mice (Figure 2E–F). To further characterize cell proliferation, we treated mice with BrdU-containing drinking water for 3 days starting at 3 wpi (Figure 2G). Over 90% of iANBs were labeled by BrdU in adult p21 KO mice when examined at 4 wpi (Figure 2H). Cell cycle exit of iANBs was determined by quantifying the fraction of KI67+BrdU+DCX+ cells among the total BrdU+DCX+ cells (Figure 2I). The p21 KO mice showed a 22.5% increase of iANBs remaining in cell cycle when compared to wild type controls (Figure 2J). Together, these data show that the p53-p21 pathway tightly regulates iANB production by restricting proliferation and cell cycle exit.
Reprogramming efficiency is not affected by the p53 pathway
The p53 pathway was shown to be a roadblock during reprogramming of somatic cells to pluripotency. Its silencing significantly increases the reprogramming efficiency of fibroblasts to pluripotent stem cells (Banito et al., 2009; Hanna et al., 2009; Hong et al., 2009; Kawamura et al., 2009; Li et al., 2009; Marion et al., 2009; Utikal et al., 2009). We examined whether the p53 pathway has a similar effect on SOX2-mediated in vivo reprogramming efficiency, which may contribute to the overall production of iANBs.
Our previous genetic lineage mapping showed that SOX2 initially reprograms astrocytes to ASCL1+ progenitors, which further differentiate into DCX+ iANBs and mature neurons (Niu et al., 2015). Therefore, the induction of ASCL1+ progenitors can be used to estimate the in vivo reprogramming efficiency. SOX2 virus was injected into the spinal cord of adult wild type or p21 KO mice, which were examined at 3 wpi. ASCL1+ cells were robustly induced; however, no difference in their number was detected between p21 KO and wild type control mice (Figure 3A–B). KI67 staining and quantification also failed to reveal any significant difference in progenitor proliferation between these groups (Figure 3C).
Figure 3. The p53 pathway specifically targets later reprogramming steps.
(A) Immunohistochemical analysis of SOX2-induced ASCL1+ progenitors at 3 wpi. Cell proliferation were analyzed by KI67 staining. Scale bar, 50 μm.
(B) p21 status has no effect on the number of SOX2-induced ASCL1+ progenitors (mean±s.d.; n=3 mice per group; n.s., not significant by Student’s t-test).
(C) p21 status has no effect on proliferation of ASCL1+ progenitors (mean±s.d.; n=3 mice per group; n.s., not significant by Student’s t-test).
(D) Immunohistochemical analysis of induced ASCL1+ progenitors transitioning to DCX+ neuroblasts. Scale bar, 50 μm.
(E) p21 status has no effect on the transition of progenitors to early ASCL1+DCX+ neuroblasts (mean±s.d.; n=3 mice per group; n.s., not significant by Student’s t-test).
(F) p21 restricts overall production of later neuroblasts (mean±s.d.; n=3 mice per group; **p=0.003 by Student’s t-test).
ASCL1+ progenitors gradually transited to early DCX+ neuroblasts, which expressed both markers (Figure 3D). Quantification of this ASCL1+DCX+ subgroup among all progenitors did not reveal any influence by p21 deletion (Figure 3E). In contrast, the ratio of ASCL1+DCX+ cells to total DCX+ cells was much lower in the p21 KO group (Figure 3F) consistent with a dramatic increase in DCX+ iANBs caused by silencing p21 (Figure 1E). Together, these results suggest that the p53 pathway does not have a major impact on the induction of ASCL1+ progenitors, their proliferation, or their transition to early DCX+ iANBs.
Spinal iANBs are halted at an immature stage
Further maturation of iANBs is important for functional integration within a neuronal network. As SOX2-mediated in vivo reprogramming passes through a proliferative stage, we used BrdU in drinking water to trace the fate of iANBs (Figure 4A). Mature neurons were identified by staining for RBFOX3 (also known as NEUN), a marker tightly associated with neuron differentiation and maturation (Mullen et al., 1992). When examined at 8 wpi, BrdU+RBFOX3+ cells were observed in SOX2 virus-injected spinal regions of both wild type and p21 KO mice (Figure 4B, C); however, quantification failed to show any increase by silencing p21 expression.
Figure 4. iANBs are arrested at an immature stage.
(A) Experimental schemes for analysis of reprogrammed spinal neurons. Both BrdU and valproic acid (VPA) were supplied in drinking water.
(B) Confocal images of new neurons with expression of RBFOX3, a marker for neuronal maturation. Scale bar, 50 μm.
(C) Reprogrammed neurons rarely become mature in p21 KO mice (mean±s.d.; n=3–4 mice per group; n.s., not significant; *p=0.03 by Student’s t-test).
(D) Immunohistochemical analysis of iANBs at 8 wpi. Scale bar, 50 μm.
(E) p21-deletion results in iANBs remained at an immature stage (mean±s.d.; n=3–4 mice per group; **p=0.006 and ***p=0.0002 by Student’s t-test).
A separate cohort of mice was also treated with valproic acid (VPA) in drinking water for 4 weeks to promote neuronal maturation (Figure 4A). Consistent with our previous results (Niu et al., 2013; Su et al., 2014), VPA treatment resulted in nearly 6,000 BrdU+RBFOX3+ cells in SOX2 virus-injected spinal regions of wild type mice; however, this number of cells was not observed in p21 KO mice (Figure 4C).
Such a low number of mature neurons in p21 KO mice might be due to a loss of iANBs during the maturation process. Immunohistochemistry was conducted to determine whether iANBs were detectable at 8 wpi. Unexpectedly, a significantly larger number of DCX+ iANBs were still observed in mice with p21 deletion (Figure 4D, E). Continuous VPA treatment in drinking water showed a minimal effect on the number of iANBs. Together, these results indicate that iANBs, though robustly generated, are halted at an immature state after silencing the p53-p21 pathway.
A neurotrophic milieu promotes maturation of spinal iANBs
During neural development, neurotrophic factors are critically important for neuronal survival and maturation. A neurotrophic milieu suitable for neuron maturation may not persist in the adult spinal cord. By using a lentiviral delivery approach, we conducted a candidate screen for factors that might enhance the maturation of SOX2-induced spinal iANBs. These included GDNF, FGF2, BDNF, and NOG. Once again, continuous BrdU treatments in drinking water were employed to trace adult-born neurons, which were examined at 8 wpi (Figure 5A).
Figure 5. Maturation of iANBs requires a neurotrophic milieu.
(A) A diagram to show experimental design. Viruses encoding SOX2 and neurotrophic factors were injected into the spinal cord of adult mice. IHC, immunohistochemistry.
(B) Neuronal maturation is greatly enhanced by neurotrophic factors (mean±s.d.; n=3–5 mice per group; F(1,26)=5.180 and p=0.031 for genotype effect, F(3,26)=6.325 and p=0.002 for treatment effect, F(3,26)=3.532 and p=0.029 for genotype-treatment interactions by two-way ANOVA; **p=0.0092 and ***p=0.0008 by post hoc Tukey’s multiple comparisons test). Ctrl, empty virus as a control.
(C) Confocal images of reprogrammed cells expressing a marker for mature neurons at 8 wpi. Scale bar, 50 μm.
(D) p53 knockdown promotes generation of SOX2-induced neurons under a neurotrophic milieu (mean±s.d.; n=5 mice per group; **p<0.004 by Student’s t-test).
(E) SOX2-induced neurons express multiple markers for mature neurons. Confocal images were taken from the virus-injected white matter. Scale bar, 50 μm.
(F) SOX2-induced neurons exhibit robust expression of the synaptic protein SYN1. Confocal images were taken from the virus-injected white matter. Scale bar, 50 μm.
The presence of either GDNF or FGF2 increased the number of BrdU+RBFOX3+ cells to roughly 6,000 in injected regions of the p21 KO spinal cord, while these two factors had minimal effect in the wild type background (Figure 5B). On the other hand, wild type mice responded to treatment with BDNF-NOG and showed about 7,000 BrdU+RBFOX3+ cells surrounding the SOX2 virus-injected spinal regions. The p21 KO mice had the most striking response to BDNF-NOG, with nearly 30,000 BrdU+RBFOX3+ cells detected in the penumbra of injected regions (Figure 5B, C). In addition to the gray matter, these cells were also robustly detectable in the white matter where the somas of endogenous neurons are not normally present (Figure 5C). The effect of BDNF-NOG on iANB maturation was also confirmed in wild type mice with shRNA-mediated knockdown of p53 (Figure 5D). Of note, BrdU+RBFOX3+ cells were undetectable in control mice when SOX2-expressing virus was not injected.
Treatments with BDNF-NOG also promoted BrdU+ new neurons to express MAP2, another well-established marker for mature neurons (Dehmelt and Halpain, 2005) (Figure 5E). Staining for SYN1, a presynaptic vesicle-associated protein, showed punctate patterns in the processes and somas of adult-born neurons indicating synaptic connections with local neurons (Figure 5F). Many of these new neurons could be detected in the white matter with potential to readily form circuits with ascending and descending axons.
Spinal iANBs mature into diverse neuronal subtypes
To facilitate subtype analysis of astrocyte-converted neurons, we created the mGfap-Cre;Thy1-STOP-YFP (GcTy) mice (Figure 6A). The mGfap-Cre transgenic line 77.6 exhibits astrocyte-restricted Cre recombinase expression under the mouse Gfap promoter in the brain and spinal cord (Gregorian et al., 2009; Su et al., 2014). In the Thy1-STOP-YFP reporter line, YFP expression is under the neuron-specific regulator elements of the mouse Thy1 promoter after Cre-mediated removal of the STOP cassette (Buffelli et al., 2003). The GcTy mice showed weak but restricted YFP expression in spinal astrocytes (Figure S4). Nonetheless, very few sparsely distributed YFP+ cells (<0.01%) were also RBFOX3+ found in the dorsal horns but not ventral regions (Figure S4); we therefore limited our analysis to the ventral spinal cord to exclude any potential contamination from endogenous neurons. Reprogramming factors were mainly delivered into the ventral spinal cord of adult GcTy mice. Immunohistochemistry was performed at 8–24 wpi.
Figure 6. iANBs mature into diverse neuronal subtypes.
(A) A genetic approach to trace astrocyte-converted neurons.
(B) Robust labeling of SOX2-dependent iANBs with the genetic reporter YFP. Scale bar, 50 μm.
(C–H) Confocal images of astrocyte-converted neurons with expression of the indicated markers. A typical cell is marked by an arrow, whereas arrowheads indicate punctate patterns of marker staining. Scale bar, 20 μm
(I) Quantification of subtypes of astrocyte-converted neurons (mean±s.d.; n=3 mice per group; n.d., not detected).
See also Figures S4 and S5.
In GcTy mice, SOX2-reprogrammed DCX+ iANBs were robustly labeled by YFP confirming an astrocyte-origin (Figure 6B). Clustered YFP+ cells expressing markers for mature neurons, such as MAP2 and RBFOX3, could be detected in both the white matter and gray matter (Figure S5A, B). These YFP+ cells were not observed in control virus-injected GcTy mice. Over 99.9% of the YFP-traced neurons also robustly expressed SYN1 with a punctate pattern (Figure S5C) indicating synaptic connections with local neurons. An extensive survey of neuronal subtypes showed astrocyte-converted neurons were diverse, including GAD6+ and GABA+ GABAergic neurons (Figure 6C, S5D), GLYT2+ glycinergic neurons (Figure 6D), 5-HT+ serotonergic neurons (Figure 6E), VGLUT2+ glutamatergic neurons (Figure 6F), CALB+ neurons (Figure 6G), and cholinergic neurons (Figure 6H). Quantification showed that the predominant subtype was glutamatergic, which represented over 80% of the reprogrammed neurons, while the other subtypes were below 20% or rarely observed (Figure 6I).
Robust generation of new neurons after contusion injury
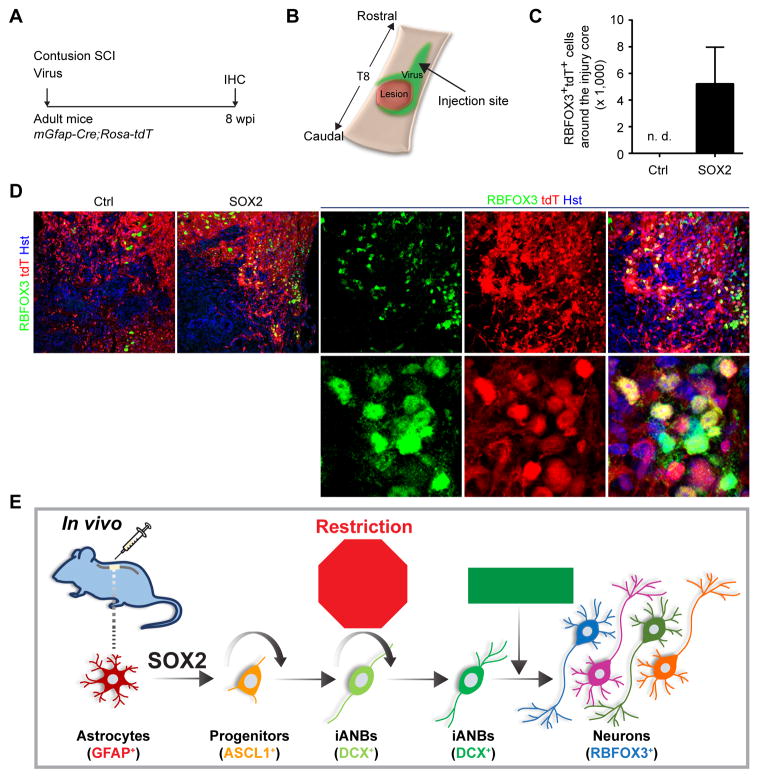
We next examined whether new neurons can be reprogrammed from reactive astrocytes after contusion injury, which is the most clinically relevant SCI type. Astrocytes were genetically traced in mGfap-Cre;Rosa-tdT mice in which the expression of tdTomato (tdT) is under the control of a CAG promoter after Cre-mediated deletion of a STOP cassette (Madisen et al., 2010). In vivo reprogramming factors were delivered through virus into the penumbra of the injury site immediately after contusion injury (Figure 7A, B). Astrocyte-converted mature neurons were examined by RBFOX3 staining and tdT fluorescence at 8 wpi. In control virus-injected mice with contusion SCI, RBFOX3+ neurons were detected in the gray matter and none were labeled with tdT confirming the glial specificity of the lineage tracing reporter even under severe injury conditions (Figure 7C, D). In sharp contrast, nearly 6,000 tdT+RBFOX3+ cells were detected surrounding the lesion core in mice injected with the reprogramming factors (Figure 7C, D). The somas of many newly reprogrammed neurons were in the white matter, a feature not observed for endogenous neurons.
Figure 7. Robust induction of new neurons after contusion injury.
(A) An experimental scheme to examine astrocyte-converted neurons in vivo.
(B) A diagram for contusion injury and virus injection.
(C) Quantification of astrocyte-converted neurons surrounding the lesion site (mean±s.d.; n=3 mice per group; n.d., not detected; Ctrl, control virus).
(D) Confocal images of astrocyte-converted neurons surrounding the lesion core. Enlarged views of the boxed regions are also shown. Arrowheads show typical astrocyte-converted neurons. Scale bar, 20 μm.
(E) A molecular and cellular roadmap from resident astrocytes to mature neurons in the adult spinal cord. The p53-p21 pathway constitutes a checkpoint restricting expansion of iANBs.
DISCUSSION
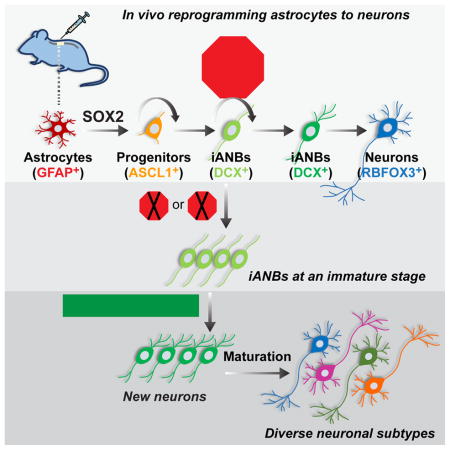
A lack of neurogenesis in the adult spinal cord may contribute to the failure of functional recovery after SCI. We now provide a molecular and cellular roadmap for robust generation of new spinal neurons after a mild or severe contusion SCI. This entails removing an inhibitory checkpoint during reprogramming of resident glial cells to mature neurons in vivo (Figure 7E).
The ectopic expression of SOX2 is sufficient to reprogram resident glial cells to neurons in the adult central nervous system (Heinrich et al., 2014; Niu et al., 2015; Niu et al., 2013; Su et al., 2014). Nonetheless, the number of reprogrammed neurons was low in the adult spinal cord (Su et al., 2014). This urged a better understanding of the reprogramming process and an improved strategy for the robust induction of new spinal neurons essential to regeneration after SCI. Among a score of factors examined in vivo, the p53-p21 pathway was uniquely revealed to be an essential checkpoint for SOX2-mediated reprogramming of spinal astrocytes in vivo. Silencing this pathway, either through genetic deletion or transient knockdown, led to a significant increase in iANB generation, which can further give rise to mature neurons in a neurotrophic milieu. Although enhanced iANB production may elevate the risk of tumorigenesis, we failed to detect any spinal tumors 15 months after p53 was conditionally silenced. Consistent with our previous results showing the number of iANBs is greatly reduced in a time-dependent manner (Niu et al., 2013), iANBs were rarely observed when examined around 6 months post virus-injection in the adult spinal cord. Additionally, transient inhibition of the p53 pathway may be achieved by the small molecule inhibitor Pifithrin-α (Komarov et al., 1999), or by AAV-mediated expression of shRNAs.
The p53 pathway is activated by reprogramming factors and acts as a roadblock to reprogramming somatic cells to pluripotency. Silencing this pathway in culture can significantly increase reprogramming efficiency for both formation of induced pluripotent stem cells and transdifferentiation of fibroblasts to induced neurons (Banito et al., 2009; Guo et al., 2014a; Hanna et al., 2009; Hong et al., 2009; Jiang et al., 2015; Kawamura et al., 2009; Li et al., 2009; Marion et al., 2009; Utikal et al., 2009; Zhao et al., 2008). In contrast, using the induction of ASCL1+ progenitors to estimate reprogramming efficiency, we showed that silencing the p53-p21 pathway has little effect on the early steps of SOX2-mediated in vivo reprogramming. Both the induction and proliferation of ASCL1+ progenitors was not significantly affected by p21 deletion. These results may not be unexpected, as we and others have previously shown that p21 expression in neural stem/progenitor cells is normally suppressed by robust expression of the orphan nuclear receptor TLX (Li et al., 2012; Niu et al., 2011; Sun et al., 2007). Accompanying naturally diminished TLX expression in DCX+ neuroblasts (Li et al., 2012), p21 expression is increased in these cells and in newly developing neurons (Pechnick et al., 2008). Consistent with a positive role in cell cycle exit, p21 deletion leads to greatly increased proliferation of both endogenous and SOX2-induced neuroblasts ((Pechnick et al., 2008) and this study). Together, these data indicate that SOX2-mediated in vivo reprogramming passes through a multistep cellular process, which resembles that of endogenous adult neurogenesis and is subject to a tight molecular regulation.
A permissive microenvironment, also known as neurogenic niche, is critical for neuronal survival and maturation during adult neurogenesis in the lateral ventricle and dentate gyrus (Jessberger and Gage, 2014; Kempermann et al., 2015; Lim and Alvarez-Buylla, 2016). The niche consists of not only cellular contexts and extracellular matrix but also secreted humoral factors. Our results indicate that an appropriate niche is also critical for further differentiation of iANBs into mature neurons in the otherwise non-neurogenic adult spinal cord. Although silencing the p53 pathway creates a large population of iANBs, only a fraction can become mature under normal or injured conditions. Our further in vivo screens revealed that locally secreted BDNF and NOG constitute important niche factors supporting robust generation of newly mature spinal neurons from iANBs. These induced neurons are often in clusters and can be established in both the white matter and gray matter. Such broader and clustered distribution may provide more opportunities for the induced neurons to form connections between themselves, with preexisting local neurons, and with descending and ascending spinal axons. Although a detailed electrophysiological analysis is needed for confirmation, the robust detection of punctate SYN1 expression in induced neurons is indicative of their ability to form synaptic connections.
The local microenvironment and/or intrinsic properties of the originating resident astrocytes are also instructive for the formation of region-specific neuronal subtypes from astrocyte-converted iANBs. iANBs in the adult spinal cord do not generate CR+ GABAergic interneurons, which are the major subtype of induced neurons in the adult striatum (Niu et al., 2015). In contrast, VGLUT2+ glutamatergic neurons are the predominant subtype of induced neurons in the adult spinal cord. Endogenous VGLUT2+ neurons are distributed throughout the spinal gray matter (Llewellyn-Smith et al., 2007). They were recently shown to be essential components of the locomotor circuitry and play an essential role for proper organization of the spinal locomotor network (Borgius et al., 2014). As such, it is conceivable that the iANB-generated VGLUT2 excitatory neurons may well be capable of forming relay circuits with ascending and descending motor pathways that are frequently disrupted by SCI (Abematsu et al., 2010; Courtine et al., 2008). Such relays, if confirmed, may contribute to functional motor recovery after SCI. In addition to glutamatergic neurons, 10–20% of the induced neurons are GABAergic, glycinergic, or serotonergic. These neuronal subtypes are thought to be essential in coordinating muscle activation during locomotion (Nishimaru and Kakizaki, 2009).
Our ability to successfully produce a large population of long-lived and diverse subtypes of new neurons in the adult spinal cord provides a cellular basis for regeneration-based therapy for SCI. Compared to cell transplantation-based approaches, in vivo reprogrammed neurons from resident glial cells are immune homologous to the host. Therefore, the reprogrammed neurons will not require immune suppression for survival and integration into the local neuronal network. Future studies are warranted to investigate their biological function after SCI.
METHODS
Animals
Wild type C57BL/6J mice and the following mutant mouse lines were obtained from The Jackson Laboratory: Ptenflox (stock # 004597) (Groszer et al., 2001), p53flox (stock # 008462) (Marino et al., 2000), p21 KO (stock # 003263) (Brugarolas et al., 1995), mGfap-Cre line 77.6 (stock # 024098) (Gregorian et al., 2009), Thy1-STOP-YFP (stock # 005630) (Buffelli et al., 2003), and Rosa-tdT (stock # 007914) (Madisen et al., 2010). Unless otherwise stated, both male and female mice at 8 weeks and older were used for all experiments. All mice were housed under a controlled temperature and a 12-h light/dark cycle with free access to water and food in an animal facility at UT Southwestern. The sample sizes were empirically determined. The experiments were not randomized and the researchers were not blinded to the allocation of animals during experiments and outcome assessment. All experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee at UT Southwestern.
Virus preparation and intraspinal injection
Lentivirus was used to deliver SOX2, GFP-T2A-Cre, Bdnf, Nog, shRNA, and the other listed genes into the adult spinal cord as previously described (Niu et al., 2013; Su et al., 2014). Gene expression was under the control of a modified human GFAP promoter, which was shown to mainly target astrocytes in the mouse spinal cord (Su et al., 2014). Briefly, the third generation replication-deficient lentivirus was generated by transient transfections of HEK293T cells with lentiviral vectors together with the packaging plasmids (pMDL, VSV-G, and pREV). The virus-containing culture supernatants were collected and concentrated by centrifugation. Viral titers were estimated by quantifying GFP-expressing cells or by immunofluorescence staining of virus-transduced U251 glioma cells at 72 h after viral infection. Using a 5 μL Hamilton syringe and a 34 gauge, 18 degree-beveled needle (Hamilton, Reno, NV), 1.5 μL of lentivirus (0.5–1 × 109 pfu/mL) was manually injected into the spinal parenchyma at each of the two locations 2 mm apart at the T8 level. The injection rate was maintained at 0.3 μL/min; and 1 min was taken to slowly withdraw the needle upon completion of the injection.
BrdU and VPA administration
When appropriate, mice were administered BrdU (B5002, Sigma; 0.5 g/L) and/or VPA (P4543, Sigma; 4 g/L) in drinking water for the indicated duration.
Contusion spinal cord injury
Adult mice were anesthetized and a laminectomy was performed at the T7–9 segments. The exposed spinal cord was subjected to a contusion injury that was introduced by using the IH impactor (Precision Systems and Instrumentation, Lexington, KY) with a 1-mm tip and a force of 60 kdyn.
Immunohistochemistry
Mice were euthanized and were perfused with intracardial injection of 4% (w/v) paraformaldehyde in phosphate-buffered saline (PBS). Spinal cords were isolated and post-fixed overnight with 4% (w/v) paraformaldehyde at 4°C. After cryoprotection with 30% sucrose in PBS for 48 h at 4 °C, transverse sections or 1.5-cm longitudinal sections of spinal cords spanning the injection/injury sites were collected on a cryostat (Leica) set at 20-μm thickness. The primary antibodies used for immunofluorescence were listed in Supplementary Table S1. When mouse primary antibodies were used, tissue sections were pretreated to block non-specific staining. Specifically, the sections were incubated with 50% formamide in 1 X SSC buffer for 1.5 h at 65 °C. For BrdU staining, the sections wer e pretreated with 2N HCl for 30 min at 37 °C. The corresponding secondary antibodies co njugated with Alexa Fluor 488, 555 or 647 dye (Jackson ImmunoResearch Laboratories) were applied at 1:2,000 dilution for indirect fluorescence. Nuclei were counterstained with Hoechst 33342 (Hst). Images were captured and examined by using a Zeiss LSM 700 confocal microscope.
TUNEL assay
TUNEL assay was performed on cryostat sections by using the R&D Systems TACS TdT In Situ Apoptosis Detection Kit (Fisher Scientific). TUNEL positive controls were set up with TACS-Nuclease provided in the kit. All TUNEL-positive cells surrounding the injection area were counted on each section.
Statistical analysis
The quantification data were expressed as mean ± s.d. from four to six mice. Statistical analysis was performed by ANOVA and Tukey’s post hoc multiple comparisons or the unpaired Student’s t-tests where appropriate. Differences were considered statistically significant at P<0.05.
Supplementary Material
Acknowledgments
We thank members of the Zhang laboratory for discussions and reagents. We also thank Jane Johnson (UT Southwestern, USA) for providing the ASCL1 antibody and Michael Brenner (University of Alabama at Birmingham, USA) for providing the hGFAP promoter. C-.L.Z. is a W. W. Caruth, Jr. Scholar in Biomedical Research. This work was supported by the Welch Foundation Award (I-1724), Texas Institute for Brain Injury and Repair, the Decherd Foundation, the Mobility Foundation, NIH Grants (NS070981, NS088095, NS092616, 1DP2OD006484, and NS093502 to C.-L.Z.), and the National Key Research and Development Program of China (2016YFA0100802 to Z.S.).
Footnotes
AUTHOR CONTRIBUTIONS
L.W., Z.S. and C.-L.Z. conceived and designed the experiments. L.W., Z.S., and Y.Z. performed the experiments. W.T. and X.-M.X. provided critical reagents and technical inputs. L.W. and C.-L.Z. analyzed data and prepared the manuscript. All authors reviewed and approved the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abematsu M, Tsujimura K, Yamano M, Saito M, Kohno K, Kohyama J, Namihira M, Komiya S, Nakashima K. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J Clin Invest. 2010;120:3255–3266. doi: 10.1172/JCI42957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banito A, Rashid ST, Acosta JC, Li S, Pereira CF, Geti I, Pinho S, Silva JC, Azuara V, Walsh M, et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 2009;23:2134–2139. doi: 10.1101/gad.1811609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgius L, Nishimaru H, Caldeira V, Kunugise Y, Low P, Reig R, Itohara S, Iwasato T, Kiehn O. Spinal glutamatergic neurons defined by EphA4 signaling are essential components of normal locomotor circuits. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:3841–3853. doi: 10.1523/JNEUROSCI.4992-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, McMahon SB. Spinal cord repair strategies: why do they work? Nature reviews Neuroscience. 2006;7:644–653. doi: 10.1038/nrn1964. [DOI] [PubMed] [Google Scholar]
- Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- Buffelli M, Burgess RW, Feng G, Lobe CG, Lichtman JW, Sanes JR. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424:430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- Chi L, Ke Y, Luo C, Li B, Gozal D, Kalyanaraman B, Liu R. Motor neuron degeneration promotes neural progenitor cell proliferation, migration, and neurogenesis in the spinal cords of amyotrophic lateral sclerosis mice. Stem cells. 2006;24:34–43. doi: 10.1634/stemcells.2005-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmelt L, Halpain S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005;6:204. doi: 10.1186/gb-2004-6-1-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- Gregorian C, Nakashima J, Le Belle J, Ohab J, Kim R, Liu A, Smith KB, Groszer M, Garcia AD, Sofroniew MV, et al. Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:1874–1886. doi: 10.1523/JNEUROSCI.3095-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- Guo S, Zi X, Schulz VP, Cheng J, Zhong M, Koochaki SH, Megyola CM, Pan X, Heydari K, Weissman SM, et al. Nonstochastic reprogramming from a privileged somatic cell state. Cell. 2014a;156:649–662. doi: 10.1016/j.cell.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell stem cell. 2014b;14:188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Saha K, Pando B, van Zon J, Lengner CJ, Creyghton MP, van Oudenaarden A, Jaenisch R. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. 2009;462:595–601. doi: 10.1038/nature08592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich C, Bergami M, Gascon S, Lepier A, Vigano F, Dimou L, Sutor B, Berninger B, Gotz M. Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem cell reports. 2014;3:1000–1014. doi: 10.1016/j.stemcr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horky LL, Galimi F, Gage FH, Horner PJ. Fate of endogenous stem/progenitor cells following spinal cord injury. The Journal of comparative neurology. 2006;498:525–538. doi: 10.1002/cne.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, Thal LJ, Gage FH. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MM, Smith DK, Niu W, Fang S, Iqbal N, Sun G, Shi Y, Zhang CL. Enhancer Analysis Unveils Genetic Interactions between TLX and SOX2 in Neural Stem Cells and In Vivo Reprogramming. Stem cell reports. 2015 doi: 10.1016/j.stemcr.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Gage FH. Adult neurogenesis: bridging the gap between mice and humans. Trends Cell Biol. 2014;24:558–563. doi: 10.1016/j.tcb.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Jiang H, Xu Z, Zhong P, Ren Y, Liang G, Schilling HA, Hu Z, Zhang Y, Wang X, Chen S, et al. Cell cycle and p53 gate the direct conversion of human fibroblasts to dopaminergic neurons. Nature communications. 2015;6:10100. doi: 10.1038/ncomms10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A, Wahl GM, Izpisua Belmonte JC. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460:1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Song H, Gage FH. Neurogenesis in the Adult Hippocampus. Cold Spring Harb Perspect Biol. 2015;7:a018812. doi: 10.1101/cshperspect.a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- Lane D, Levine A. p53 Research: the past thirty years and the next thirty years. Cold Spring Harb Perspect Biol. 2010;2:a000893. doi: 10.1101/cshperspect.a000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Collado M, Villasante A, Strati K, Ortega S, Canamero M, Blasco MA, Serrano M. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460:1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Sun G, Murai K, Ye P, Shi Y. Characterization of TLX expression in neural stem cells and progenitor cells in adult brains. PLoS One. 2012;7:e43324. doi: 10.1371/journal.pone.0043324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Alvarez-Buylla A. The Adult Ventricular-Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) Neurogenesis. Cold Spring Harb Perspect Biol. 2016 doi: 10.1101/cshperspect.a018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Miao Q, Yuan J, Han S, Zhang P, Li S, Rao Z, Zhao W, Ye Q, Geng J, et al. Ascl1 Converts Dorsal Midbrain Astrocytes into Functional Neurons In Vivo. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35:9336–9355. doi: 10.1523/JNEUROSCI.3975-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Martin CL, Fenwick NM, Dicarlo SE, Lujan HL, Schreihofer AM. VGLUT1 and VGLUT2 innervation in autonomic regions of intact and transected rat spinal cord. J Comp Neurol. 2007;503:741–767. doi: 10.1002/cne.21414. [DOI] [PubMed] [Google Scholar]
- Lugert S, Vogt M, Tchorz JS, Muller M, Giachino C, Taylor V. Homeostatic neurogenesis in the adult hippocampus does not involve amplification of Ascl1(high) intermediate progenitors. Nature communications. 2012;3:670. doi: 10.1038/ncomms1670. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature neuroscience. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 2000;14:994–1004. [PMC free article] [PubMed] [Google Scholar]
- Marion RM, Strati K, Li H, Murga M, Blanco R, Ortega S, Fernandez-Capetillo O, Serrano M, Blasco MA. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460:1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nishimaru H, Kakizaki M. The role of inhibitory neurotransmission in locomotor circuits of the developing mammalian spinal cord. Acta Physiol (Oxf) 2009;197:83–97. doi: 10.1111/j.1748-1716.2009.02020.x. [DOI] [PubMed] [Google Scholar]
- Niu W, Zang T, Smith DK, Vue TY, Zou Y, Bachoo R, Johnson JE, Zhang CL. SOX2 reprograms resident astrocytes into neural progenitors in the adult brain. Stem cell reports. 2015;4:780–794. doi: 10.1016/j.stemcr.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, Zhang CL. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nature cell biology. 2013;15:1164–1175. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu W, Zou Y, Shen C, Zhang CL. Activation of postnatal neural stem cells requires nuclear receptor TLX. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:13816–13828. doi: 10.1523/JNEUROSCI.1038-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohori Y, Yamamoto S, Nagao M, Sugimori M, Yamamoto N, Nakamura K, Nakafuku M. Growth factor treatment and genetic manipulation stimulate neurogenesis and oligodendrogenesis by endogenous neural progenitors in the injured adult spinal cord. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:11948–11960. doi: 10.1523/JNEUROSCI.3127-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechnick RN, Zonis S, Wawrowsky K, Pourmorady J, Chesnokova V. p21Cip1 restricts neuronal proliferation in the subgranular zone of the dentate gyrus of the hippocampus. Proc Natl Acad Sci U S A. 2008;105:1358–1363. doi: 10.1073/pnas.0711030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Schwab M, Schwartz M, Fehlings MG. Spinal cord injury: time to move? The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:11782–11792. doi: 10.1523/JNEUROSCI.3444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Sellers DL, Maris DO, Horner PJ. Postinjury niches induce temporal shifts in progenitor fates to direct lesion repair after spinal cord injury. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:6722–6733. doi: 10.1523/JNEUROSCI.4538-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, Ziv Y, Schwartz M. New GABAergic interneurons supported by myelin-specific T cells are formed in intact adult spinal cord. Stem cells. 2007;25:2277–2282. doi: 10.1634/stemcells.2006-0705. [DOI] [PubMed] [Google Scholar]
- Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Niu W, Liu ML, Zou Y, Zhang CL. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nature communications. 2014;5:3338. doi: 10.1038/ncomms4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Yu RT, Evans RM, Shi Y. Orphan nuclear receptor TLX recruits histone deacetylases to repress transcription and regulate neural stem cell proliferation. Proc Natl Acad Sci U S A. 2007;104:15282–15287. doi: 10.1073/pnas.0704089104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nature reviews Neuroscience. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- Torper O, Ottosson DR, Pereira M, Lau S, Cardoso T, Grealish S, Parmar M. In Vivo Reprogramming of Striatal NG2 Glia into Functional Neurons that Integrate into Local Host Circuitry. Cell Rep. 2015;12:474–481. doi: 10.1016/j.celrep.2015.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torper O, Pfisterer U, Wolf DA, Pereira M, Lau S, Jakobsson J, Bjorklund A, Grealish S, Parmar M. Generation of induced neurons via direct conversion in vivo. Proc Natl Acad Sci U S A. 2013;110:7038–7043. doi: 10.1073/pnas.1303829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utikal J, Polo JM, Stadtfeld M, Maherali N, Kulalert W, Walsh RM, Khalil A, Rheinwald JG, Hochedlinger K. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 2009;460:1145–1148. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessal M, Aycock A, Garton MT, Ciferri M, Darian-Smith C. Adult neurogenesis in primate and rodent spinal cord: comparing a cervical dorsal rhizotomy with a dorsal column transection. Eur J Neurosci. 2007;26:2777–2794. doi: 10.1111/j.1460-9568.2007.05871.x. [DOI] [PubMed] [Google Scholar]
- Yang H, Lu P, McKay HM, Bernot T, Keirstead H, Steward O, Gage FH, Edgerton VR, Tuszynski MH. Endogenous neurogenesis replaces oligodendrocytes and astrocytes after primate spinal cord injury. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:2157–2166. doi: 10.1523/JNEUROSCI.4070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yin X, Qin H, Zhu F, Liu H, Yang W, Zhang Q, Xiang C, Hou P, Song Z, et al. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell stem cell. 2008;3:475–479. doi: 10.1016/j.stem.2008.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.